








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (8): 69-76.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1062
Previous Articles Next Articles
LI Jia-le1( ), LIN Sheng-hao2, XU Wen-tao1,2(
), LIN Sheng-hao2, XU Wen-tao1,2( )
)
Received:2021-08-19
Online:2022-08-26
Published:2022-09-14
Contact:
XU Wen-tao
E-mail:815403750@qq.com;xuwentao@cau.edu.cn
LI Jia-le, LIN Sheng-hao, XU Wen-tao. Construction of an Ultra-sensitive Colorimetric Biosensor for Insect Resistance Genes Based on Loop-mediated Isothermal Amplification[J]. Biotechnology Bulletin, 2022, 38(8): 69-76.
| 品种 Variety | 测试样品 Testing sample | Cry1A基因信息 Cry1A gene information | 研发单位 R & D unit |
|---|---|---|---|
| 转基因水稻 | Kefeng 6 | Cry1Ac | 中国科学院遗传与发育研究所、福建省农业科学院 |
| Kefeng 8 | Cry1Ac | 中国科学院遗传与发育生物学研究所、福建省农业科学院生物技术研究所、山西省农业科学院棉花研究所 | |
| TT51 | Cry1Ab/Ac | 华中农业大学 | |
| 转基因玉米 | Bt11 | Cry1Ab | 先正达种子有限公司 |
| Bt176 | Cry1Ab | 先正达种子有限公司 | |
| MON 810 | Cry1Ab | 美国孟山都公司 | |
| 转基因棉花 | MON 15985 | Cry1Ac | 美国孟山都公司 |
| 转基因甜菜 | H7-1 | - | 美国孟山都公司 |
| 非转基因作物 | 五优稻4号 | - | 五常市龙凤山长粒香水稻研究所 |
| 粳稻糙米 | - | 五常市龙凤山长粒香水稻研究所 |
Table 1 Test specific information of Cry1A gene in GMO and non-GMO samples
| 品种 Variety | 测试样品 Testing sample | Cry1A基因信息 Cry1A gene information | 研发单位 R & D unit |
|---|---|---|---|
| 转基因水稻 | Kefeng 6 | Cry1Ac | 中国科学院遗传与发育研究所、福建省农业科学院 |
| Kefeng 8 | Cry1Ac | 中国科学院遗传与发育生物学研究所、福建省农业科学院生物技术研究所、山西省农业科学院棉花研究所 | |
| TT51 | Cry1Ab/Ac | 华中农业大学 | |
| 转基因玉米 | Bt11 | Cry1Ab | 先正达种子有限公司 |
| Bt176 | Cry1Ab | 先正达种子有限公司 | |
| MON 810 | Cry1Ab | 美国孟山都公司 | |
| 转基因棉花 | MON 15985 | Cry1Ac | 美国孟山都公司 |
| 转基因甜菜 | H7-1 | - | 美国孟山都公司 |
| 非转基因作物 | 五优稻4号 | - | 五常市龙凤山长粒香水稻研究所 |
| 粳稻糙米 | - | 五常市龙凤山长粒香水稻研究所 |
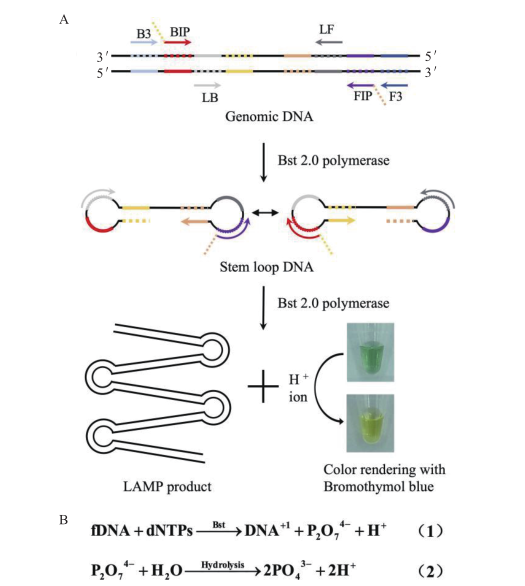
Fig. 1 Detection principle diagram A:Schematic diagram of the experiment. B:Schematic diagram of the chemical equation generated by the LAMP reaction H+
| 引物名称 Primer name | 序列 Sequence(5'-3') | |
|---|---|---|
| Cry1Ac&Ab/Ac -1 | F3 | GGTGGAAGACAAGGTTCTGT |
| B3 | TCTACACCGATGCTCACAGA | |
| FIP | GGAACTATGGGAAACGCCGCT- AGACACCCTGACCTAGTTGA | |
| BIP | GAGGAAAGGTAAACTCGGGCCC- TGGTCTGGACACCAGATCA | |
| LF | CACAACAACGTATCGTTGC | |
| LB | GCTGAATCCAACTGGAGAGGC | |
| Cry1Ac&Ab/Ac -2 | F3 | TGATGCTCACGGAACTGTTG |
| B3 | TCGCCTATGGAACCTCTTCT | |
| FIP | CCACCCAGGCAAGGATTCTCC- TGAATCCGGAACGGAACATG | |
| BIP | TGTGGTGGGATTTCGTCCAAGG- AACTTGCCATCCGCTGTT | |
| LF | ACAGGTTGAGCCACGTGTC | |
| LB | AATCAACGGTACCGCTCTTTC | |
| Cry1Ac&Ab/Ac -3 | F3 | GGAGCTCTGATGATGCTCAC |
| B3 | TCGCCTATGGAACCTCTTCT | |
| FIP | CAGGCAAGGATTCTCCCACAGG- GGAACTGTTGCTGAATCCGG | |
| BIP | TGTGGTGGGATTTCGTCCAAGG- AACTTGCCATCCGCTGTT | |
| LF | CCACGTGTCCATGTTCCGTT | |
| LB | ACGGTACCGCTCTTTCTGT | |
Table 2 LAMP primer design table
| 引物名称 Primer name | 序列 Sequence(5'-3') | |
|---|---|---|
| Cry1Ac&Ab/Ac -1 | F3 | GGTGGAAGACAAGGTTCTGT |
| B3 | TCTACACCGATGCTCACAGA | |
| FIP | GGAACTATGGGAAACGCCGCT- AGACACCCTGACCTAGTTGA | |
| BIP | GAGGAAAGGTAAACTCGGGCCC- TGGTCTGGACACCAGATCA | |
| LF | CACAACAACGTATCGTTGC | |
| LB | GCTGAATCCAACTGGAGAGGC | |
| Cry1Ac&Ab/Ac -2 | F3 | TGATGCTCACGGAACTGTTG |
| B3 | TCGCCTATGGAACCTCTTCT | |
| FIP | CCACCCAGGCAAGGATTCTCC- TGAATCCGGAACGGAACATG | |
| BIP | TGTGGTGGGATTTCGTCCAAGG- AACTTGCCATCCGCTGTT | |
| LF | ACAGGTTGAGCCACGTGTC | |
| LB | AATCAACGGTACCGCTCTTTC | |
| Cry1Ac&Ab/Ac -3 | F3 | GGAGCTCTGATGATGCTCAC |
| B3 | TCGCCTATGGAACCTCTTCT | |
| FIP | CAGGCAAGGATTCTCCCACAGG- GGAACTGTTGCTGAATCCGG | |
| BIP | TGTGGTGGGATTTCGTCCAAGG- AACTTGCCATCCGCTGTT | |
| LF | CCACGTGTCCATGTTCCGTT | |
| LB | ACGGTACCGCTCTTTCTGT | |
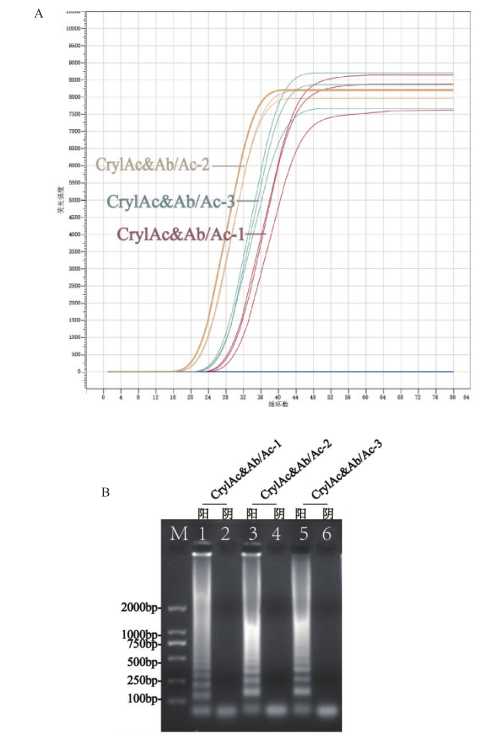
Fig.2 Primer screening diagram A:Real-time amplification curve of 3 sets of primers. B:Electropherogram of the end point of 3 sets of primers amplification
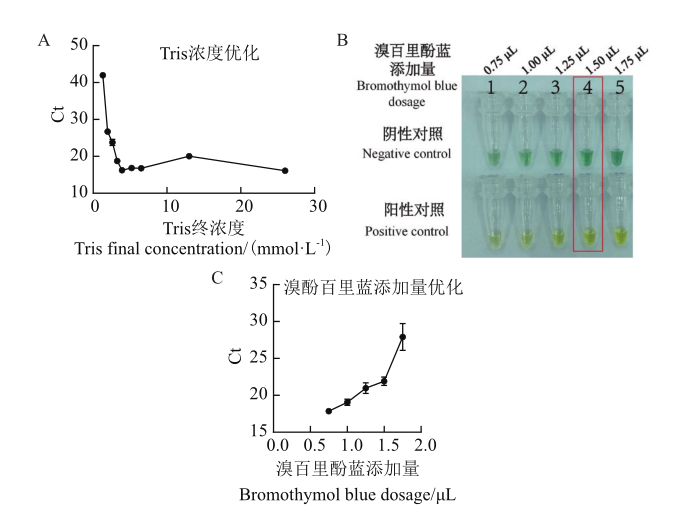
Fig. 3 Feasibility and optimization of colorimetric method A:Tris concentration optimization. B:Comparison of indication effects under different volumes of bromothymol blue addition. C:Influence diagram of different addition amounts of bromothymol blue
| [1] | 李葱葱, 闫伟, 夏蔚, 等. 应用简并PCR方法检测转cry1A基因作物[J]. 食品科学, 2018, 39(14):317-322. |
|
Li CC, Yan W, Xia, et al. Detection of genetically modified crops with cry1A gene by PCR with degenerate primers[J]. Food Sci, 2018, 39(14):317-322.
doi: 10.1111/j.1365-2621.1974.tb02884.x URL |
|
| [2] | H·安德森, J·杜格拉斯, J·格罗亚特, 等. 对应于转基因事件MON89034的玉米植物和种子及其检测和使用方法:中国, CN101495635[P]. 2009-07-29. |
| Anderson H, Duglas J, Groat J, et al. Maize plants and seeds corresponding to the transgenic event MON89034 and methods for their detection and use:China, CN101495635[P]. 2009-07-29. | |
| [3] | 赖锦盛, 董永彬, 宋伟彬, 等. 人工合成用于转基因抗虫植物的Bt杀虫基因:中国, CN101580843[P]. 2009-11-18. |
| Lai JS, Dong YB, Song WB, et al. Artificial synthesis of Bt insecticide genes for transgenic insect-resistant plants:China, CN101580843[P]. 2009-11-18. | |
| [4] |
Brookes G, Barfoot P. GM crop technology use 1996-2018:farm income and production impacts[J]. GM Crops Food, 2020, 11(4):242-261.
doi: 10.1080/21645698.2020.1779574 pmid: 32706314 |
| [5] |
Kumar K, Gambhir G, Dass A, et al. Genetically modified crops:current status and future prospects[J]. Planta, 2020, 251(4):91.
doi: 10.1007/s00425-020-03372-8 URL |
| [6] | 张大兵, 郭金超. 转基因生物及其产品检测技术和标准化[J]. 生命科学, 2011, 23(2):195-204. |
| Zhang DB, Guo JC. The development and standardization of testing approaches for genetically modified organisms and their derived products[J]. Chin Bull Life Sci, 2011, 23(2):195-204. | |
| [7] |
Zhao ZY, Chen YS, Xu WZ, et al. Surface plasmon resonance detection of transgenic Cry1Ac cotton(Gossypium spp. )[J]. J Agric Food Chem, 2013, 61(12):2964-2969.
doi: 10.1021/jf3050439 URL |
| [8] |
Rupula K, Kosuri T, Gul MZ, et al. Immuno-analytical method development for detection of transgenic Cry1Ac protein and its validation[J]. J Sci Food Agric, 2019, 99(15):6903-6910.
doi: 10.1002/jsfa.9976 URL |
| [9] | Jambagi P, Shankergoud J, Nidagundi A, et al. Detecting cry1Ac by loop mediated isothermal amplification by SYBR green-I[J]. 2018, 7(2):2176-2180. |
| [10] | Grohmann L, Reiting R, Mäde D, et al. Collaborative trial validation of cry1Ab/Ac and Pubi-cry TaqMan-based real-time PCR assays for detection of DNA derived from genetically modified Bt plant products[J]. Accreditation Qual Assur, 2015, 20(2):85-96. |
| [11] |
Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA[J]. Nucleic Acids Res, 2000, 28(12):E63.
doi: 10.1093/nar/28.12.e63 pmid: 10871386 |
| [12] |
Qin A, Fu LT, Wong JK, et al. Precipitation of PEG/carboxyl-modified gold nanoparticles with magnesium pyrophosphate:a new platform for real-time monitoring of loop-mediated isothermal amplification[J]. ACS Appl Mater Interfaces, 2017, 9(12):10472-10480.
doi: 10.1021/acsami.7b00046 URL |
| [13] |
Liu H, Wu W, Tan J, et al. Development and evaluation of a one-step reverse transcription loop-mediated isothermal amplification for detection of Citrus leaf blotch virus[J]. J Virol Methods, 2019, 270:150-152.
doi: 10.1016/j.jviromet.2019.05.009 URL |
| [14] | 王晨光, 许文涛, 黄昆仑, 等. 转基因食品分析检测技术研究进展[J]. 食品科学, 2014, 35(21):297-305. |
| Wang CG, Xu WT, Huang KL, et al. Recent progress in techniques for the detection and analysis of genetically modified foods[J]. Food Sci, 2014, 35(21):297-305. | |
| [15] |
Chen K, Han H, Luo Z, et al. A practicable detection system for genetically modified rice by SERS-barcoded nanosensors[J]. Biosens Bioelectron, 2012, 34(1):118-124.
doi: 10.1016/j.bios.2012.01.029 pmid: 22342698 |
| [16] |
Sarkes A, Fu H, Feindel D, et al. Development and evaluation of a loop-mediated isothermal amplification(LAMP)assay for the detection of Tomato brown rugose fruit virus(ToBRFV)[J]. PLoS One, 2020, 15(6):e0230403.
doi: 10.1371/journal.pone.0230403 URL |
| [17] |
Lam P, Keri RA, Steinmetz NF. A bioengineered positive control for rapid detection of the Ebola virus by reverse transcription loop-mediated isothermal amplification(RT-LAMP)[J]. ACS Biomater Sci Eng, 2017, 3(3):452-459.
doi: 10.1021/acsbiomaterials.6b00769 URL |
| [18] |
Tao Y, Yun J, Wang J, et al. High-performance detection of Mycobacterium bovis in milk using digital LAMP[J]. Food Chem, 2020, 327:126945.
doi: 10.1016/j.foodchem.2020.126945 URL |
| [19] |
Tanner NA, Zhang Y, Evans TC. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes[J]. Biotechniques, 2015, 58(2):59-68.
doi: 10.2144/000114253 URL |
| [20] | Charoenpanich P, Mungkung A, Seeviset N, et al. A pH sensitive, loop-mediated isothermal amplification assay for detection of Salmonella in food[J]. Science, Engineering and Health Studies, 2020, 14(3):160-168. |
| [21] |
Ferrara M, Logrieco AF, Moretti A, et al. A loop-mediated isothermal amplification(LAMP)assay for rapid detection of fumonisin producing Aspergillus species[J]. Food Microbiol, 2020, 90:103469.
doi: 10.1016/j.fm.2020.103469 URL |
| [22] |
Xiong J, Huang B, Xu JS, et al. A closed-tube loop-mediated isothermal amplification assay for the visual detection of Staphylococcus aureus[J]. Appl Biochem Biotechnol, 2020, 191(1):201-211.
doi: 10.1007/s12010-020-03278-x pmid: 32103471 |
| [23] |
Tatulli G, Cecere P, Maggioni D, et al. A rapid colorimetric assay for on-site authentication of cephalopod species[J]. Biosensors, 2020, 10(12):190.
doi: 10.3390/bios10120190 URL |
| [24] |
Li Y, Wang Y, Song K, et al. A rapid and sensitive colorimetric assay for the determination of adenosine kinase activity[J]. Biochem Biophys Res Commun, 2018, 502(2):250-254.
doi: 10.1016/j.bbrc.2018.05.152 URL |
| [25] |
Magnaghi LR, Alberti G, Capone F, et al. Development of a dye-based device to assess the poultry meat spoilage. part II:array on act[J]. J Agric Food Chem, 2020, 68(45):12710-12718.
doi: 10.1021/acs.jafc.0c03771 URL |
| [26] |
Stocker MK, Sanson ML, Bernardes AA, et al. Acid-base sensor based on sol-gel encapsulation of bromothymol blue in silica:application for milk spoilage detection[J]. J Sol Gel Sci Technol, 2021, 98(3):568-579.
doi: 10.1007/s10971-021-05529-7 URL |
| [27] |
Ong SA, Wu JC. A simple method for rapid screening of biosurfactant-producing strains using bromothymol blue alone[J]. Biocatal Agric Biotechnol, 2018, 16:121-125.
doi: 10.1016/j.bcab.2018.07.027 URL |
| [28] |
Ramadan AA, Zeino S. Development and validation of spectrophotometric determination of glimepiride in pure and tablet dosage forms through ion-pair complex formation using bromothymol blue[J]. Rese Jour Pharm And Technol, 2018, 11(7):3049.
doi: 10.5958/0974-360X.2018.00561.9 URL |
| [29] |
Gul I, Bogale TF, Deng J, et al. Enzyme-based detection of epoxides using colorimetric assay integrated with smartphone imaging[J]. Biotechnol Appl Biochem, 2020, 67(4):685-692.
doi: 10.1002/bab.1898 URL |
| [1] | ZHANG Ya-han, ZHU Li-xia, HU Jing, ZHU Ya-jing, ZHANG Xue-jing, CAO Ye-zhong. Opportunities and Challenges of Glyphosate in the Application of Biotechnology Breeding in China [J]. Biotechnology Bulletin, 2022, 38(11): 1-9. |
| [2] | SHI Xin-yue, SHANG Xiao-yao, ZHOU Ling-fang, ZHANG Tie-jun, CHAO Yue-hui. Cloning and Transformation of MsAP2 Gene in Medicago sativa [J]. Biotechnology Bulletin, 2021, 37(12): 13-21. |
| [3] | WANG Qi-wen, LI Pan, Pan Cui-yun, HAN Fen-xia. Effect of Ethylene Glycol on the Expression of Exogenous Genes in Vivo [J]. Biotechnology Bulletin, 2019, 35(4): 64-68. |
| [4] | LIU Cha, HAN Li-hong, WANG Hai-bo, GAO Yong, TANG Li-zhou. Research Advances on Plant Thaumatin-like Protein Family [J]. Biotechnology Bulletin, 2018, 34(3): 9-17. |
| [5] | XIE Wen-ping, ZHAO Hui-jun, ZHANG Lin, JIANG Hong-xu, WU Bin, SUN Hao. Rapid Diagnosis of Brucella by Loop-mediated Isothermal Amplification [J]. Biotechnology Bulletin, 2017, 33(3): 186-192. |
| [6] | Jing Zhaobin, Lei Yushan, Li Yongwu. Biotechnology and Kiwifruit Breeding in China [J]. Biotechnology Bulletin, 2015, 31(7): 1-10. |
| [7] | He Qian, Wang Yanping, Chen Yuzhen, Lu Cunfu. Ectopic-overexpression of AmHsa32 from Ammopiptanthus mongolicus Promoted Heat Tolerance in Nicotiana benthamiana [J]. Biotechnology Bulletin, 2015, 31(5): 100-105. |
| [8] | Lu Baorong. Analysis of Fitness Effect and Its Application in Assessing Environmental Risk Caused by Transgene Flow [J]. Biotechnology Bulletin, 2015, 31(4): 7-16. |
| [9] | Wang Hefei, Liu Dongjun. Transgenic Technology: Establishment of Animal Models and Treatment of Diabetes Mellitus [J]. Biotechnology Bulletin, 2015, 31(10): 89-98. |
| [10] | Lu Rui, Song Shaozheng, Qi Zhengqiang, Ge Xin, Shao Bin, Cheng Yong. Preparation of Recombinant Human Plasminogen Activator in Rabbit Mammary Gland and Detection of Expressed Products [J]. Biotechnology Bulletin, 2015, 31(10): 216-221. |
| [11] | Li Ji, Lu Xu, Huang Tiandai, Hua Yuwei, Huang Huasun. Optimization of Digoxigenin Based Southern Blot for Transgenic Hevea brasiliensis Analysis [J]. Biotechnology Bulletin, 2014, 0(8): 76-81. |
| [12] | Dong Ying, Hu Hongxia, Wang Wei, Tian Zhaohui. Construction of Transgenic Goldfish Based on Tol2 Transposable Element [J]. Biotechnology Bulletin, 2014, 0(5): 122-128. |
| [13] | Kuang Xiaoshan, Hu Songnan, Wang Xiaoyu, Tang Shiming, Cheng Xiaowei, Feng Jiawang. Development of a Real-time Turbidimeter-based LAMP Method for Detection of NPTⅡ Gene in the Genetically Modified Plant [J]. Biotechnology Bulletin, 2014, 0(3): 60-64. |
| [14] | Gan Yimei, Zhang Shuzhen, Zeng Fanyun, Feng Cuilian, Yang Benpeng. Advance in Sugarcane Transgenic Breeding [J]. Biotechnology Bulletin, 2013, 0(3): 1-9. |
| [15] | Chen Sijie, Zhang Hefei, Zhang Cuizhen, Peng Gang. Fluorescence-activated Cell Sorting(FACS)of Fluorescently Tagged Cells from Transgenic Zebrafish Larvae for Gene Expression Analysis [J]. Biotechnology Bulletin, 2013, 0(11): 112-116. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||