








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (11): 112-121.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0016
Previous Articles Next Articles
DONG Ya-ru( ), ZHAO Dong-xiao, GENG Bing, LI Yun-zhi, WANG Zhao-hong(
), ZHAO Dong-xiao, GENG Bing, LI Yun-zhi, WANG Zhao-hong( )
)
Received:2022-01-05
Online:2022-11-26
Published:2022-12-01
Contact:
WANG Zhao-hong
E-mail:dongyaru2013@126.com;wzh1788@163.com
DONG Ya-ru, ZHAO Dong-xiao, GENG Bing, LI Yun-zhi, WANG Zhao-hong. Expression Analysis of MnERF2 in Mulberry[J]. Biotechnology Bulletin, 2022, 38(11): 112-121.
| 引物名称 Primer name | 序列 Sequence(5'-3') | 用途 Application |
|---|---|---|
| MnERF2-F | ATGGCGACCATAAACGAAGTC | 基因克隆 Gene cloning |
| MnERF2-R | TCACACAACCATTAGTTGTGG | |
| MnERF2-F | TCCCGTTGGAAGCCGGGA | 实时荧光定量PCR Quantitative real-time PCR |
| MnERF2-R | CGACAGAGGCGGGACGTT | |
| MnRPL15-F | GGCTATGTGATTTACCGTGTT | |
| MnRPL15-R | TTGGTCCAGTATGAGTTGAGAA | |
| β-actin-F | AGCAACTGGGATGACATGGAGA | |
| β-actin-R | CGACCACTGGCGTAAAGGGA |
Table 1 Primers used in this study
| 引物名称 Primer name | 序列 Sequence(5'-3') | 用途 Application |
|---|---|---|
| MnERF2-F | ATGGCGACCATAAACGAAGTC | 基因克隆 Gene cloning |
| MnERF2-R | TCACACAACCATTAGTTGTGG | |
| MnERF2-F | TCCCGTTGGAAGCCGGGA | 实时荧光定量PCR Quantitative real-time PCR |
| MnERF2-R | CGACAGAGGCGGGACGTT | |
| MnRPL15-F | GGCTATGTGATTTACCGTGTT | |
| MnRPL15-R | TTGGTCCAGTATGAGTTGAGAA | |
| β-actin-F | AGCAACTGGGATGACATGGAGA | |
| β-actin-R | CGACCACTGGCGTAAAGGGA |

Fig. 2 Multiple sequence alignments of MnERF2 with other group B-3 ERFs from A. thaliana * indicates positions which have a single,fully conserved residue.:indicates that one of the following ‘strong’ groups is fully conserved.. indicates that one of the following ‘weaker’ groups is fully conserved,and arrow indicates the amino acids at the position of 9 and 14 of the AP2/ERF domain respectively
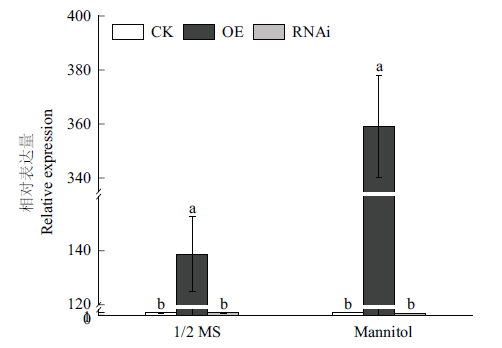
Fig. 3 RT-qPCR detection of mulberry transiently transfo-rmed with MnERF2 Different lowercase letters indicate significant difference at 0.05 level among treatments. The same below
| [1] |
Hilker M, Schmülling T. Stress priming, memory, and signalling in plants[J]. Plant Cell Environ, 2019, 42(3):753-761.
doi: 10.1111/pce.13526 |
| [2] |
Faraji S, Filiz E, Kazemitabar SK, et al. The AP2/ERF gene family in Triticum durum:genome-wide identification and expression analysis under drought and salinity stresses[J]. Genes, 2020, 11(12):1464.
doi: 10.3390/genes11121464 URL |
| [3] |
Ma ZM, Wu T, Huang K, et al. A novel AP2/ERF transcription factor, OsRPH1, negatively regulates plant height in rice[J]. Front Plant Sci, 2020, 11:709.
doi: 10.3389/fpls.2020.00709 URL |
| [4] | 董亚茹, 赵东晓, 杜建勋, 等. 外源NO对NaCl胁迫下桑树种子萌发及幼苗生理生化特性的影响[J]. 蚕业科学, 2018, 44(6):821-827. |
| Dong YR, Zhao DX, Du JX, et al. Effects of exogenous nitric oxide on mulberry seed germination and physiological characteristics of mulberry seedling upon salt stress[J]. Sci Seric, 2018, 44(6):821-827. | |
| [5] | Zhu M, Wang ZJ, He YJ, et al. Bioguided isolation, identification and bioactivity evaluation of anti-MRSA constituents from Morus alba Linn[J]. J Ethnopharmacol, 2021, 281:114542. |
| [6] |
Liu D, Zeng Y, Qiu C, et al. Molecular cloning and adversity stress expression analysis of SPDS genes in mulberry(Morus notabilis)[J]. Russ J Plant Physiol, 2021, 68(6):1186-1193.
doi: 10.1134/S1021443721060108 URL |
| [7] | 周宏. 桑树抗旱相关4个转录因子家族鉴定与表达分析[D]. 镇江: 江苏科技大学, 2017. |
| Zhou H. Identification and expression analysis of drought-resistant related 4 transcription factor families in mulberry(Morus L.)[D]. Zhenjiang: Jiangsu University of Science and Technology, 2017. | |
| [8] | 何宁佳, 向仲怀. 桑树基因组[M]. 北京: 中国林业出版社, 2016. |
| He NJ, Xiang ZH. Mulberry genome[M]. Beijing: China Forestry Publishing House, 2016. | |
| [9] |
Feng K, Hou XL, Xing GM, et al. Advances in AP2/ERF super-family transcription factors in plant[J]. Crit Rev Biotechnol, 2020, 40(6):750-776.
doi: 10.1080/07388551.2020.1768509 pmid: 32522044 |
| [10] |
Chen LH, Han JP, Deng XM, et al. Expansion and stress responses of AP2/EREBP superfamily in Brachypodium distachyon[J]. Sci Rep, 2016, 6:21623.
doi: 10.1038/srep21623 URL |
| [11] | Sharma R, Singh G, Bhattacharya S, et al. Comparative transcriptome meta-analysis of Arabidopsis thaliana under drought and cold stress[J]. PLoS One, 2018, 13(9):e0203266. |
| [12] |
Zhang HN, Pan XL, Liu SH, et al. Genome-wide analysis of AP2/ERF transcription factors in pineapple reveals functional divergence during flowering induction mediated by ethylene and floral organ development[J]. Genomics, 2021, 113(2):474-489.
doi: 10.1016/j.ygeno.2020.10.040 pmid: 33359830 |
| [13] | Lv KW, Li J, Zhao K, et al. Overexpression of an AP2/ERF family gene, BpERF13, in birch enhances cold tolerance through upregulating CBF genes and mitigating reactive oxygen species[J]. Plant Sci, 2020, 292:110375. |
| [14] | Kavas M, Gökdemir G, Seçgin Z, et al. Ectopic expression of common bean ERF transcription factor PvERF35 promotes salt stress tolerance in tobacco[J]. Plant Biol(Stuttg), 2020, 22(6):1102-1112. |
| [15] |
An JP, Zhang XW, Bi SQ, et al. The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple[J]. Plant J, 2020, 101(3):573-589.
doi: 10.1111/tpj.14555 URL |
| [16] |
Djemal R, Khoudi H. The barley SHN1-type transcription factor HvSHN1 imparts heat, drought and salt tolerances in transgenic tobacco[J]. Plant Physiol Biochem, 2021, 164:44-53.
doi: 10.1016/j.plaphy.2021.04.018 URL |
| [17] |
Li Z, Wang G, Liu XH, et al. Genome-wide identification and expression profiling of DREB genes in Saccharum spontaneum[J]. BMC Genomics, 2021, 22(1):456.
doi: 10.1186/s12864-021-07799-5 URL |
| [18] |
Zhao Q, Hu RS, Liu D, et al. The AP2 transcription factor NtERF172 confers drought resistance by modifying NtCAT[J]. Plant Biotechnol J, 2020, 18(12):2444-2455.
doi: 10.1111/pbi.13419 URL |
| [19] | Ji XY, Nie XG, Liu YJ, et al. A bHLH gene from Tamarix hispida improves abiotic stress tolerance by enhancing osmotic potential and decreasing reactive oxygen species accumulation[J]. Tree Physiol, 2016, 36(2):193-207. |
| [20] |
He ZH, Wang ZB, Nie XG, et al. UNFERTILIZED EMBRYO SAC 12 phosphorylation plays a crucial role in conferring salt tolerance[J]. Plant Physiol, 2022, 188(2):1385-1401.
doi: 10.1093/plphys/kiab549 URL |
| [21] |
Chai GH, Qi G, Cao YP, et al. Poplar PdC3H17 and PdC3H18 are direct targets of PdMYB3 and PdMYB21, and positively regulate secondary wall formation in Arabidopsis and poplar[J]. New Phytol, 2014, 203(2):520-534.
doi: 10.1111/nph.12825 URL |
| [22] |
赵东晓, 施新琴, 董亚茹, 等. 60Co-γ辐射及PEG胁迫对桑树幼苗生理特性和相关基因表达的影响[J]. 核农学报, 2021, 35(7):1485-1494.
doi: 10.11869/j.issn.100-8551.2021.07.1485 |
| Zhao DX, Shi XQ, Dong YR, et al. Effects of 60Co-γ radiation and PEG stress on physiological characteristics and related gene expression of mulberry seedlings[J]. J Nucl Agric Sci, 2021, 35(7):1485-1494. | |
| [23] | 陈建勋, 王晓峰. 植物生理学实验指导[M]. 广州: 华南理工大学出版社, 2002. |
| Chen JX, Wang XF. Plant physiology experiment guide[M]. Guangzhou: South China University of Technology Press, 2002. | |
| [24] |
Elstner EF, Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride:a simple assay for superoxide dismutase[J]. Anal Biochem, 1976, 70(2):616-620.
pmid: 817618 |
| [25] |
Patterson BD, MacRae EA, Ferguson IB. Estimation of hydrogen peroxide in plant extracts using titanium(IV)[J]. Anal Biochem, 1984, 139(2):487-492.
pmid: 6476384 |
| [26] |
Pandey P, Srivastava RK, Rajpoot R, et al. Water deficit and aluminum interactive effects on generation of reactive oxygen species and responses of antioxidative enzymes in the seedlings of two rice cultivars differing in stress tolerance[J]. Environ Sci Pollut Res Int, 2016, 23(2):1516-1528.
doi: 10.1007/s11356-015-5392-8 URL |
| [27] |
董亚茹, 张艳波, 赵东晓, 等. 外源24-表油菜素内酯对NaCl胁迫下桑树幼苗的缓解效应[J]. 核农学报, 2021, 35(6):1466-1475.
doi: 10.11869/j.issn.100-8551.2021.06.1466 |
|
Dong YR, Zhang YB, Zhao DX, et al. Alleviation effect of exogenous 24-epigenolide on mulberry seedlings under NaCl stress[J]. J Nucl Agric Sci, 2021, 35(6):1466-1475.
doi: 10.11869/j.issn.100-8551.2021.06.1466 |
|
| [28] |
Fuerst EP, Irzyk GP, Miller KD. Partial characterization of glutathione S-transferase isozymes induced by the herbicide safener benoxacor in maize[J]. Plant Physiol, 1993, 102(3):795-802.
pmid: 12231867 |
| [29] |
Singh N, Ma LQ, Srivastava M, et al. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L and Pteris ensiformis L[J]. Plant Sci, 2006, 170(2):274-282.
doi: 10.1016/j.plantsci.2005.08.013 URL |
| [30] |
de Vos CH, Vonk MJ, Vooijs R, et al. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene Cucubalus[J]. Plant Physiol, 1992, 98(3):853-858.
doi: 10.1104/pp.98.3.853 pmid: 16668756 |
| [31] |
Xie ZL, Nolan TM, Jiang H, et al. AP2/ERF transcription factor regulatory networks in hormone and abiotic stress responses in Arabidopsis[J]. Front Plant Sci, 2019, 10:228.
doi: 10.3389/fpls.2019.00228 URL |
| [32] |
Klay I, Gouia S, Liu MC, et al. Ethylene Response Factors(ERF)are differentially regulated by different abiotic stress types in tomato plants[J]. Plant Sci, 2018, 274:137-145.
doi: 10.1016/j.plantsci.2018.05.023 URL |
| [33] | Zhang J, Shi SZ, Jiang YN, et al. Genome-wide investigation of the AP2/ERF superfamily and their expression under salt stress in Chinese willow(Salix matsudana)[J]. PeerJ, 2021, 9:e11076. |
| [34] |
Kirienko DR, Luo AD, Sylvester AW. Reliable transient transformation of intact maize leaf cells for functional genomics and experimental study[J]. Plant Physiol, 2012, 159(4):1309-1318.
doi: 10.1104/pp.112.199737 pmid: 22706447 |
| [35] |
Chen XL, Equi R, Baxter H, et al. A high-throughput transient gene expression system for switchgrass(Panicum virgatum L.)seedlings[J]. Biotechnol Biofuels, 2010, 3:9.
doi: 10.1186/1754-6834-3-9 URL |
| [36] | Lu YM, Chen X, Wu YX, et al. Directly transforming PCR-amplified DNA fragments into plant cells is a versatile system that facilitates the transient expression assay[J]. PLoS One, 2013, 8(2):e57171. |
| [37] | He ZH, Li ZY, Lu HJ, et al. The NAC protein from Tamarix hispida, ThNAC7, confers salt and osmotic stress tolerance by increasing reactive oxygen species scavenging capability[J]. Plants(Basel), 2019, 8(7):221. |
| [38] | Liu YJ, Ji XY, Nie XG, et al. Arabidopsis AtbHLH112 regulates the expression of genes involved in abiotic stress tolerance by binding to their E-box and GCG-box motifs[J]. New Phytol, 2015, 207 |
| 3):692-709. | |
| [39] | 张群, 及晓宇, 贺子航, 等. 白桦BpGRAS1基因的克隆及耐盐功能分析[J]. 南京林业大学学报:自然科学版, 2021, 45(5):38-46. |
| Zhang Q, Ji XY, He ZH, et al. Cloning and salt tolerance analysis of BpGRAS1 gene in Betula platyphylla[J]. J Nanjing For Univ Nat Sci Ed, 2021, 45(5):38-46. | |
| [40] |
Qin LP, Wang LQ, Guo Y, et al. An ERF transcription factor from Tamarix hispida, ThCRF1, can adjust osmotic potential and reactive oxygen species scavenging capability to improve salt tolerance[J]. Plant Sci, 2017, 265:154-166.
doi: 10.1016/j.plantsci.2017.10.006 URL |
| [41] |
Gao Y, Han D, Jia W, et al. Molecular characterization and systematic analysis of NtAP2/ERF in tobacco and functional determination of NtRAV-4 under drought stress[J]. Plant Physiol Biochem, 2020, 156:420-435.
doi: 10.1016/j.plaphy.2020.09.027 URL |
| [42] |
Wu DD, Sun YH, Wang HF, et al. The SlNAC8 gene of the halophyte Suaeda liaotungensis enhances drought and salt stress tolerance in transgenic Arabidopsis thaliana[J]. Gene, 2018, 662:10-20.
doi: 10.1016/j.gene.2018.04.012 URL |
| [43] |
Choudhury FK, Rivero RM, Blumwald E, et al. Reactive oxygen species, abiotic stress and stress combination[J]. Plant J, 2017, 90(5):856-867.
doi: 10.1111/tpj.13299 URL |
| [44] |
Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants[J]. Plant Physiol Biochem, 2010, 48(12):909-930.
doi: 10.1016/j.plaphy.2010.08.016 URL |
| [45] | Yang GY, Peng SB, Wang TY, et al. Walnut ethylene response factor JrERF2-2 interact with JrWRKY7 to regulate the GSTs in plant drought tolerance[J]. Ecotoxicol Environ Saf, 2021, 228:112945. |
| [46] |
Zhu JK. Abiotic stress signaling and responses in plants[J]. Cell, 2016, 167(2):313-324.
doi: 10.1016/j.cell.2016.08.029 URL |
| [47] | Ren YR, Yang YY, Zhao Q, et al. MdCIB1, an apple bHLH transcription factor, plays a positive regulator in response to drought stress[J]. Environ Exp Bot, 2021, 188:104523. |
| [48] |
Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance[J]. Environ Exp Bot, 2007, 59(2):206-216.
doi: 10.1016/j.envexpbot.2005.12.006 URL |
| [1] | WANG Zi-ying, LONG Chen-jie, FAN Zhao-yu, ZHANG Lei. Screening of OsCRK5-interacted Proteins in Rice Using Yeast Two-hybrid System [J]. Biotechnology Bulletin, 2023, 39(9): 117-125. |
| [2] | LIU Wen-jin, MA Rui, LIU Sheng-yan, YANG Jiang-wei, ZHANG Ning, SI Huai-jun. Cloning of StCIPK11 Gene and Analysis of Its Response to Drought Stress in Solanum tuberosum [J]. Biotechnology Bulletin, 2023, 39(9): 147-155. |
| [3] | DING Kai-xin, WANG Li-chun, TIAN Guo-kui, WANG Hai-yan, LI Feng-yun, PAN Yang, PANG Ze, SHAN Ying. Research Progress in Uniconazole Alleviating Plant Drought Damage [J]. Biotechnology Bulletin, 2023, 39(6): 1-11. |
| [4] | WANG Chun-yu, LI Zheng-jun, WANG Ping, ZHANG Li-xia. Physiological and Biochemical Analysis of Drought Resistance in Sorghum Cuticular Wax-deficient Mutant sb1 [J]. Biotechnology Bulletin, 2023, 39(5): 160-167. |
| [5] | WANG Hai-long, LI Yu-qian, WANG Bo, XING Guo-fang, ZHANG Jie-wei. Isolation and Expression Analysis of SiMAPK3 in Setaria italica L. [J]. Biotechnology Bulletin, 2023, 39(3): 123-132. |
| [6] | WANG Qi, HU Zhe, FU Wei, LI Guang-zhe, HAO Lin. Regulation of Burkholderia sp. GD17 on the Drought Tolerance of Cucumber Seedlings [J]. Biotechnology Bulletin, 2023, 39(3): 163-175. |
| [7] | JIANG Min-xuan, LI Kang, LUO Liang, LIU Jian-xiang, LU Hai-ping. Advances on the Expressions of Foreign Proteins in Plants [J]. Biotechnology Bulletin, 2023, 39(11): 110-122. |
| [8] | YU Bo, QIN Xiao-hui, ZHAO Yang. Mechanisms of Plant Sensing Drought Signals [J]. Biotechnology Bulletin, 2023, 39(11): 6-17. |
| [9] | CHEN Chu-yi, YANG Xiao-mei, CHEN Sheng-yan, CHEN Bin, YUE Li-ran. Expression Analysis of the ZF-HD Gene Family in Chrysanthemum nankingense Under Drought and ABA Treatment [J]. Biotechnology Bulletin, 2023, 39(11): 270-282. |
| [10] | FENG Ce-ting, JIANG Lyu, LIU Xing-ying, LUO Le, PAN Hui-tang, ZHANG Qi-xiang, YU Chao. Identification of the NAC Gene Family in Rosa persica and Response Analysis Under Drought Stress [J]. Biotechnology Bulletin, 2023, 39(11): 283-296. |
| [11] | YAN Meng-yu, WEI Xiao-wei, CAO Jing, LAN Hai-yan. Cloning of Basic Helix-loop-helix(bHLH)Transcription Factor Gene SabHLH169 in Suaeda aralocaspica and Analysis of Its Resistances to Drought Stress [J]. Biotechnology Bulletin, 2023, 39(11): 328-339. |
| [12] | SHI Guang-zhen, WANG Zhao-ye, SUN Qi, ZHU Xin-xia. Cloning and Activity Analysis of SikCDPK1 Promoter from Saussurea involucrata [J]. Biotechnology Bulletin, 2022, 38(9): 191-197. |
| [13] | GUAN Zhi-xiu, WANG Yan, LIANG Cheng-gang, WEI Chun-yu, HUANG Juan, CHEN Qing-fu. Identification of FtCBL Genes in Fagopyrum tataricum and Their Stress Responses to Drought and High Calcium [J]. Biotechnology Bulletin, 2022, 38(8): 101-109. |
| [14] | CHEN Jia-min, LIU Yong-jie, MA Jin-xiu, LI Dan, GONG Jie, ZHAO Chang-ping, GENG Hong-wei, GAO Shi-qing. Expression Pattern Analysis of Histone Methyltransferase Under Drought Stress in Hybrid Wheat [J]. Biotechnology Bulletin, 2022, 38(7): 51-61. |
| [15] | YU Guo-hong, LIU Peng-cheng, LI Lei, LI Ming-zhe, CUI Hai-ying, HAO Hong-bo, GUO An-qiang. Physiological Responses of Potato in Different Genotypes to Drought Stress [J]. Biotechnology Bulletin, 2022, 38(5): 56-63. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||