








生物技术通报 ›› 2025, Vol. 41 ›› Issue (7): 117-127.doi: 10.13560/j.cnki.biotech.bull.1985.2024-1266
收稿日期:2024-12-27
出版日期:2025-07-26
发布日期:2025-07-22
通讯作者:
杨秀丽,女,硕士,副研究员,研究方向 :作物遗传育种及栽培;E-mail: yangxiuli1234@163.com作者简介:张泽,男,博士,助理研究员,研究方向 :植物基因工程与分子生物学;E-mail: zhangzelab3027@163.com
基金资助:
ZHANG Ze( ), YANG Xiu-li(
), YANG Xiu-li( ), NING Dong-xian(
), NING Dong-xian( )
)
Received:2024-12-27
Published:2025-07-26
Online:2025-07-22
摘要:
目的 分析花生4-香豆酸:CoA连接酶(4-coumarate:CoA ligase, 4CL)基因家族成员基本特性及其对干旱和盐胁迫的响应,为培育花生耐旱抗盐品种提供重要的目标基因。 方法 通过HMM文件及NCBI CDD和Pfam数据库在全基因组水平鉴定花生4CL基因家族成员;利用ExPASy-ProtParam工具进行蛋白理化性质分析;通过MEGA7及itol工具进行系统进化分析;通过MEME及NCBI中的CD-search工具进行蛋白保守基序和保守结构域分析;通过PlantCARE及TBtools进行启动子元件分析及可视化;通过RNA-seq数据及RT-qPCR分析花生4CLs转录水平变化。 结果 借助花生Tifrunner基因组参考数据,鉴定到56个花生4CL基因,氨基酸长度介于239‒1 208之间,pI介于5.5‒9.22之间,蛋白脂肪系数介于80.2‒103.13之间,不稳定性指数在25.51‒48.79之间,GRAVY值在-0.367‒0.139之间;花生A与B基因组的20条染色体上,Ah4CLs基因不均匀地分布,5和15号染色体Ah4CLs基因密度最高;同一个聚类分支,Ah4CLs具有相似的保守基序组成及相似的内含子‒外显子分布结构,外显子数量在1‒18之间;Ah4CLs启动子区域富含光、非生物胁迫、激素及生长发育响应元件;Ah4CLs表达具有组织特异性,在根、花及种子中表达量较高;在ABA、盐与干旱胁迫处理下,部分Ah4CLs转录水平显著增加,特别是Ah4CL28在ABA、干旱及盐处理下均明显转录上调,这些基因在花生应对非生物胁迫中可能发挥着重要作用。 结论 鉴定到的56个花生4CL基因家族成员有不同结构与特性,在部分基序及结构域上具有保守型,Ah4CLs在影响花生生长发育的同时,还参与非生物胁迫响应。
张泽, 杨秀丽, 宁东贤. 花生4CL基因家族鉴定及对干旱与盐胁迫响应分析[J]. 生物技术通报, 2025, 41(7): 117-127.
ZHANG Ze, YANG Xiu-li, NING Dong-xian. Identification of 4CL Gene Family in Arachis hypogaea L. and Expression Analysis in Response to Drought and Salt Stress[J]. Biotechnology Bulletin, 2025, 41(7): 117-127.
基因名称 Gene name | 正向引物 Forward primer (5′‒3′) | 反向引物 Reverse primer (5′‒3′) |
|---|---|---|
| Ahactin7 | TTGGAATGGGTCAGAAGGATGC | AGTGGTGCCTCAGTAAGAAGC |
| Ah4CL21 | GAGAGAGCGAGGATGAAGGC | AGCTCCTCTGACCACGATCT |
| Ah4CL22 | TCTTGGACAGAGCGGGGATA | GTGTCCTCTTCCAATGCCGA |
| Ah4CL28 | GCTACACTTGGACCCTTGCT | GGCAGAGGAAGGATGGTGTC |
| Ah4CL37 | AGCCCTTGTGCCTTTCTCTC | CATGGCCCTCAACTGAACCT |
| Ah4CL49 | CGAGAATTTGAGCAGCGTGG | ACTCCACCATCTCCTCCTCC |
| Ah4CL53 | CTCTGCACTCCCTCAACCTG | GCCTGGTTTACGCTCTCCTT |
表1 RT-qPCR所用基因引物
Table 1 Genetic primers for RT-qPCR
基因名称 Gene name | 正向引物 Forward primer (5′‒3′) | 反向引物 Reverse primer (5′‒3′) |
|---|---|---|
| Ahactin7 | TTGGAATGGGTCAGAAGGATGC | AGTGGTGCCTCAGTAAGAAGC |
| Ah4CL21 | GAGAGAGCGAGGATGAAGGC | AGCTCCTCTGACCACGATCT |
| Ah4CL22 | TCTTGGACAGAGCGGGGATA | GTGTCCTCTTCCAATGCCGA |
| Ah4CL28 | GCTACACTTGGACCCTTGCT | GGCAGAGGAAGGATGGTGTC |
| Ah4CL37 | AGCCCTTGTGCCTTTCTCTC | CATGGCCCTCAACTGAACCT |
| Ah4CL49 | CGAGAATTTGAGCAGCGTGG | ACTCCACCATCTCCTCCTCC |
| Ah4CL53 | CTCTGCACTCCCTCAACCTG | GCCTGGTTTACGCTCTCCTT |

图1 花生与拟南芥4CL蛋白的系统进化分析不同区域颜色表示不同分类亚组分支,包括Clade 4CL与Clade A‒F
Fig. 1 Phylogenetic analysis of 4CL protein in peanut (Arachis hypogaea L.) and Arabidopsis thalianaDifferent regional colors indicate different classification subgroup branches, including Clade 4CL and Clade A‒F

图3 花生4CL基因家族成员共线性分析粉红色框表示花生染色体,灰色线条指示花生4CL基因位置,蓝色线条连接的Ah4CL基因之间存在共线性
Fig. 3 Syntenic relationship of peanut 4CL gene family membersThe pink box indicates the peanut chromosomes, while the gray lines denote the positions of the 4CL genes in peanuts. The blue lines illustrate the collinearity observed between the Ah4CL genes

图4 花生4CL家族成员进化关系(A)、保守基序(B)、保守结构域(C)及基因结构(D)分析
Fig. 4 Phylogenetic relationship (A), conserved motif (B), conserved domain (C), and gene structure (D) analysis of peanut 4CL family members
| 基序 Motif | 序列 Sequence | E值 E_value |
|---|---|---|
| Motif 1 | GWLHTGDLGYIDEDGYJFIVDRLKELIKYKGEQVAPAELEAVLYSHP | 3.8e-1 534 |
| Motif 2 | LLYSSGTTGLPKGVVLTHRGL | 1.8e-653 |
| Motif 3 | GEICIRGPTIMKGYLKBPEAT | 2.1e-581 |
| Motif 4 | DAAVVPRPDEEAGEVPCAFVV | 4.8e-540 |
| Motif 5 | VVFIDSJPKTSTGKILRKDLR | 2.8e-488 |
| Motif 6 | KSEDVYLWTLPMFHVNGLCFP | 2.8e-448 |
| Motif 7 | SPAFYELHLAVPMAGAVLTTANP | 7.2e-402 |
| Motif 8 | AVGGTNVCMRKFDAKAILEAIEKHKVT | 5.4e-457 |
| Motif 9 | PGAIVSQGYGMTETG | 6.9e-322 |
| Motif 10 | ITEEEIIEFCAKQVAPYKRPK | 3.5e-402 |
表2 花生4CL家族蛋白保守基序序列
Table 2 Conserved motif sequences of peanut 4CL family proteins
| 基序 Motif | 序列 Sequence | E值 E_value |
|---|---|---|
| Motif 1 | GWLHTGDLGYIDEDGYJFIVDRLKELIKYKGEQVAPAELEAVLYSHP | 3.8e-1 534 |
| Motif 2 | LLYSSGTTGLPKGVVLTHRGL | 1.8e-653 |
| Motif 3 | GEICIRGPTIMKGYLKBPEAT | 2.1e-581 |
| Motif 4 | DAAVVPRPDEEAGEVPCAFVV | 4.8e-540 |
| Motif 5 | VVFIDSJPKTSTGKILRKDLR | 2.8e-488 |
| Motif 6 | KSEDVYLWTLPMFHVNGLCFP | 2.8e-448 |
| Motif 7 | SPAFYELHLAVPMAGAVLTTANP | 7.2e-402 |
| Motif 8 | AVGGTNVCMRKFDAKAILEAIEKHKVT | 5.4e-457 |
| Motif 9 | PGAIVSQGYGMTETG | 6.9e-322 |
| Motif 10 | ITEEEIIEFCAKQVAPYKRPK | 3.5e-402 |

图6 花生4CL基因在不同组织(A)及在干旱、盐及ABA处理后(B)的表达分析
Fig. 6 Expression analysis of peanut 4CL genes in different tissues (A) and after drought, salt and ABA treatment (B)

图7 模拟干旱处理后Ah4CL28及Ah4CL37表达水平变化通过Student’s t检验进行显著性分析,*表示P<0.05,**表示P<0.01。下同
Fig. 7 Ah4CL28 and Ah4CL37 expression alterations after simulated drought treatmentSignificance analysis is conducted by Student's t-test, with * indicating P<0.05 and ** indicating P<0.01. The same below
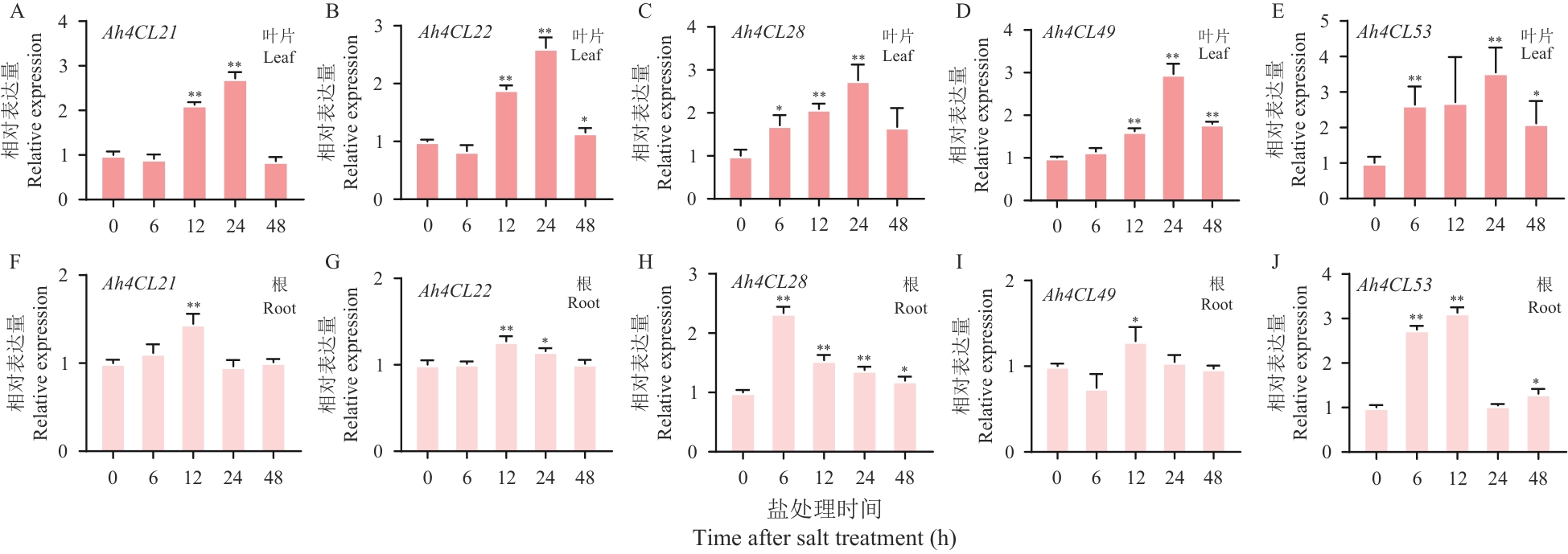
图8 盐处理后Ah4CL21、 Ah4CL22、 Ah4CL28、 Ah4CL49及Ah4CL53表达水平变化
Fig. 8 Ah4CL21, Ah4CL22, Ah4CL28, Ah4CL49, and Ah4CL53 expression alterations after salt treatment
| [1] | 刘福星, 汪可欣, 张璐, 等. 国内油料作物市场整合关系研究——以油菜籽、花生和芝麻为例 [J]. 中国油料作物学报, 2022, 44(5): 957-965. |
| Liu FX, Wang KX, Zhang L, et al. Study on domestic market integration of oil crops—Taking rapeseed, peanut and sesame for examples [J]. Chin J Oil Crop Sci, 2022, 44(5): 957-965. | |
| [2] | 刘海东, 陈庆政, 林秀芳, 等. 干旱胁迫对花生生理特性与产质量的影响[J]. 贵州农业科学, 2022, 50: 25-34. |
| Liu HD, Chen QZ, Lin XF, et al. Effects of drought stress on physiological characteristics, yield and quality of peanut[J]. Guizhou Agricultural Sciences, 2022, 50: 25-34. | |
| [3] | 朱统国, 高华援, 周玉萍, 等. 花生耐盐性鉴定研究进展 [J]. 中国农学通报, 2014, 30(21): 19-23. |
| Zhu TG, Gao HY, Zhou YP, et al. Research advances on salt tolerance identification of peanut [J]. Chin Agric Sci Bull, 2014, 30(21): 19-23. | |
| [4] | 徐扬, 丁红, 张冠初, 等. 盐胁迫下花生种子萌发期代谢组学分析 [J]. 生物技术通报, 2023, 39(1): 199-213. |
| Xu Y, Ding H, Zhang GC, et al. Metabolomics analysis of germinating peanut seed under salt stress [J]. Biotechnol Bull, 2023, 39(1): 199-213. | |
| [5] | 徐扬, 张瑞英, 戴良香, 等. 盐胁迫下氮素对花生种子萌发和种子际细菌菌群结构的调控 [J]. 生物技术通报, 2024, 40(2): 253-265. |
| Xu Y, Zhang RY, Dai LX, et al. Regulation of nitrogen application on peanut seed germination and spermosphere bacterial community structure under salt stress [J]. Biotechnol Bull, 2024, 40(2): 253-265. | |
| [6] | Dong NQ, Lin HX. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions [J]. J Integr Plant Biol, 2021, 63(1): 180-209. |
| [7] | Schneider K, Hövel K, Witzel K, et al. The substrate specificity-determining amino acid code of 4-coumarate: CoA ligase [J]. Proc Natl Acad Sci USA, 2003, 100(14): 8601-8606. |
| [8] | Hahlbrock K, Scheel D. Physiology and molecular biology of phenylpropanoid metabolism [J]. Annu Rev Plant Physiol Plant Mol Biol, 1989, 40: 347-369. |
| [9] | Fulda M, Heinz E, Wolter FP. The fadD gene of Escherichia coli K12 is located close to rnd at 39.6 min of the chromosomal map and is a new member of the AMP-binding protein family [J]. Mol Gen Genet, 1994, 242(3): 241-249. |
| [10] | Stuible H, Büttner D, Ehlting J, et al. Mutational analysis of 4-coumarate: CoA ligase identifies functionally important amino acids and verifies its close relationship to other adenylate-forming enzymes [J]. FEBS Lett, 2000, 467(1): 117-122. |
| [11] | De Azevedo Souza C, Barbazuk B, Ralph SG, et al. Genome-wide analysis of a land plant-specific acyl: coenzyme A synthetase (ACS) gene family in Arabidopsis, poplar, rice and Physcomitrella [J]. New Phytol, 2008, 179(4): 987-1003. |
| [12] | Costa MA, Eric Collins R, Anterola AM, et al. An in silico assessment of gene function and organization of the phenylpropanoid pathway metabolic networks in Arabidopsis thaliana and limitations thereof [J]. Phytochemistry, 2003, 64(6): 1097-1112. |
| [13] | Shockey JM, Fulda MS, Browse J. Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme a synthetases [J]. Plant Physiol, 2003, 132(2): 1065-1076. |
| [14] | Lavhale SG, Kalunke RM, Giri AP. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants [J]. Planta, 2018, 248(5): 1063-1078. |
| [15] | Chowdhury MEK, Choi B, Cho BK, et al. Regulation of 4CL, encoding 4-coumarate: coenzyme a ligase, expression in kenaf under diverse stress conditions [J]. Plant OMICS, 2013, 6(4): 254-262. |
| [16] | Nie TK, Sun XX, Wang SL, et al. Genome-wide identification and expression analysis of the 4-coumarate: CoA ligase gene family in Solanum tuberosum [J]. Int J Mol Sci, 2023, 24(2): 1642. |
| [17] | Sun SC, Xiong XP, Zhang XL, et al. Characterization of the Gh4CL gene family reveals a role of Gh4CL7 in drought tolerance [J]. BMC Plant Biol, 2020, 20(1): 125. |
| [18] | Zhong J, Qing J, Wang Q, et al. Genome-wide identification and expression analyses of the 4-coumarate: CoA ligase (4CL) gene family in Eucommia ulmoides [J]. Forests, 2022, 13(8): 1253. |
| [19] | Bertioli DJ, Jenkins J, Clevenger J, et al. The genome sequence of segmental allotetraploid peanut Arachis hypogaea [J]. Nat Genet, 2019, 51(5): 877-884. |
| [20] | Zhao XB, Li CJ, Wan SB, et al. Transcriptomic analysis and discovery of genes in the response of Arachis hypogaea to drought stress [J]. Mol Biol Rep, 2018, 45(2): 119-131. |
| [21] | Zhang H, Zhao XB, Sun QX, et al. Comparative transcriptome analysis reveals molecular defensive mechanism of Arachis hypogaea in response to salt stress [J]. Int J Genomics, 2020, 2020: 6524093. |
| [22] | Chen CJ, Chen H, Zhang Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data [J]. Mol Plant, 2020, 13(8): 1194-1202. |
| [23] | Sahraeian SME, Mohiyuddin M, Sebra R, et al. Gaining comprehensive biological insight into the transcriptome by performing a broad-spectrum RNA-seq analysis [J]. Nat Commun, 2017, 8(1): 59. |
| [24] | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR [J]. Nucleic Acids Res, 2001, 29(9): e45. |
| [25] | Douglas CJ. Phenylpropanoid metabolism and lignin biosynthesis: from weeds to trees [J]. Trends Plant Sci, 1996, 1(6): 171-178. |
| [26] | Vogt T. Phenylpropanoid biosynthesis [J]. Mol Plant, 2010, 3(1): 2-20. |
| [27] | Sun HY, Li Y, Feng SQ, et al. Analysis of five rice 4-coumarate: coenzyme A ligase enzyme activity and stress response for potential roles in lignin and flavonoid biosynthesis in rice [J]. Biochem Biophys Res Commun, 2013, 430(3): 1151-1156. |
| [28] | Cheng Y, Ahammed GJ, Yao ZP, et al. Comparative genomic analysis reveals extensive genetic variations of WRKYs in Solanaceae and functional variations of CaWRKYs in pepper [J]. Front Genet, 2019, 10: 492. |
| [29] | 陈雷, 杨明达, 贺群岭, 等. 基于不同生育时期干旱对花生抗旱性的综合评定 [J]. 花生学报, 2024, 53(2): 31-38, 46. |
| Chen L, Yang MD, He QL, et al. Comprehensive evaluation on drought resistance of peanuts at different growth stages [J]. J Peanut Sci, 2024, 53(2): 31-38, 46. | |
| [30] | 吴兰荣, 陈静, 许婷婷, 等. 花生全生育期耐盐鉴定研究 [J]. 花生学报, 2005, 34(1): 20-24. |
| Wu LR, Chen J, Xu TT, et al. Identification of salt tolerance in peanut growth duration [J]. J Peanut Sci, 2005, 34(1): 20-24. | |
| [31] | Chen XH, Wang HT, Li XY, et al. Molecular cloning and functional analysis of 4-coumarate: CoA ligase 4 (4CL-like 1) from Fraxinus mandshurica and its role in abiotic stress tolerance and cell wall synthesis [J]. BMC Plant Biol, 2019, 19(1): 231. |
| [32] | Wang CH, Yu J, Cai YX, et al. Characterization and functional analysis of 4-coumarate: CoA ligase genes in mul-berry [J]. PLoS One, 2016, 11(5): e0155814. |
| [33] | 陈烨, 单思杰, 钟磊坚, 等. 甜橙过氧化氢酶基因CsCAT2启动子克隆及活性分析 [J]. 农业生物技术学报, 2024, 32(12): 2755-2763. |
| Chen Y, Shan SJ, Zhong LJ, et al. Cloning and activity analysis of the CsCAT2 promoter in Citrus sinensis [J]. J Agric Biotechnol, 2024, 32(12): 2755-2763. | |
| [34] | Danquah A, de Zelicourt A, Colcombet J, et al. The role of ABA and MAPK signaling pathways in plant abiotic stress responses [J]. Biotechnol Adv, 2014, 32(1): 40-52. |
| [35] | Liu QQ, Luo L, Zheng LQ. Lignins: biosynthesis and biological functions in plants [J]. Int J Mol Sci, 2018, 19(2): 335. |
| [36] | Shomali A, Das S, Arif N, et al. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance [J]. Plants, 2022, 11(22): 3158. |
| [1] | 李新妮, 李俊怡, 马雪华, 何卫, 李佳丽, 于佳, 曹晓宁, 乔治军, 刘思辰. 谷子果胶甲酯酶抑制子PMEI基因家族鉴定及其对非生物胁迫的响应分析[J]. 生物技术通报, 2025, 41(7): 150-163. |
| [2] | 付博晗, 毛华, 赵薪程, 陆虹, 欧庸彬, 姚银安. 不同杨树SOS1基因启动子的克隆及盐胁迫响应分析[J]. 生物技术通报, 2025, 41(7): 205-213. |
| [3] | 李凯月, 邓晓霞, 殷缘, 杜亚彤, 徐元静, 王竞红, 于耸, 蔺吉祥. 蓖麻LEA基因家族的鉴定和铝胁迫响应分析[J]. 生物技术通报, 2025, 41(7): 128-138. |
| [4] | 王芳, 乔帅, 宋伟, 崔鹏娟, 廖安忠, 谭文芳, 杨松涛. 甘薯IbNRT2基因家族全基因组鉴定和表达分析[J]. 生物技术通报, 2025, 41(7): 193-204. |
| [5] | 魏雨佳, 李岩, 康语涵, 弓晓楠, 杜敏, 涂岚, 石鹏, 于子涵, 孙彦, 张昆. 白颖苔草CrMYB4基因的克隆和表达分析[J]. 生物技术通报, 2025, 41(7): 248-260. |
| [6] | 程珊, 王会伟, 陈晨, 朱雅婧, 李春鑫, 别海, 王树峰, 陈献功, 张向歌. 油莎豆MYB转录因子基因CeMYB154克隆及耐盐功能分析[J]. 生物技术通报, 2025, 41(6): 218-228. |
| [7] | 王苗苗, 赵相龙, 王召明, 刘志鹏, 闫龙凤. 花苜蓿TCP基因家族的鉴定及其在干旱胁迫下的表达模式分析[J]. 生物技术通报, 2025, 41(6): 179-190. |
| [8] | 黄丹, 彭兵阳, 张盼盼, 焦悦, 吕佳斌. 油茶HD-Zip基因家族鉴定及其在非生物胁迫下的表达分析[J]. 生物技术通报, 2025, 41(6): 191-207. |
| [9] | 宗建伟, 邓海芳, 蔡沅原, 常雅雯, 朱雅琦, 杨雨华. AM真菌对干旱胁迫下文冠果根系形态和叶片结构耦合的影响[J]. 生物技术通报, 2025, 41(6): 167-178. |
| [10] | 瞿美玲, 周思敏, 张惊宇, 何佳蔚, 朱佳源, 刘笑蓉, 童巧珍, 周日宝, 刘湘丹. 灰毡毛忍冬bHLH转录基因家族的鉴定与表达分析[J]. 生物技术通报, 2025, 41(6): 256-268. |
| [11] | 宋慧洋, 苏宝杰, 李京昊, 梅超, 宋倩娜, 崔福柱, 冯瑞云. 马铃薯StAS2-15基因的克隆及盐胁迫下功能分析[J]. 生物技术通报, 2025, 41(5): 119-128. |
| [12] | 何卫, 李俊怡, 李新妮, 马雪华, 邢媛, 曹晓宁, 乔治军, 刘思辰. 谷子泛素连接酶U-box E3基因家族的鉴定及响应非生物胁迫分析[J]. 生物技术通报, 2025, 41(5): 104-118. |
| [13] | 李志强, 罗正乾, 徐琳黎, 周国慧, 屈丝雨, 刘恩良, 顼东婷. 基于T2T基因组鉴定大豆R2R3-MYB基因家族及干旱和盐胁迫下的表达分析[J]. 生物技术通报, 2025, 41(5): 141-152. |
| [14] | 吴娅, 姚润, 杨含婷, 刘微, 杨帅, 宋驰, 陈士林. 凤梨薄荷SDR基因家族全基因组鉴定及表达分析[J]. 生物技术通报, 2025, 41(5): 175-185. |
| [15] | 罗嗣芳, 张祖铭, 谢丽芳, 郭紫晶, 陈兆星, 杨月华, 严翔, 张洪铭. 山金柑GATA基因家族全基因组鉴定及在果实发育中的表达分析[J]. 生物技术通报, 2025, 41(5): 218-230. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||