








Biotechnology Bulletin ›› 2021, Vol. 37 ›› Issue (4): 245-250.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0875
Previous Articles Next Articles
JIN Qiu-xia1( ), WANG Si-hong2, JIN Li-hua1(
), WANG Si-hong2, JIN Li-hua1( )
)
Received:2020-07-15
Online:2021-04-26
Published:2021-05-13
Contact:
JIN Li-hua
E-mail:15104659650@163.com;lhjin2000@hotmail.com
JIN Qiu-xia, WANG Si-hong, JIN Li-hua. Research Progress on Drosophila Intestinal Stem Cells and Intestinal Microflora[J]. Biotechnology Bulletin, 2021, 37(4): 245-250.
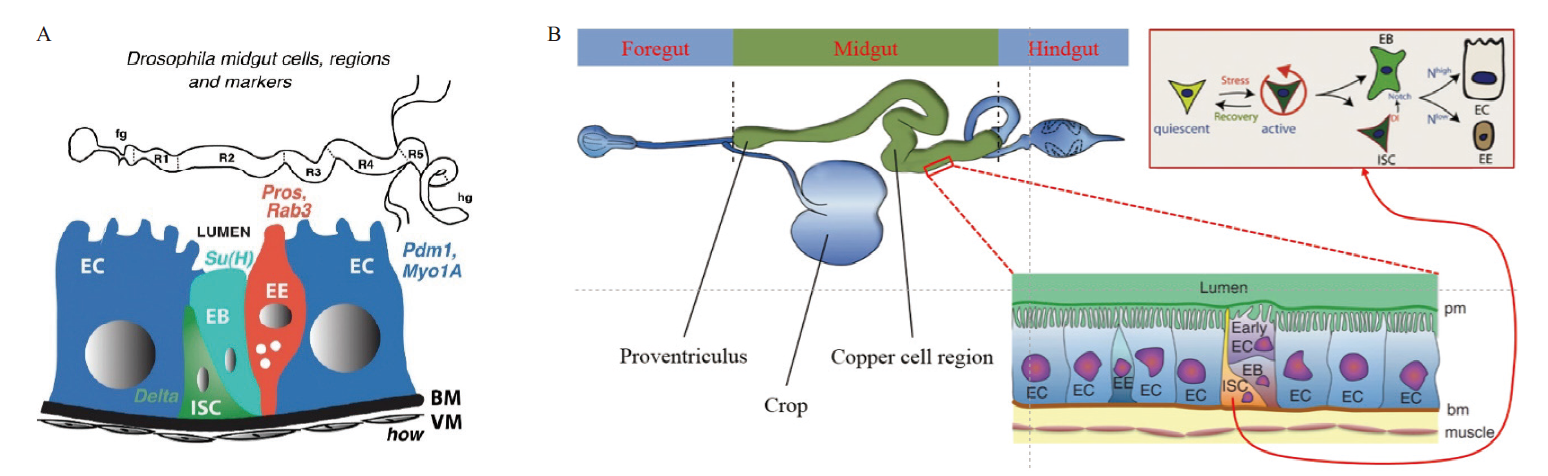
Fig.1 Adult Drosophila midgut cell types (A) and proliferation and differentiation of Drosophila intestinal stem cells(B) R1-R5 : Midgut region. fg: Foregut. hg: Hindgut. BM: Basement membrane
| [1] |
Buchon N, Broderick NA, Chakrabarti S, et al. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila[J]. Genes Dev, 2009,23(19):2333-2344.
doi: 10.1101/gad.1827009 URL |
| [2] | 刘利英. 纳米二氧化钛对果蝇肠道菌群影响的研究[D]. 南京:南京农业大学, 2015. |
| Liu LY. Effects of titanium dioxide nanoparticles on intestinal commensal bacteria[D]. Nanjing:Nanjing Agricultural University, 2015. | |
| [3] |
Peterson DA, Frank DN, Pace NR, et al. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases[J]. Cell Host Microbe, 2008,3(6):417-427.
doi: 10.1016/j.chom.2008.05.001 pmid: 18541218 |
| [4] |
Lee WJ, Brey PT. How microbiomes influence metazoan development:insights from history and Drosophila modeling of gut-microbe interactions[J]. Annu Rev Cell Dev Biol, 2013,29:571-592.
doi: 10.1146/annurev-cellbio-101512-122333 URL |
| [5] |
Zhai Z, Boquete JP, Lemaitre B. Cell-specific Imd-NF-κB responses enable simultaneous antibacterial immunity and intestinal epithelial cell shedding upon bacterial infection[J]. Immunity, 2018, 48(5):897-910. e7.
doi: 10.1016/j.immuni.2018.04.010 URL |
| [6] |
Nászai M, Carroll LR, Cordero JB. Intestinal stem cell proliferation and epithelial homeostasis in the adult Drosophila midgut[J]. Insect Biochem Mol Biol, 2015,67:9-14.
doi: 10.1016/j.ibmb.2015.05.016 URL |
| [7] |
Chen J, Xu N, Wang C, et al. Transient scute activation via a self-stimulatory loop directs enteroendocrine cell pair specification from self-renewing intestinal stem cells[J]. Nat Cell Biol, 2018,20(2):152-161.
doi: 10.1038/s41556-017-0020-0 URL |
| [8] |
Miguel-Aliaga I, Jasper H, Lemaitre B. Anatomy and physiology of the digestive tract of Drosophila melanogaster[J]. Genetics, 2018,210(2):357-396.
doi: 10.1534/genetics.118.300224 pmid: 30287514 |
| [9] | Park JS, Na HJ, Pyo JH, et al. Requirement of ATR for maintenance of intestinal stem cells in aging Drosophila[J]. Aging(Albany NY), 2015,7(5):307-318. |
| [10] |
Ferrandon D. The complementary facets of epithelial host defenses in the genetic model organism Drosophila melanogaster:from resistance to resilience[J]. Curr Opin Immunol, 2013,25(1):59-70.
doi: 10.1016/j.coi.2012.11.008 URL |
| [11] | 薛迪. 影响果蝇肠道干细胞增殖与分化的lncRNAs的筛选及其功能分析[D]. 大连:大连医科大学, 2018. |
| Xue D. Screening and function analysis of InRNAs regulating proliferation and differentiation of Drosophila intestinal stem cells[D]. Dalian:Dalian Medical University, 2018. | |
| [12] |
Jiang H, Patel PH, Kohlmaier A, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut[J]. Cell, 2009,137(7):1343-1355.
doi: 10.1016/j.cell.2009.05.014 URL |
| [13] |
Guo X, Yin C, Yang F, et al. The cellular diversity and transcription factor code of Drosophila enteroendocrine Cells[J]. Cell Rep, 2019, 29(12):4172-4185. e5.
doi: 10.1016/j.celrep.2019.11.048 URL |
| [14] |
Biteau B, Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila[J]. Cell Rep, 2014,7(6):1867-1875.
doi: 10.1016/j.celrep.2014.05.024 URL |
| [15] | 李玉梅. Escargot和Scute调控肠道内分泌细胞分化的机制研究[D]. 北京:清华大学, 2017. |
| Li YM. Antagonism between transcription factors escargot and scute controls intestinal enteroendocrine cell specification[D]. Beijing:Tsinghua University, 2017. | |
| [16] |
Korzelius J, Azami S, Ronnen-Oron T, et al. The WT1-like transcription factor Klumpfuss maintains lineage commitment of enterocyte progenitors in the Drosophila intestine[J]. Nat Commun, 2019,10(1):4123.
doi: 10.1038/s41467-019-12003-0 pmid: 31511511 |
| [17] | Carmena A, Baylies M. Development of the larval somatic musculature[M]// Sink H. Muscle development in Drosophila. New York:Springer, 2006: 79-91. |
| [18] |
Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell:mechanism and applications[J]. Science, 2013,340(6137):1190-1194.
doi: 10.1126/science.1234852 URL |
| [19] |
Liu Q, Jin LH. The zinc finger protein CG12744 is essential for differentiation and regeneration after infection in the adult Drosophila midgut[J]. Exp Cell Res, 2017,361(2):225-235.
doi: 10.1016/j.yexcr.2017.10.021 URL |
| [20] | Park JS, Jeon HJ, Pyo JH, et al. Deficiency in DNA damage response of enterocytes accelerates intestinal stem cell aging in Drosophila[J]. Aging(Albany NY), 2018,10(3):322-338. |
| [21] |
Houtz P, Bonfini A, Liu X, et al. Hippo, TGF-β, and Src-MAPK pathways regulate transcription of the upd3 cytokine in Drosophila enterocytes upon bacterial infection[J]. PLoS Genet, 2017,13(11):e1007091.
doi: 10.1371/journal.pgen.1007091 URL |
| [22] | 郭彤. SdBP在Hippo信号通路中的功能研究及在果蝇肠干细胞系统中的作用[D]. 北京:中国科学院大学, 2014. |
| Guo T. The function of SdBP in the Hippo signaling pathway and its role in the Drosophila intestinal stem cell system[D]. Beijing:University of Chinese Academy of Sciences, 2014. | |
| [23] |
Hao X, et al. Lola regulates Drosophila adult midgut homeostasis via non-canonical hippo signaling[J]. eLife, 2020,9:e47542.
doi: 10.7554/eLife.47542 URL |
| [24] |
Kučerová L, Kubrak OI, et al. Slowed aging during reproductive dormancy is reflected in genome-wide transcriptome changes in Drosophila melanogaster[J]. BMC Genomics, 2016,17:50.
doi: 10.1186/s12864-016-2383-1 pmid: 26758761 |
| [25] |
Zhang S, Chen C, Wu C, et al. The canonical Wg signaling modulates Bsk-mediated cell death in Drosophila[J]. Cell Death Dis, 2015,6(4):e1713.
doi: 10.1038/cddis.2015.85 URL |
| [26] |
Wang S, Yin B, Li H, et al. MKK4 from Litopenaeus vannamei is a regulator of p38 MAPK kinase and involved in anti-bacterial response[J]. Dev Comp Immunol, 2018,78:61-70.
doi: 10.1016/j.dci.2017.09.015 URL |
| [27] | 彭琳, 姜北海, 苏向前. 表皮生长因子样重复序列糖基化对Notch信号通路的作用[J]. 中国生物化学与分子生物学报, 2019,35(8):850-855. |
| Peng L, Jiang BH, Su XQ. Effect of glycosylation of EGF-R on Notch signaling pathway[J]. Chinese Journal of Biochemistry and Molecular Biology, 2019,35(8):850-855. | |
| [28] |
Chen CL, Fu XF, Wang LQ, et al. Primordial follicle assembly was regulated by Notch signaling pathway in the mice[J]. Mol Biol Rep, 2014,41(3):1891-1899.
doi: 10.1007/s11033-014-3038-4 URL |
| [29] |
Cordero JB, Stefanatos RK, Scopelliti A, et al. Inducible progenitor-derived Wingless regulates adult midgut regeneration in Drosophila[J]. EMBO J, 2012,31(19):3901-3917.
doi: 10.1038/emboj.2012.248 URL |
| [30] |
Xu N, Wang SQ, Tan D, et al. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells[J]. Dev Biol, 2011,354(1):31-43.
doi: 10.1016/j.ydbio.2011.03.018 URL |
| [31] | 赵璐, 花蕾, 白芃, 等. 肠道微生物群加重黑腹果蝇盐胁迫反应[J]. 微生物学通报, 2020,47(6):1867-1875. |
| Zhao L, Hua L, Bai P, et al. Intestinal microbiota aggravates the salt stress response of Drosophila melanogaster[J]. Bulletin of Microbiology, 2020,47(6):1867-1875. | |
| [32] |
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity[J]. Nat Rev Immunol, 2016,16(6):341-352.
doi: 10.1038/nri.2016.42 URL |
| [33] |
Tripathi A, Debelius J, Brenner DA, et al. The gut-liver axis and the intersection with the microbiome[J]. Nat Rev Gastroenterol Hepatol, 2018,15(7):397-411.
doi: 10.1038/s41575-018-0011-z URL |
| [34] |
Komaroff AL. The microbiome and risk for atherosclerosis[J]. JAMA, 2018,319(23):2381-2382.
doi: 10.1001/jama.2018.5240 pmid: 29800043 |
| [35] |
Jia B. Commentary:Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells[J]. Front Immunol, 2019,10:282.
doi: 10.3389/fimmu.2019.00282 URL |
| [36] | Broderick NA, Buchon N, Lemaitre B. Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology[J]. mBio, 2014; 5(3):e01117-14. |
| [37] |
Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world:insights from Drosophila melanogaster[J]. Nat Rev Microbiol, 2013,11(9):615-626.
doi: 10.1038/nrmicro3074 URL |
| [38] |
Guo L, Karpac J, Tran SL, et al. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan[J]. Cell, 2014,156(1-2):109-122.
doi: 10.1016/j.cell.2013.12.018 URL |
| [39] |
Hou Q, Ye L, Huang L, Yu Q. The research progress on intestinal stem cells and its relationship with intestinal microbiota[J]. Front Immunol, 2017,8:599.
doi: 10.3389/fimmu.2017.00599 URL |
| [40] | Chmiel JA, Daisley BA, Burton JP, et al. Deleterious effects of neonicotinoid pesticides on Drosophila melanogaster immune pathways[J]. mBio, 2019,10(5):e01395-19. |
| [41] |
Iatsenko I, Boquete JP, Lemaitre B. Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase nox and shortensDrosophila lifespan[J]. Immunity, 2018, 49(5):929-942. e5.
doi: S1074-7613(18)30432-1 pmid: 30446385 |
| [42] |
Obata F, Fons CO, Gould AP. Early-life exposure to low-dose oxidants can increase longevity via microbiome remodelling in Drosophila[J]. Nat Commun, 2018,9(1):975.
doi: 10.1038/s41467-018-03070-w URL |
| [43] |
Zhou J, Boutros M. JNK-dependent intestinal barrier failure disrupts host-microbe homeostasis during tumorigenesis[J]. Proc Natl Acad Sci USA, 2020,117(17):9401-9412.
doi: 10.1073/pnas.1913976117 URL |
| [44] |
Chen K, Luan X, Liu Q, et al. Drosophila histone demethylase KDM5 regulates social behavior through immune control and gut microbiota maintenance[J]. Cell Host Microbe, 2019, 25(4): 537-552. e8.
doi: S1931-3128(19)30099-X pmid: 30902578 |
| [45] |
Schretter CE, Vielmetter J, Bartos I, et al. A gut microbial factor modulates locomotor behaviour in Drosophila[J]. Nature, 2018,563(7731):402-406.
doi: 10.1038/s41586-018-0634-9 URL |
| [46] |
Tremlett H, Bauer KC, Appel-Cresswell S, et al. The gut microbiome in human neurological disease:A review[J]. Ann Neurol, 2017,81(3):369-382.
doi: 10.1002/ana.v81.3 URL |
| [47] |
Chen PB, Black AS, Sobel AL, et al. Directed remodeling of the mouse gut microbiome inhibits the development of atherosclerosis[J]. Nat Biotechnol, 2020,38(1):1228-1297.
doi: 10.1038/s41587-020-0736-4 URL |
| [48] | 董娇娥, 刘洁, 胡璇, 等. 高脂饮食对肠道功能的影响[J]. 生命的化学, 2019,39(6):1098-1106. |
| Dong JE, Liu J, Hu X, et al. The effect of high-fat diet on intestinal function[J]. Chemisty of Life, 2019,39(6):1098-1106. | |
| [49] |
Buffington SA, Di Prisco GV, Auchtung TA, et al. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring[J]. Cell, 2016,165(7):1762-1775.
doi: S0092-8674(16)30730-9 pmid: 27315483 |
| [50] |
von Frieling J, Faisal MN, Sporn F, et al. A high-fat diet induces a microbiota-dependent increase in stem cell activity in the Drosophila intestine[J]. PLoS Genet, 2020,16(5):e1008789.
doi: 10.1371/journal.pgen.1008789 URL |
| [51] |
Campbell C, McKenney PT, Konstantinovsky D, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells[J]. Nature, 2020,581(7809):475-479.
doi: 10.1038/s41586-020-2193-0 URL |
| [52] |
You H, Lee WJ, Lee WJ. Homeostasis between gut-associated microorganisms and the immune system in Drosophila[J]. Curr Opin Immunol, 2014,30:48-53.
doi: 10.1016/j.coi.2014.06.006 URL |
| [53] |
Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions[J]. Science, 2012,336(6086):1262-1267.
doi: 10.1126/science.1223813 URL |
| [54] |
Wang L, Ravichandran V, Yin Y, et al. Natural products from mammalian gut microbiota[J]. Trends Biotechnol, 2019,37(5):492-504.
doi: 10.1016/j.tibtech.2018.10.003 URL |
| [55] | Newell PD, Douglas AE. Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster[J]. Appl Environ Microbiol, 2014,2:788-796. |
| [1] | WANG Song, JIAN Xiao-ping, PAN Wan-shu, ZHANG Yong-guang, WANG Tao, YOU Ling. Effects of Fermented Corn Xiaoqu Distiller's Grains Feed on the Intestinal Microbiota of Growing-Finishing Pigs [J]. Biotechnology Bulletin, 2022, 38(9): 248-257. |
| [2] | CHEN Tian-ci, WU Shao-lan, YANG Guo-hui, JIANG Dan-xia, JIANG Yu-ji, CHEN Bing-zhi. Effects of Ganoderma resinaceum Alcohol Extract on Sleep and Intestinal Microbiota in Mice [J]. Biotechnology Bulletin, 2022, 38(8): 225-232. |
| [3] | XIE Guo-zhen, TANG Yuan, WU Yi, HUANG Li-li, TAN Zhou-jin. Effects of Total Glycosides of Qiwei Baizhu Powder on Intestinal Microbiota and Enzyme Activities in Diarrhea Mice [J]. Biotechnology Bulletin, 2021, 37(12): 124-131. |
| [4] | ZHAO Xu, XU Qun, HOU Yan-ru, LI Ming-yu, ZHANG Ya-ning, WANG Hai. Effects of ANGPTL4 on Intestinal Microbiota Affecting Lipid Metabolism of Animals [J]. Biotechnology Bulletin, 2020, 36(6): 230-235. |
| [5] | WANG Jing, DAI Dong, WU Shu-geng, ZHANG Hai-jun, QI Guang-hai. Advances in Successional Development and Early Establishment of the Chicken Intestinal Microbiota [J]. Biotechnology Bulletin, 2020, 36(2): 1-8. |
| [6] | Li Dongping, Guo Mingzhang, Xu Wentao. Advances and Applications on Methodology of 16S rRNA Sequencing in Gut Microbiota Analysis [J]. Biotechnology Bulletin, 2015, 31(2): 71-77. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||