








生物技术通报 ›› 2025, Vol. 41 ›› Issue (11): 62-74.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0032
张纪娇1,2( ), 王会颖2, 房欢2,3,4,5(
), 王会颖2, 房欢2,3,4,5( ), 张大伟1,2,3,4,5,6(
), 张大伟1,2,3,4,5,6( )
)
收稿日期:2025-01-08
出版日期:2025-11-26
发布日期:2025-12-09
通讯作者:
房欢,男,博士,副研究员,研究方向 :维生素B12生物合成;E-mail: fang_h@tib.cas.cn作者简介:张纪娇,女,硕士研究生,研究方向 :维生素B12生物合成;E-mail: zhangjijiao@tib.cas.cn
基金资助:
ZHANG Ji-jiao1,2( ), WANG Hui-ying2, FANG Huan2,3,4,5(
), WANG Hui-ying2, FANG Huan2,3,4,5( ), ZHANG Da-wei1,2,3,4,5,6(
), ZHANG Da-wei1,2,3,4,5,6( )
)
Received:2025-01-08
Published:2025-11-26
Online:2025-12-09
摘要:
维生素B12是人体、动物及多种微生物必需的小分子化合物,广泛参与一碳代谢、同型半胱氨酸的甲基化及脂肪酸代谢等生理过程。近年来,随着微生物学与代谢工程的快速发展,微生物发酵法合成维生素B12的研究取得了显著进展。本文首先简要介绍了维生素B12合成途径,然后回顾了近十年几种主要合成维生素B12的微生物,包括工业生产菌株Pseudomonasdenitrificans、Sinorhizobium meliloti、Ensifer adhaerens、Propionibacterium freudenreichii和近几年新出现的人工菌种异源合成维生素B12方面的研究进展,探讨了这些微生物的代谢工程策略、发酵工艺以及在工业化生产中的应用潜力。此外,还分析了当前研究的挑战与未来发展方向,如基因工程改造与代谢调控策略在提高维生素B12生产中的应用。通过对这些微生物的合成途径的深入了解,有望为工业规模维生素B12生产提供新的理论依据和技术支持。
张纪娇, 王会颖, 房欢, 张大伟. 维生素B12生物合成领域的十年回顾与技术进展[J]. 生物技术通报, 2025, 41(11): 62-74.
ZHANG Ji-jiao, WANG Hui-ying, FANG Huan, ZHANG Da-wei. A Decade Review and Technological Advances in the Field of Vitamin B 12 Biosysthesis[J]. Biotechnology Bulletin, 2025, 41(11): 62-74.
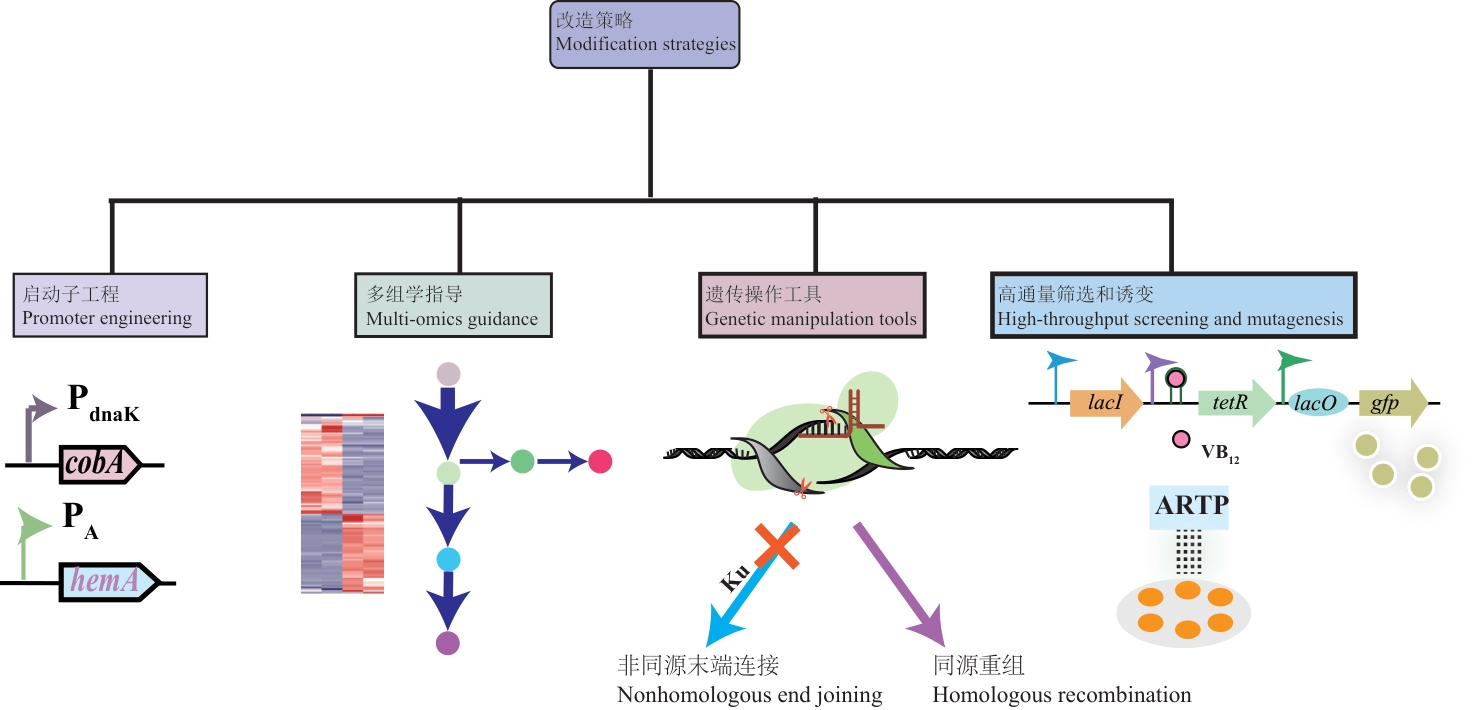
图2 维生素B12合成改造策略lacl:乳糖操纵子抑制蛋白编码基因;tetR:四环素抗性阻遏蛋白编码基因;lacO:乳糖操纵子操作子序列;gfp:绿色荧光蛋白;ARTP:大气与室温等离子体诱变
Fig. 2 Modification strategies for vitamin B12 synthesislacI: the gene encoding the lactose operon repressor protein; tetR: the gene encoding the tetracycline resistance repressor protein; lacO: the operator sequence of the lactose operon; gfp: green fluorescent protein; ARTP: atmospheric and room temperature plasma mutagenesis
| 微生物 Microorganism | 主要策略 Main strategy | 产量 Yield | 参考文献Reference |
|---|---|---|---|
| Pseudomonas denitrificans | 响应面法优化培养基中麦芽糖浆、玉米浆、甜菜碱 | 198.80 mg/L | [ |
| 代谢组学分析培养基中添加甜菜碱对维生素B12合成作用 | (58.61 ± 3.21) mg/L | [ | |
| 表达透明颤菌vgb基因 | 比产物合成速率提高52% | [ | |
| 调节氧气供应改变细胞形态 | (239.7 ± 8) mg/L | [ | |
| 筛选强启动子表达维生素B12合成途径关键基因cobA | 75.5 mg/L | [ | |
| 过表达b12fla基因的突变株提高维生素B12产量 | 比野生型提高8.09% | [ | |
| Sinorhizobium meliloti | 开发基于Cas12k的染色体整合与转录调控工具,整合hemA、hemB、hemC、hemD、cobA,阻断cysG | 92 mg/L | [ |
| 以ARTP诱变为核心,结合基因改造(过表达关键突变基因),实现维生素B12产量阶梯式提升 | 104.54 mg/L | [ | |
| 挖掘木糖诱导启动子,表达hemA | 提高11% | [ | |
| 基于核糖开关的维生素B12高通量筛选技术与ARTP联合应用 | (156±4.2) mg/L | [ | |
| 开发正向响应的维生素B12高通量筛选技术,筛选紫外诱变菌种 | 10%菌株维生素B12产量提高 | [ | |
| Ensifer adhaerens | ARTP诱变、流式细胞仪分选 | 110.25 mg /L | [ |
| 转录组挖掘内源启动子,精准表达维生素B12合成基因cobSV、cobQ和cobW | 171.2 mg/L | [ | |
| 敲除西罗血红素合成途径基因cysG、弱化血红素合成途径基因hemE | (114.17±5.77) mg/L | [ | |
| 通过动力学分析不同碳源(麦芽糖、蔗糖、葡萄糖、果糖)对发酵的影响,发现蔗糖为最佳碳源,可显著提升维生素B₁₂产量 | 115 mg/L | [ | |
| 比较转录组分析不同维生素B12合成菌株,过表达维生素B12合成途径基因cobA、cobT | (245.6±4.36) mg/L | [ | |
| Propionibacterium freudenreichii | 发酵过程中控制丙酸生成量和DMBI补料策略 | 58.8 mg/L | [ |
| 扩展床吸附生物反应器中以玉米秸秆水解液为碳源分批补料发酵联产维生素B12和丙酸 | 47.6 mg/L | [ | |
| 采用半连续发酵工艺,膜分离菌体使腺苷钴啉醇酰胺与DMBI反应,非原位合成维生素B12 | (56.76±3.86) mg/L | [ | |
| 使用大豆液态酸性蛋白渣进行发酵,Plackett-Burman实验和响应面法优化培养基配方 | 0.6 mg/g cells | [ | |
| Plackett-Burman实验和响应面法优化培养基配方 | (8.32±0.02) mg/L | [ | |
| 丙酸作碳源,微好氧发酵 | 184 μg/g DCW | [ | |
| 核糖体工程调节细菌基础代谢 | 单位细胞维生素B12产量提高5.2倍 | [ | |
| Escherichia coli | 染色体上整合前体模块基因hemOBCD、钴吸收基因cbiMNQO,3个质粒上分别表达HBA合成基因cobAIGJMFKLH、钴(II)啉酸a,c-二酰胺合成基因cobNSTW、腺苷咕啉醇酰胺磷酸合成基因cobR、cobA、cbiP、pduX、cobD、cbiB,sRNA弱化hemF、hemG,敲除乙酸合成基因ackA-pta、敲除乳酸合成基因ldhA、敲除endA | 307 μg/g DCW | [ |
| 筛选不同来源cobB,双顺反正优化cobN表达,精准调节cobS、cobT表达,筛选不同来源的腺苷咕啉醇酰胺磷酸合成基因组合,正交实验优化培养基中碳氮比 | 530.29 μg/g DCW | [ | |
| 敲除metE构建维生素B12营养缺陷型菌株,调节metH表达优化生物量和维生素B12合成 | 13.2 μg/L | [ | |
| 开发标准化基因编辑工具,将维生素B12合成途径基因整合在染色体上,构建无质粒菌株。筛选腺苷咕啉醇酰胺磷酸合成基因组合,将途径基因以单、多顺反子表达,改变途径基因染色体整合位点 | 1.49 mg/L | [ | |
| 多元模块化代谢工程优化钴(II)啉酸a,c-二酰胺、腺苷咕啉醇酰胺磷酸合成模块,添加不同种类有机氮到培养基中,5 L反应器放大 | 2.89 mg/L | [ | |
| 通过基因工程(整合异源基因、表达vgb基因、引入ED途径改造碳代谢)与发酵优化(单因素及Taguchi法优化培养基),提升大肠杆菌合成维生B12的产量,最终在5 L发酵罐中放大培养时产量达到21.09 mg/L | 21.09 mg/L | [ |
表1 主要合成维生素B12菌种的优化策略
Table 1 Optimization strategies for major vitamin B12-producing strains
| 微生物 Microorganism | 主要策略 Main strategy | 产量 Yield | 参考文献Reference |
|---|---|---|---|
| Pseudomonas denitrificans | 响应面法优化培养基中麦芽糖浆、玉米浆、甜菜碱 | 198.80 mg/L | [ |
| 代谢组学分析培养基中添加甜菜碱对维生素B12合成作用 | (58.61 ± 3.21) mg/L | [ | |
| 表达透明颤菌vgb基因 | 比产物合成速率提高52% | [ | |
| 调节氧气供应改变细胞形态 | (239.7 ± 8) mg/L | [ | |
| 筛选强启动子表达维生素B12合成途径关键基因cobA | 75.5 mg/L | [ | |
| 过表达b12fla基因的突变株提高维生素B12产量 | 比野生型提高8.09% | [ | |
| Sinorhizobium meliloti | 开发基于Cas12k的染色体整合与转录调控工具,整合hemA、hemB、hemC、hemD、cobA,阻断cysG | 92 mg/L | [ |
| 以ARTP诱变为核心,结合基因改造(过表达关键突变基因),实现维生素B12产量阶梯式提升 | 104.54 mg/L | [ | |
| 挖掘木糖诱导启动子,表达hemA | 提高11% | [ | |
| 基于核糖开关的维生素B12高通量筛选技术与ARTP联合应用 | (156±4.2) mg/L | [ | |
| 开发正向响应的维生素B12高通量筛选技术,筛选紫外诱变菌种 | 10%菌株维生素B12产量提高 | [ | |
| Ensifer adhaerens | ARTP诱变、流式细胞仪分选 | 110.25 mg /L | [ |
| 转录组挖掘内源启动子,精准表达维生素B12合成基因cobSV、cobQ和cobW | 171.2 mg/L | [ | |
| 敲除西罗血红素合成途径基因cysG、弱化血红素合成途径基因hemE | (114.17±5.77) mg/L | [ | |
| 通过动力学分析不同碳源(麦芽糖、蔗糖、葡萄糖、果糖)对发酵的影响,发现蔗糖为最佳碳源,可显著提升维生素B₁₂产量 | 115 mg/L | [ | |
| 比较转录组分析不同维生素B12合成菌株,过表达维生素B12合成途径基因cobA、cobT | (245.6±4.36) mg/L | [ | |
| Propionibacterium freudenreichii | 发酵过程中控制丙酸生成量和DMBI补料策略 | 58.8 mg/L | [ |
| 扩展床吸附生物反应器中以玉米秸秆水解液为碳源分批补料发酵联产维生素B12和丙酸 | 47.6 mg/L | [ | |
| 采用半连续发酵工艺,膜分离菌体使腺苷钴啉醇酰胺与DMBI反应,非原位合成维生素B12 | (56.76±3.86) mg/L | [ | |
| 使用大豆液态酸性蛋白渣进行发酵,Plackett-Burman实验和响应面法优化培养基配方 | 0.6 mg/g cells | [ | |
| Plackett-Burman实验和响应面法优化培养基配方 | (8.32±0.02) mg/L | [ | |
| 丙酸作碳源,微好氧发酵 | 184 μg/g DCW | [ | |
| 核糖体工程调节细菌基础代谢 | 单位细胞维生素B12产量提高5.2倍 | [ | |
| Escherichia coli | 染色体上整合前体模块基因hemOBCD、钴吸收基因cbiMNQO,3个质粒上分别表达HBA合成基因cobAIGJMFKLH、钴(II)啉酸a,c-二酰胺合成基因cobNSTW、腺苷咕啉醇酰胺磷酸合成基因cobR、cobA、cbiP、pduX、cobD、cbiB,sRNA弱化hemF、hemG,敲除乙酸合成基因ackA-pta、敲除乳酸合成基因ldhA、敲除endA | 307 μg/g DCW | [ |
| 筛选不同来源cobB,双顺反正优化cobN表达,精准调节cobS、cobT表达,筛选不同来源的腺苷咕啉醇酰胺磷酸合成基因组合,正交实验优化培养基中碳氮比 | 530.29 μg/g DCW | [ | |
| 敲除metE构建维生素B12营养缺陷型菌株,调节metH表达优化生物量和维生素B12合成 | 13.2 μg/L | [ | |
| 开发标准化基因编辑工具,将维生素B12合成途径基因整合在染色体上,构建无质粒菌株。筛选腺苷咕啉醇酰胺磷酸合成基因组合,将途径基因以单、多顺反子表达,改变途径基因染色体整合位点 | 1.49 mg/L | [ | |
| 多元模块化代谢工程优化钴(II)啉酸a,c-二酰胺、腺苷咕啉醇酰胺磷酸合成模块,添加不同种类有机氮到培养基中,5 L反应器放大 | 2.89 mg/L | [ | |
| 通过基因工程(整合异源基因、表达vgb基因、引入ED途径改造碳代谢)与发酵优化(单因素及Taguchi法优化培养基),提升大肠杆菌合成维生B12的产量,最终在5 L发酵罐中放大培养时产量达到21.09 mg/L | 21.09 mg/L | [ |
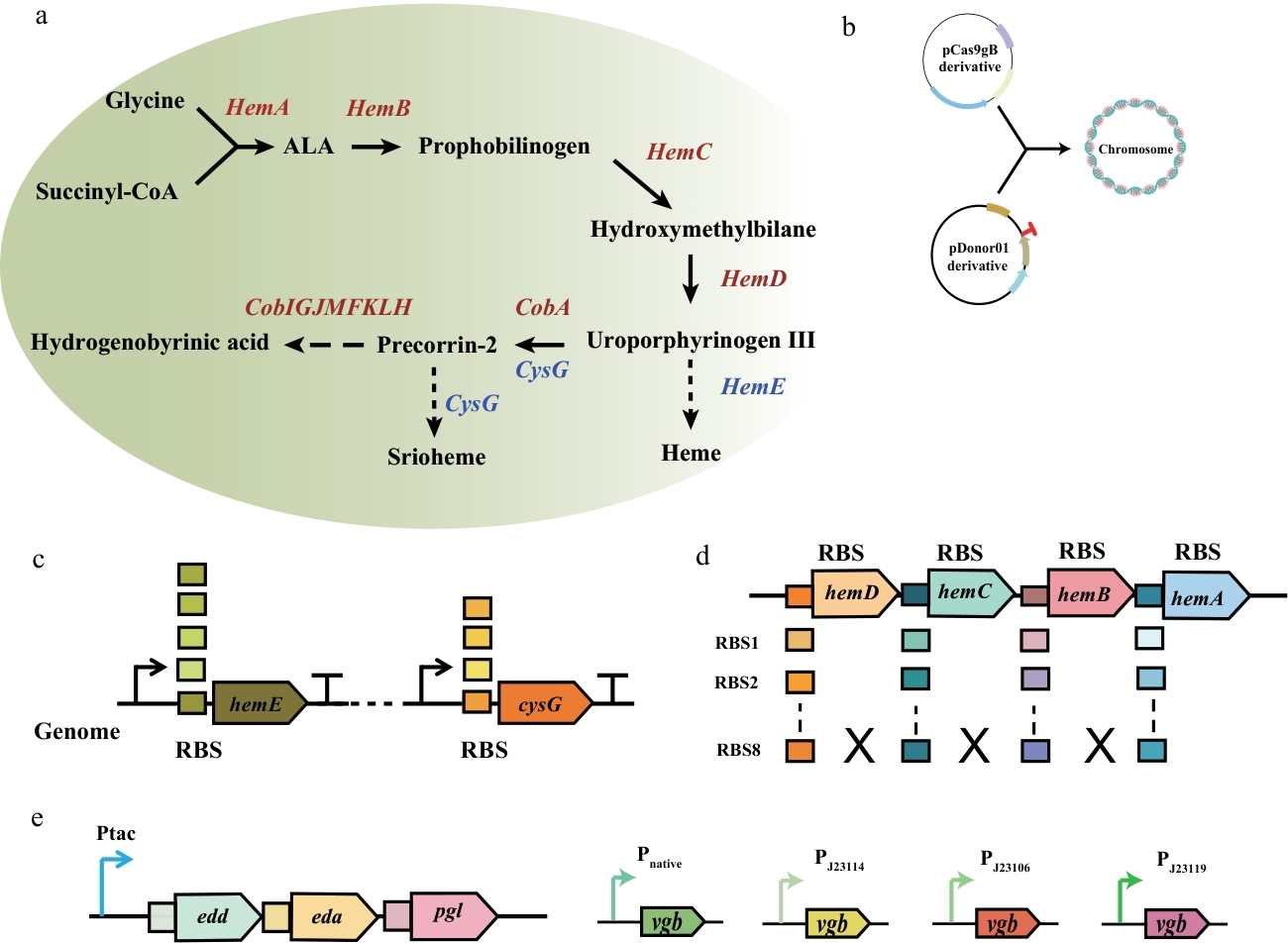
图3 维生素 B12 合成基因与调控元件图(a):维生素B12合成途径前体模块(precursor module)与HBA模块(HBA module)示意图。展示了从甘氨酸(Glycine)和琥珀酰辅酶A(succinyl-CoA)起始,经hemA、hemB、hemC、hemD等基因参与的反应,生成尿卟啉原III(uroporphyrinogen III),并分别向血红素(heme)和维生素B12合成分支的代谢路径;(b):pCas9gB衍生物和pDonor01衍生物与染色体(chromosome)相互作用示意图。描绘了两种衍生物参与基因编辑的过程;(c):基因组中hemE和cysG基因及其核糖体结合位点(RBS)示意图。展示了这两个基因在基因组中的位置及RBS分布,体现其在维生素B12合成途径中的潜在调控关系;(d):hemA、hemB、hemC、hemD基因及其相关核糖体结合位点(RBS)示意图。呈现了不同RBS(RBS1-RBS8)与对应基因的组合情况,用于研究其对基因表达及维生素B12合成的调控作用;(e):ED途径模块及不同启动子调控vgb基因表达的示意图。通过tac启动子驱动edd、eda、pgl操纵子的表达,重构ED途径关键酶;分别采用Native启动子(内源)、J23114、J23106、J23119等人工启动子驱动vgb基因
Fig. 3 Diagram of genes and regulatory elements involved in vitamin B12 synthesis(a): Schematic diagram of the precursor module and HBA module in the vitamin B12 biosynthesis pathway. This illustrates the metabolic steps starting from Glycine and succinyl-CoA, involving the genes hemA, hemB, hemC, and hemD, leading to the formation of uroporphyrinogen III, which then branches into the heme biosynthesis and vitamin B12 biosynthesis pathways. (b): Diagram of interactions between pCas9gB derivatives, pDonor01 derivatives, and the chromosome. It depicts how these derivatives participate in gene editing. (c): Schematic diagram of the hemE and cysG genes and their ribosome binding sites (RBS) in the genome. It shows the genomic locations of these genes and the distribution of their RBSs, highlighting their potential regulatory roles in the vitamin B12 biosynthetic pathway. (d): Diagram of hemA, hemB, hemC, and hemD genes and their associated ribosome binding sites (RBS). It presents the combinations of different RBS (RBS1-RBS8) with these genes, designed to study their impact on gene expression and regulation of vitamin B12 biosynthesis. (e): Schematic diagram of ED pathway module and different promoters regulating vgb gene expression. The enzymes of the ED pathway were remodeled by driving the expression of edd, eda, and pgl operon through the tac promoter. The vgb gene was driven by the native promoter (endogenous), artificial promoters such as J23114, J23106, and J23119, respectively
| [1] | Sukumar N. Crystallographic studies on B12 binding proteins in eukaryotes and prokaryotes [J]. Biochimie, 2013, 95(5): 976-988. |
| [2] | Watanabe F. Vitamin B12 sources and bioavailability [J]. Exp Biol Med, 2007, 232(10): 1266-1274. |
| [3] | Infante M, Leoni M, Caprio M, et al. Long-term metformin therapy and vitamin B12 deficiency: an association to bear in mind [J]. World J Diabetes, 2021, 12(7): 916-931. |
| [4] | Banerjee R. B12 trafficking in mammals: a for coenzyme escort service [J]. ACS Chem Biol, 2006, 1(3): 149-159. |
| [5] | Matthews RG. Cobalamin-dependent methyltransferases [J]. Acc Chem Res, 2001, 34(8): 681-689. |
| [6] | Bryant DA, Hunter CN, Warren MJ. Biosynthesis of the modified tetrapyrroles—the pigments of life [J]. J Biol Chem, 2020, 295(20): 6888-6925. |
| [7] | Brown KL. Chemistry and enzymology of vitamin B12 [J]. Chem Rev, 2005, 105(6): 2075-2149. |
| [8] | Hankey GJ. B vitamins for stroke prevention [J]. Stroke Vasc Neurol, 2018, 3(2): 51-58. |
| [9] | Sadeghian S, Fallahi F, Salarifar M, et al. Homocysteine, vitamin B12 and folate levels in premature coronary artery disease [J]. BMC Cardiovasc Disord, 2006, 6: 38. |
| [10] | Cheng Y, Lu XF, Zhao FX, et al. The effects of serum folic acid and vitamin B12 on the risk of gestational diabetes mellitus [J]. Diabetes Metab Syndr Obes, 2022, 15: 3891-3899. |
| [11] | Fedosov SN. New insights into mechanisms of vitamin B12 uptake and conversion [J]. Am J Clin Nutr, 2023, 118(6): 1073-1074. |
| [12] | Fang H, Kang J, Zhang DW. Microbial production of vitamin B12: a review and future perspectives [J]. Microb Cell Fact, 2017, 16(1): 15. |
| [13] | Stolz A. Basic and applied aspects in the microbial degradation of azo dyes [J]. Appl Microbiol Biotechnol, 2001, 56(1-2): 69-80. |
| [14] | Liu ZQ, Dong HN, Wu XY, et al. Identification of a xylose-inducible promoter and its application for improving vitamin B12 production in Sinorhizobium meliloti [J]. Biotechnol Appl Biochem, 2021, 68(4): 856-864. |
| [15] | Silano V, Barat Baviera JM, et al. Safety evaluation of the food enzyme α-amylase from the Parageobacillus thermoglucosidasius strain DP-Gzb47 [J]. EFSA J, 2020, 18(5): e06129. |
| [16] | Dank A, Biel G, Abee T, et al. Microaerobic metabolism of lactate and propionate enhances vitamin B12 production in Propionibacterium freudenreichii [J]. Microb Cell Fact, 2022, 21(1): 225. |
| [17] | Fang H, Li D, Kang J, et al. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12 [J]. Nat Commun, 2018, 9(1): 4917. |
| [18] | Calvillo Á, Pellicer T, Carnicer M, et al. Bioprocess strategies for vitamin B12 production by microbial fermentation and its market applications [J]. Bioengineering, 2022, 9(8): 365. |
| [19] | Cui YL, Dong HN, Tong BS, et al. A versatile Cas12k-based genetic engineering toolkit (C12KGET) for metabolic engineering in genetic manipulation-deprived strains [J]. Nucleic Acids Res, 2022, 50(15): 8961-8973. |
| [20] | Smith A D, Warren M J, Refsum H. Vitamin B12 [J]. Advances in Food and Nutrition Research, 2018, 83: 215-279. |
| [21] | 李芳芳. 大肠杆菌血红素合成途径的改造与调控对5-氨基乙酰丙酸积累和菌体代谢的影响 [D]. 济南: 山东大学, 2014. |
| Li FF. The Accompanying 5-aminolevulinic acid accumulation and metabolism shifts of engineering and regulating heme biosynthesis pathway in Escherichia coli [D]. Jinan: Shandong University, 2014. | |
| [22] | Yi YC, Shih IT, Yu TH, et al. Challenges and opportunities of bioprocessing 5-aminolevulinic acid using genetic and metabolic engineering: a critical review [J]. Bioresour Bioprocess, 2021, 8(1): 100. |
| [23] | 蒲伟, 陈久洲, 孙村民, 等. 琥珀酸脱氢酶或琥珀酰辅酶A合成酶缺失促进大肠杆菌积累5-氨基乙酰丙酸 [J]. 生物工程学报, 2013, 29(10): 1494-1503. |
| Pu W, Chen JZ, Sun CM, et al. Deficiency of succinic dehydrogenase or succinyl-CoA synthetase enhances the production of 5-aminolevulinic acid in recombinant Escherichia coli [J]. Chin J Biotechnol, 2013, 29(10): 1494-1503. | |
| [24] | Ge FL, Li XK, Ge QR, et al. Modular control of multiple pathways of Corynebacterium glutamicum for 5-aminolevulinic acid production [J]. AMB Express, 2021, 11(1): 179. |
| [25] | Blanche F, Debussche L, Thibaut D, et al. Purification and characterization of S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase from Pseudomonas denitrificans [J]. J Bacteriol, 1989, 171(8): 4222-4231. |
| [26] | Wienhausen G, Moraru C, Bruns S, et al. Ligand cross-feeding resolves bacterial vitamin B12 auxotrophies [J]. Nature, 2024, 629(8013): 886-892. |
| [27] | Xia W, Chen W, Peng WF, et al. Industrial vitamin B12 production by Pseudomonas denitrificans using maltose syrup and corn steep liquor as the cost-effective fermentation substrates [J]. Bioprocess Biosyst Eng, 2015, 38(6): 1065-1073. |
| [28] | Xia W, Peng WF, Chen W, et al. Interactive performances of betaine on the metabolic processes of Pseudomonas denitrificans [J]. J Ind Microbiol Biotechnol, 2015, 42(2): 273-278. |
| [29] | 施慧琳, 王泽建, 吴杰群, 等. 透明颤菌vgb基因在脱氮假单胞菌中的表达及对维生素B12合成的碳中心代谢流分析 [J]. 中国生物工程杂志, 2016, 36(9): 21-30. |
| Shi HL, Wang ZJ, Wu JQ, et al. Expression of vitreosicilla hemoglobin gene (vgb) in Pseudomonas denitrificans and the central carbon metabolic flux analysis on vitamin B12 production [J]. China Biotechnol, 2016, 36(9): 21-30. | |
| [30] | Wang ZJ, Shi HL, Wang P. The online morphology control and dynamic studies on improving vitamin B12 production by Pseudomonas denitrificans with online capacitance and specific oxygen consumption rate [J]. Appl Biochem Biotechnol, 2016, 179(6): 1115-1127. |
| [31] | 王玲玲, 夏苗苗, 董会娜, 等. 筛选脱氮假单胞菌启动子提高维生素B12产量 [J]. 生物技术通报, 2017, 33(8): 159-166. |
| Wang LL, Xia MM, Dong HN, et al. Enhanced production of vitamin B12 by screening the promoter in Pseudomonas denitrificans [J]. Biotechnol Bull, 2017, 33(8): 159-166. | |
| [32] | 吴杰群, 王博伦, 王泽建. 一种氧调控基因及其过表达突变株和用于维生素B12工业生产中的应用: CN202310798366.7 [P]. 2023-11-07. |
| Wu JQ, Wang BL, Wang ZJ. An oxygen-regulated gene, its overexpression mutant strain, and its application in industrial production of vitamin B12: CN202310798366.7 [P]. 2023-11-07. | |
| [33] | Wang Q, Liu YH, Zhu TT, et al. Advancing VB12 production: insights into enhancing VB12 titer in Ensifer adhaerens casida a through ARTP mutagenesis and multiomics analysis [J]. ACS Synth Biol, 2025, 14(4): 1264-1276. |
| [34] | Cai YY, Xia MM, Dong HN, et al. Engineering a vitamin B12 high-throughput screening system by riboswitch sensor in Sinorhizobium meliloti [J]. BMC Biotechnol, 2018, 18(1): 27. |
| [35] | Yang X, Wang HY, Ding DQ, et al. A hybrid RNA-protein biosensor for high-throughput screening of adenosylcobalamin biosynthesis [J]. Synth Syst Biotechnol, 2024, 9(3): 513-521. |
| [36] | 肖志强, 朱永明, 李江华, 等. 高通量筛选高产维生素B12的诱变菌株——黏着剑菌 [J]. 食品与发酵工业, 2021, 47(19): 7-11. |
| Xiao ZQ, Zhu YM, Li JH, et al. High-throughput screening of Ensifer adhaerens with improved vitamin B12 yield [J]. Food Ferment Ind, 2021, 47(19): 7-11. | |
| [37] | Xu S, Xiao ZQ, Yu SQ, et al. Enhanced cobalamin biosynthesis in Ensifer adhaerens by regulation of key genes with gradient promoters [J]. Synth Syst Biotechnol, 2022, 7(3): 941-948. |
| [38] | Liu YH, Chang YY, Wang Q, et al. Effect of blocking the haem synthesis pathway and weakening the haem synthesis pathway for sirohaem on the growth of and vitamin B12 synthesis in Ensifer adhaerens Casida A [J]. Bioprocess Biosyst Eng, 2023, 46(12): 1825-1835. |
| [39] | 陈欣怡, 栗波, 赵杜娟,等. 不同碳源对E. adhaerens产维生素B12发酵代谢动力学分析 [J]. 华东理工大学学报: 自然科学版, 2024, 50(1): 80-87. |
| Chen XY, Li B, Zhao DJ, et al. Kinetic analysis of fermentation metabolism of E.adhaerens producing vitamin B12 with different carbon sources [J]. Journal of East China University of Science and Technology, 2024, 50(1): 80-87. | |
| [40] | Liu YH, Huang W, Wang Q, et al. Research on the targeted improvement of the yield of a new VB12-producing strain, Ensifer adhaerens S305, based on genomic and transcriptomic analysis [J]. BMC Biotechnol, 2023, 23(1): 53. |
| [41] | Wang P, Zhang ZW, Jiao YJ, et al. Improved propionic acid and 5, 6-dimethylbenzimidazole control strategy for vitamin B12 fermentation by Propionibacterium freudenreichii [J]. J Biotechnol, 2015, 193: 123-129. |
| [42] | Wang P, Shen C, Li LW, et al. Simultaneous production of propionic acid and vitamin B12 from corn stalk hydrolysates by Propionibacterium freudenreichii in an expanded bed adsorption bioreactor [J]. Prep Biochem Biotechnol, 2020, 50(8): 763-767. |
| [43] | Wang ZQ, Xu GX, Du W, et al. Efficient ex-situ biosynthesis of vitamin B12 by Propionibacterium freudenreichii using membrane separation coupling technology [J]. Biochem Eng J, 2020, 161: 107688. |
| [44] | Assis DA, Matte C, Aschidamini B, et al. Biosynthesis of vitamin B12 by Propionibacterium freudenreichii subsp. shermanii ATCC 13673 using liquid acid protein residue of soybean as culture medium [J]. Biotechnol Prog, 2020, 36(5): e3011. |
| [45] | Liu J, Liu YF, Wu J, et al. Metabolic profiling analysis of the vitamin B12 producer Propionibacterium freudenreichii [J]. Microbiologyopen, 2021, 10(3): e1199. |
| [46] | Tanaka Y, Kasahara K, Izawa M, et al. Applicability of ribosome engineering to vitamin B12 production by Propionibacterium shermanii [J]. Biosci Biotechnol Biochem, 2017, 81(8): 1636-1641. |
| [47] | Li D, Fang H, Gai YM, et al. Metabolic engineering and optimization of the fermentation medium for vitamin B12 production in Escherichia coli [J]. Bioprocess Biosyst Eng, 2020, 43(10): 1735-1745. |
| [48] | Noh MH, Lim HG, Moon D, et al. Auxotrophic selection strategy for improved production of coenzyme B12 in Escherichia coli [J]. iScience, 2020, 23(3): 100890. |
| [49] | Fang H, Zhao JH, Zhao XF, et al. Standardized iterative genome editing method for Escherichia coli based on CRISPR-Cas9 [J]. ACS Synth Biol, 2024, 13(2): 613-623. |
| [50] | Chen FT, Fang H, Zhao JH, et al. Multivariate modular metabolic engineering and medium optimization for vitamin B12 production by Escherichia coli [J]. Synth Syst Biotechnol, 2024, 9(3): 453-461. |
| [51] | Zhao XF, Fang H, Dong N, et al. Enhancing vitamin B12 production in engineered Escherichia coli through cofactor engineering and fermentation media optimization [J]. J Agric Food Chem, 2025, 73(16): 9732-9742. |
| [52] | Prieto-de Lima TS, Rojas-Jimenez K, Vaglio C. Strategy for optimizing vitamin B12 production in Pseudomonas putida KT2440 using metabolic modeling [J]. Metabolites, 2024, 14(11): 636. |
| [53] | Nguyen-Vo TP, Ainala SK, Kim JR, et al. Analysis and characterization of coenzyme B12 biosynthetic gene clusters and improvement of B12 biosynthesis in Pseudomonas denitrificans ATCC 13867 [J]. FEMS Microbiol Lett, 2018, 365(21). DOI: 10.1093/femsle/fny211 . |
| [54] | Liu GQ, Wang HY, Tong BS, et al. An efficient CRISPR/Cas12e system for genome editing in Sinorhizobium meliloti [J]. ACS Synth Biol, 2023, 12(3): 898-903. |
| [55] | Li KT, Yang Y, Cheng X. Revealing the promoting effect of betaine on vitamin B12 biosynthetic pathway of Pseudomonas denitrificans by using a proteomics analysis [J]. Curr Pharm Biotechnol, 2022, 23(3): 466-475. |
| [56] | Wang ZJ, Wang P, Liu YW, et al. Metabolic flux analysis of the central carbon metabolism of the industrial vitamin B12 producing strain Pseudomonas denitrificans using 13C-labeled glucose [J]. J Taiwan Inst Chem Eng, 2012, 43(2): 181-187. |
| [57] | Li B, Chen XY, Zhao DJ, et al. Physiological metabolic analysis of VB12 accumulation in Ensifer adhaerens casida a enhanced by oxygen limitation [J]. Biotechnol J, 2024, 19(9): e202400305. |
| [58] | Zhang Y, Liu JZ, Huang JS, et al. Genome shuffling of Propionibacterium shermanii for improving vitamin B12 production and comparative proteome analysis [J]. J Biotechnol, 2010, 148(2-3): 139-143. |
| [59] | Balabanova L, Averianova L, Marchenok M, et al. Microbial and genetic resources for cobalamin (vitamin B12) biosynthesis: from ecosystems to industrial biotechnology [J]. Int J Mol Sci, 2021, 22(9): 4522. |
| [60] | Piwowarek K, Lipińska E, Hać-Szymańczuk E, et al. Research on the ability of propionic acid and vitamin B12 biosynthesis by Propionibacterium freudenreichii strain T82 [J]. Antonie Van Leeuwenhoek, 2018, 111(6): 921-932. |
| [61] | Jiang PT, Fang H, Zhao J, et al. Optimization of hydrogenobyrinic acid biosynthesis in Escherichia coli using multi-level metabolic engineering strategies [J]. Microb Cell Fact, 2020, 19(1): 118. |
| [62] | 董宁, 房欢, 赵莹, 等. 代谢工程改造大肠杆菌生产氢咕啉酸 [J]. 食品与发酵工业, 2023, 49(19): 44-52. |
| Dong N, Fang H, Zhao Y, et al. Metabolic engineering of Escherichia coli for hydrogenobyrinic acid production [J]. Food Ferment Ind, 2023, 49(19): 44-52. | |
| [63] | Kong KZ, Chen FT, Fang H, et al. Production of vitamin B12 in Escherichia coli using a thermal switch to control pathway genes [J]. J Microbiol Biotechnol, 2025, 35: e2412068. |
| [64] | Jiang WC, Men SS, Wen XY, et al. A preliminary study for the establishment of a reference interval for vitamin B12 in China after performance verification of a second-generation ECLIA kit [J]. J Clin Lab Anal, 2020, 34(5): e23165. |
| [1] | 彭钿钿, 马云龙, 许沛冬, 陈迪, 谢丙炎, 李彦. 芽胞杆菌防治植物病害作用机制与应用[J]. 生物技术通报, 2025, 41(8): 42-52. |
| [2] | 张津浩, 邓辉, 张清壮, 陶禹, 周池, 李鑫. 贝莱斯芽胞杆菌XY40-1对百合球茎生长、品质及镉含量的调控作用[J]. 生物技术通报, 2025, 41(7): 281-291. |
| [3] | 弥春霞, 许澍, 王守现, 刘宇, 宋庆港, 宋爽. 糙皮侧耳覆土栽培对土壤中抗生素抗性基因的影响[J]. 生物技术通报, 2025, 41(6): 335-343. |
| [4] | 陈才锭, 宋云洁, 田梦青. 四大主粮作物不同生育期根际微生物菌群变化研究进展[J]. 生物技术通报, 2025, 41(6): 49-60. |
| [5] | 张钧杰, 刘爽, 胡明珠, 石雪瑞, 代金霞. 荒漠植物根际土壤固氮微生物的筛选及其抗逆促生特性[J]. 生物技术通报, 2025, 41(6): 317-326. |
| [6] | 叶柳健, 蒙健宗, 覃福方, 何双, 朱绮霞, 王小虎, 韦圣博, 周礼芹. 古茶树林菌株D2的鉴定、酶学特性及基因组学分析[J]. 生物技术通报, 2025, 41(5): 267-279. |
| [7] | 赵春夺, 李玉娥, 刘友杰, 王新航, 赵伟, 黄永成, 李虎林, 姬文秀. 轮作与连作对烟草根际土壤养分、酶活性及微生物群落结构的影响[J]. 生物技术通报, 2025, 41(4): 312-322. |
| [8] | 宋奋奋, 段艳雪, 桑愉, 王继朋, 彭锐, 孙年喜, 李勇. 患病和健康羊肚菌菌丝际土壤微生物群落特征[J]. 生物技术通报, 2025, 41(4): 323-334. |
| [9] | 鲁天怡, 李爱朋, 费强. 生物合成聚乳酸研究进展[J]. 生物技术通报, 2025, 41(4): 47-60. |
| [10] | 何听雨, 逄雨, 张远洋, 孙雪, 李玉, 路福平, 李庆刚. 高产乳酰-N-三糖Ⅱ大肠杆菌菌株的构建[J]. 生物技术通报, 2025, 41(11): 143-152. |
| [11] | 魏敏华, 李晓童, 姜亚文, 周飘飘, 汪凯, 孙浩, 芦楠, 张成林. |
| [12] | 杨熠辰, 朱宏宇, 苏小运, 王苑, 罗会颖, 田健, 姚斌, 黄火清, 张杰. 以果葡糖浆为底物高效合成肌醇细胞工厂的构建[J]. 生物技术通报, 2025, 41(11): 121-133. |
| [13] | 张雨珊, 张雯雯, 刘岩, 申玉璞, 孙鲁, 黄伟红, 李中媛. 伏马毒素的污染现状、毒性作用机制及防控策略研究进展[J]. 生物技术通报, 2025, 41(10): 129-142. |
| [14] | 饶峻, 赵晨, 李端华, 廖豪, 黄加雨, 王辂. 自诱导策略在麦角硫因生物合成中的应用[J]. 生物技术通报, 2025, 41(1): 333-346. |
| [15] | 温绍福, 江润海, 朱城强, 张梅, 余小琴, 杨杰惠, 杨小容, 侯秀丽. 铅污染土壤中解磷菌对玉米根际土壤性质和微生物群落结构的影响[J]. 生物技术通报, 2024, 40(9): 225-237. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||