








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (9): 74-81.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0441
Previous Articles Next Articles
ZHAO Ping-juan1,2( ), LIN Chen-yu1, WANG Meng-yue1, ZHANG Xiu-chun1,2, LI Shu-xia1,2, RUAN Meng-bin1,2(
), LIN Chen-yu1, WANG Meng-yue1, ZHANG Xiu-chun1,2, LI Shu-xia1,2, RUAN Meng-bin1,2( )
)
Received:2024-05-11
Online:2024-09-26
Published:2024-10-12
Contact:
RUAN Meng-bin
E-mail:zhaopingjuan@itbb.org.cn;ruanmmmmmengbin@itbb.org.cn
ZHAO Ping-juan, LIN Chen-yu, WANG Meng-yue, ZHANG Xiu-chun, LI Shu-xia, RUAN Meng-bin. Sequence Analysis of MeSDH Protein and Its Relationship with MeH1.2 in Cassava[J]. Biotechnology Bulletin, 2024, 40(9): 74-81.
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 目的Purpose |
|---|---|---|
| MeSDH-F | atgggtaaaggagggatgtctc | 基因克隆 Gene cloning |
| MeSDH-R | cagattaaacatgaccttaatg | |
| 1300-MeSDH-F | tgatacatatgcccgtcgac atgggtaaaggagggatgtctc | 植物表达载体 Plant expression vector |
| 1300-MeSDH-R | ctcaccatggatccggtacc cagattaaacatgaccttaatg | |
| Actin1-F | tggattctggtgatggtgtgagt | 内参基因 Reference gene |
| Actin1-R | ccgttcagcagtggtggtga | |
| MeSDH-Q-F | gtgaaggagccgatggtga | MeSDH定量PCR MeSDH RT-qPCR |
| MeSDH-Q-R | ccacacggtctccaggtaaaa | |
| H1.2-PGBKT7-X | tctagaatggccgactctgaagttcaggct | 酵母文库筛选 Yeast library screening |
| H1.2-PGBKT7-B | ggatcctttcttcgccttcttcgctgtc | |
| MeSDH-AD-F | ccatggaggccagtgaattcatgggtaaaggagggatgtc | Y2H载体 Y2H vector |
| MeSDH-AD-R | agctcgagctcgatggatccttacagattaaacatgacctt | |
| MeSDH-C-F | tctagaatggccgactctgaagttcaggct | BiFC载体 BiFC vector |
| MeSDH-C-R | ggatcctttcttcgccttcttcgctgtc |
Table 1 Primers’ sequences used in the study
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 目的Purpose |
|---|---|---|
| MeSDH-F | atgggtaaaggagggatgtctc | 基因克隆 Gene cloning |
| MeSDH-R | cagattaaacatgaccttaatg | |
| 1300-MeSDH-F | tgatacatatgcccgtcgac atgggtaaaggagggatgtctc | 植物表达载体 Plant expression vector |
| 1300-MeSDH-R | ctcaccatggatccggtacc cagattaaacatgaccttaatg | |
| Actin1-F | tggattctggtgatggtgtgagt | 内参基因 Reference gene |
| Actin1-R | ccgttcagcagtggtggtga | |
| MeSDH-Q-F | gtgaaggagccgatggtga | MeSDH定量PCR MeSDH RT-qPCR |
| MeSDH-Q-R | ccacacggtctccaggtaaaa | |
| H1.2-PGBKT7-X | tctagaatggccgactctgaagttcaggct | 酵母文库筛选 Yeast library screening |
| H1.2-PGBKT7-B | ggatcctttcttcgccttcttcgctgtc | |
| MeSDH-AD-F | ccatggaggccagtgaattcatgggtaaaggagggatgtc | Y2H载体 Y2H vector |
| MeSDH-AD-R | agctcgagctcgatggatccttacagattaaacatgacctt | |
| MeSDH-C-F | tctagaatggccgactctgaagttcaggct | BiFC载体 BiFC vector |
| MeSDH-C-R | ggatcctttcttcgccttcttcgctgtc |
| A 实验组1 | B 实验组2 | C 实验组3 | D 实验组4 |
|---|---|---|---|
| pGADT7-T | pGADT7-T | pGADT7-MeSDH | pGADT7 |
| pGBKT7-53 | pGBKT7-lam | pGBKT7-MeH1.2 | pGBKT7-MeH1.2 |
Table 2 Sample design
| A 实验组1 | B 实验组2 | C 实验组3 | D 实验组4 |
|---|---|---|---|
| pGADT7-T | pGADT7-T | pGADT7-MeSDH | pGADT7 |
| pGBKT7-53 | pGBKT7-lam | pGBKT7-MeH1.2 | pGBKT7-MeH1.2 |
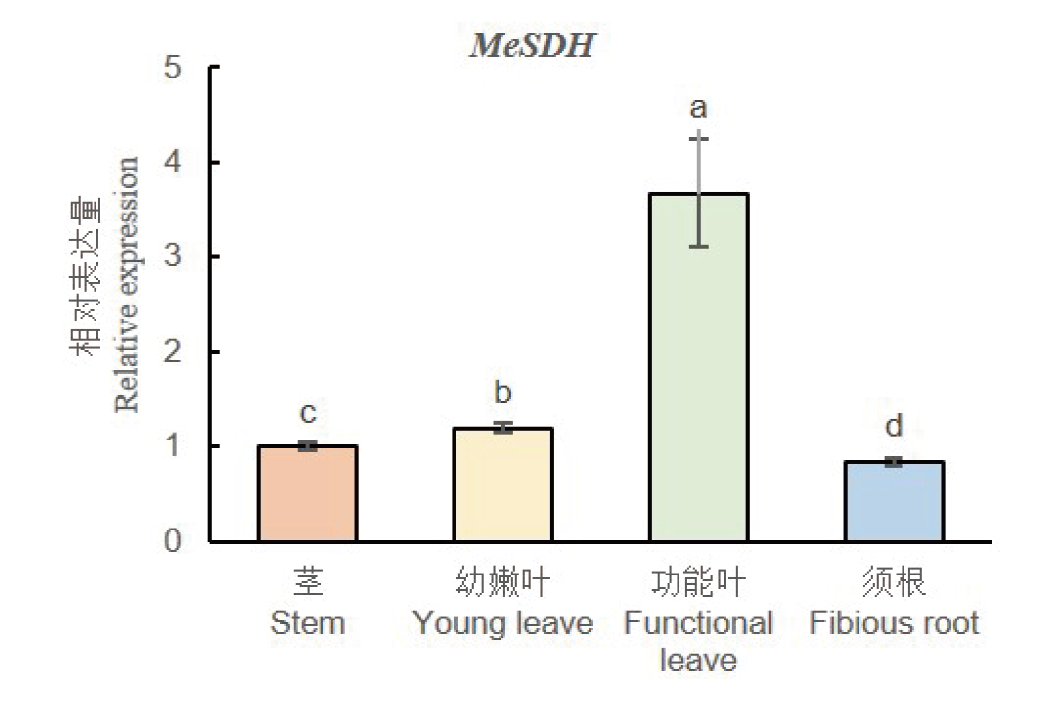
Fig. 3 Tissue-specific expressions of MeSDH gene in cassava Different letters show that gene expression is significant difference among other tissues on P<0.05
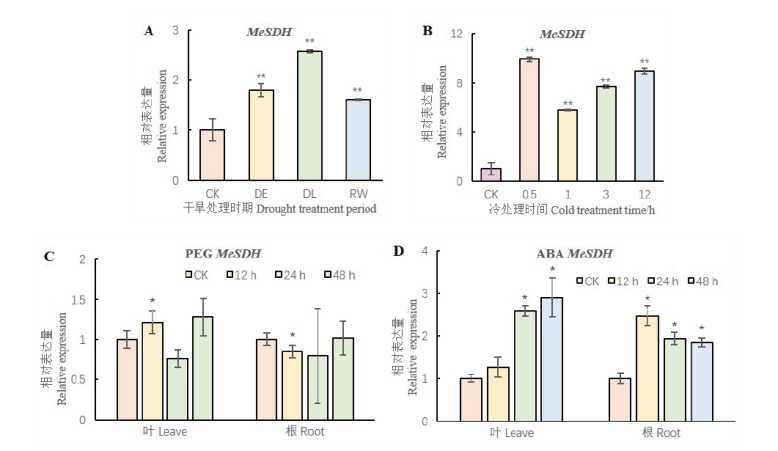
Fig. 5 Expression patterns of MeSDH in the leaves and roots under different treatment *, ** indicate gene expression is significant difference between treatment and control groups in P<0.05 and P<0.01 level
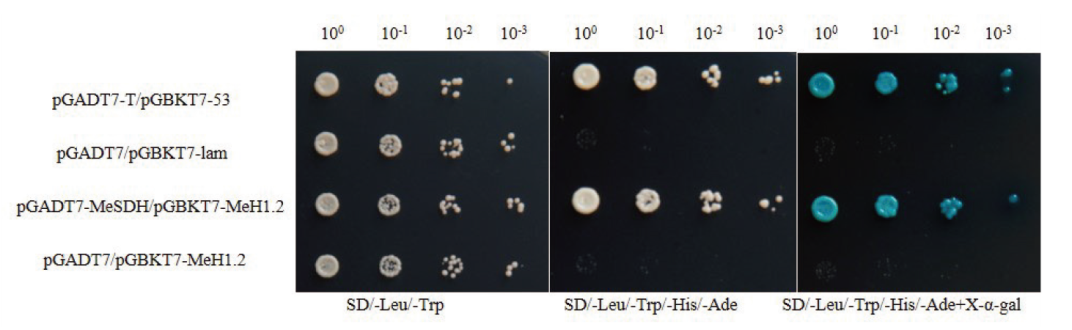
Fig. 6 Relationship between MeSDH and MeH1.2 proteins verified by Y2H pGADT7-T/pGBKT7-53 is positive control; pGADT7-T/pGBKT7-Lam and pGADT7-T/pGBKT7-MeH1.2 are negative control; 100, 10-1, 10-2, and 10-3 refers to the dilution gradients of bacterial solution

Fig. 7 Relationship between MeSDH and MeH1.2 proteins verified by BiFC MeTGA2-nYFP/MeHistone3-cYFP is positive control; MeH12-nYFP/cYFP和-nYFP/MeSDH-cYFP are negative control
| [1] | Khumaida N, Ardie S, Dianasari M, et al. Cassava(Manihot esculenta crantz.) improvement through gamma irradiation[J]. Procedia Food Sci, 2015, 3: 27-34. |
| [2] | 付丹丹, 韩昕儒, 问锦尚, 等. 基于Rotterdam模型的中国热带农产品进口市场格局研究[J]. 中国农业资源与区划, 2022, 43(11):168-177. |
| Fu DD, Han XR, Wen JS, et al. China's tropical agricultural product imports: A rotterdam model anlysis[J]. Chinese Journal of Agricultural Resources and Rrgional Planning, 2022, 43(11):168-177. | |
| [3] | Muiruri SK, Ntui VO, Tripathi L, et al. Mechanisms and approaches towards enhanced drought tolerance in cassava(Manihot esculenta)[J]. Curr Plant Biol, 2021, 28: 100227. |
| [4] |
Jia Y, Wong DCJ, Sweetman C, et al. New insights into the evolutionary history of plant sorbitol dehydrogenase[J]. BMC Plant Biol, 2015, 15: 101.
doi: 10.1186/s12870-015-0478-5 pmid: 25879735 |
| [5] |
Chen T, Zhang ZQ, Li BQ, et al. Molecular basis for optimizing sugar metabolism and transport during fruit development[J]. aBIOTECH, 2021, 2(3): 330-340.
doi: 10.1007/s42994-021-00061-2 pmid: 36303881 |
| [6] | Dai MS, Shi ZB, Xu CJ. Genome-wide analysis of sorbitol dehydrogenase(SDH)genes and their differential expression in two sand pear(Pyrus pyrifolia)fruits[J]. Int J Mol Sci, 2015, 16(6): 13065-13083. |
| [7] |
Nosarzewski M, Archbold DD. Tissue-specific expression of SORBITOL DEHYDROGENASE in apple fruit during early development[J]. J Exp Bot, 2007, 58(7): 1863-1872.
pmid: 17404378 |
| [8] | Wanek W, Richter A. L-Iditol: NAD+5-oxidoreductase in Viscum album: utilization of host-derived sorbitol[J]. Plant Physiol Biochem, 1993, 31: 205-211. |
| [9] |
Kuo TM, Doehlert DC, Crawford CG. Sugar metabolism in germinating soybean seeds: evidence for the sorbitol pathway in soybean axes[J]. Plant Physiol, 1990, 93(4): 1514-1520.
doi: 10.1104/pp.93.4.1514 pmid: 16667649 |
| [10] |
Nosarzewski M, Downie AB, Wu BH, et al. The role of SORBITOL DEHYDROGENASE in Arabidopsis thaliana[J]. Funct Plant Biol, 2012, 39(6): 462-470.
doi: 10.1071/FP12008 pmid: 32480797 |
| [11] |
Ohta K, Moriguchi R, Kanahama K, et al. Molecular evidence of sorbitol dehydrogenase in tomato, a Non-Rosaceae plant[J]. Phytochemistry, 2005, 66(24): 2822-2828.
doi: 10.1016/j.phytochem.2005.09.033 pmid: 16289145 |
| [12] |
Nadwodnik J, Lohaus G. Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens[J]. Planta, 2008, 227(5): 1079-1089.
doi: 10.1007/s00425-007-0682-0 pmid: 18188589 |
| [13] | 刘政, 安莉园, 林世华, 等. 梨树山梨醇代谢及其调控因子研究进展[J]. 中国南方果树, 2018, 47(4): 165-168. |
| Liu Z, An LY, Lin SH, et al. Research progress on sorbitol metabolism and its regulatory factors in pear trees[J]. South China Fruits, 2018, 47(4): 165-168. | |
| [14] |
聂佩显, 王来平, 韩雪平, 等. 杏山梨醇脱氢酶基因克隆及其在果实发育过程中的表达与酶活性分析[J]. 核农学报, 2021, 35(2): 291-297.
doi: 10.11869/j.issn.100-8551.2021.02.0291 |
|
Nie PX, Wang LP, Han XP, et al. Cloning of NAD-dependent sorbitol dehydrogenase gene from apricot fruit and analysis of its expression and enzyme activity[J]. J Nucl Agric Sci, 2021, 35(2): 291-297.
doi: 10.11869/j.issn.100-8551.2021.02.0291 |
|
| [15] |
Yamada K, Oura Y, Mori H, et al. Cloning of NAD-dependent sorbitol dehydrogenase from apple fruit and gene expression[J]. Plant Cell Physiol, 1998, 39(12): 1375-1379.
pmid: 10050321 |
| [16] | 任秋萍, 周淑梅, 王秀玲. 苹果山梨醇脱氢酶的基因表达、组织分布和活性调控[J]. 中国细胞生物学学报, 2010, 32(4): 668-672. |
| Ren QP, Zhou SM, Wang XL. Gene expression, tissue distribution and activity regulation of NAD+-dependent sorbitol dehydrogenase in apple[J]. Chin J Cell Biol, 2010, 32(4): 668-672. | |
| [17] | Bantog NA, Yamada K, Niwa N, et al. Gene expression of NAD+-dependent sorbitol dehydrogenase and NADP+-dependent sorbitol-6-phosphate dehydrogenase during development of loquat(Eriobotrya japonica lindl.) fruit[J]. Engei Gakkai Zasshi, 2000, 69(3): 231-236. |
| [18] |
Kelker NE, Anderson RL. Sorbitol metabolism in Aerobacter aerogenes[J]. J Bacteriol, 1971, 105(1): 160-164.
doi: 10.1128/jb.105.1.160-164.1971 pmid: 5541002 |
| [19] |
Sarthy AV, Schopp C, Idler KB. Cloning and sequence determination of the gene encoding sorbitol dehydrogenase from Saccharomyces cerevisiae[J]. Gene, 1994, 140(1): 121-126.
pmid: 8125328 |
| [20] | Yamaki S, Ishikawa K. Roles of four sorbitol related enzymes and invertase in the seasonal alteration of sugar metabolism in apple tissue[J]. J Amer Soc Hort Sci, 1986, 111(1): 134-137. |
| [21] | Loescher WH. Physiology and metabolism of sugar alcohols in higher plants[J]. Physiol Plant, 1987, 70(3): 553-557. |
| [22] | Wu BH, Li SH, Nosarzewski M, et al. Sorbitol dehydrogenase gene expression and enzyme activity in apple: tissue specificity during bud development and response to rootstock vigor and growth manipulation[J]. J Amer Soc Hort Sci, 2010, 135(4): 379-387. |
| [23] |
Brown PH, Bellaloui N, Hu H, et al. Transgenically enhanced sorbitol synthesis facilitates phloem boron transport and increases tolerance of tobacco to boron deficiency[J]. Plant Physiol, 1999, 119(1): 17-20.
doi: 10.1104/pp.119.1.17 pmid: 9880341 |
| [24] |
Bellaloui N, Brown PH, Dandekar AM. Manipulation of in vivo sorbitol production alters boron uptake and transport in tobacco[J]. Plant Physiol, 1999, 119(2): 735-742.
doi: 10.1104/pp.119.2.735 pmid: 9952470 |
| [25] | Wang T, Hou M, Zhao N, et al. Cloning and expression of the sorbitol dehydrogenase gene during embryonic development and temperature stress in Artemia sinica[J]. Gene, 2013, 521(2): 296-302. |
| [26] |
El-Kabbani O, Darmanin C, Chung RPT. Sorbitol dehydrogenase: structure, function and ligand design[J]. Curr Med Chem, 2004, 11(4): 465-476.
pmid: 14965227 |
| [27] | Wu T, Wang Y, Zheng Y, et al. Suppressing sorbitol synthesis substantially alters the global expression profile of stress response genes in apple(Malus domestica)leaves[J]. Plant Cell Physiol, 2015, 56(9): 1748-1761. |
| [28] | Aguayo MF, Ampuero D, Mandujano P, et al. Sorbitol dehydrogenase is a cytosolic protein required for sorbitol metabolism in Arabidopsis thaliana[J]. Plant Sci, 2013, 205-206: 63-75. |
| [29] | Bellaloui N, Yadavc RC, Chern MS, et al. Transgenically enhanced sorbitol synthesis facilitates phloem-boron mobility in rice[J]. Physiol Plant, 2003, 117(1): 79-84. |
| [30] |
Hergeth SP, Schneider R. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle[J]. EMBO Rep, 2015, 16(11): 1439-1453.
doi: 10.15252/embr.201540749 pmid: 26474902 |
| [31] | Andrés M, García-Gomis D, Ponte I, et al. Histone H1 post-translational modifications: update and future perspectives[J]. Int J Mol Sci, 2020, 21(16): 5941. |
| [32] | Wu X, Xu JN, Meng XN, et al. Linker histone variant HIS1-3 and WRKY1 oppositely regulate salt stress tolerance in Arabidopsis[J]. Plant Physiol, 2022, 189(3): 1833-1847. |
| [33] | Zhao PJ, Guo X, Wang B, et al. Overexpression of MeH1.2 gene inhibited plant growth and increased branch root differentiation in transgenic cassava[J]. Crop Sci, 2021, 61(4): 2639-2650. |
| [34] |
Zhao PJ, Liu P, Shao JF, et al. Analysis of different strategies adapted by two cassava cultivars in response to drought stress: ensuring survival or continuing growth[J]. J Exp Bot, 2015, 66(5): 1477-1488.
doi: 10.1093/jxb/eru507 pmid: 25547914 |
| [35] |
薛满德, 赵峰月, 李洁, 等. 组蛋白变体在植物表观遗传调控中的研究进展[J]. 生物技术通报, 2022, 38(7): 1-12.
doi: 10.13560/j.cnki.biotech.bull.1985.2022-0071 |
| Xue MD, Zhao FY, Li J, et al. Advances in histone variants in plant epigenetic regulation[J]. Biotechnol Bull, 2022, 38(7): 1-12. | |
| [36] |
Kalashnikova AA, Rogge RA, Hansen JC. Linker histone H1 and protein-protein interactions[J]. Biochimica et Biophysica Acta 2016, 1859(3):455-461.
doi: 10.1016/j.bbagrm.2015.10.004 pmid: 26455956 |
| [37] | 凡慧明. 砂梨山梨醇脱氢酶基因PpSDH3和PpSDH9调控水心病发生的分子机制研究[D]. 扬州: 扬州大学, 2021. |
| Fan HM. Study on the molecular mechanism of sorbitol dehydrogenase genes PpSDH3 and PpSDH9 regulating the occurrence of water heart disease in Pyrus pyrifolia[D]. Yangzhou: Yangzhou University, 2021. |
| [1] | TONG Wei-jing, LUO Shu, LU Xin-lu, SHEN Jian-fu, LU Bai-yi, LI Kai-mian, MA Qiu-xiang, ZHANG Peng. CRISPR/Cas9 Editing MeHNL Gene to Generate Cassava Plants with Low Cyanogenic Glycoside [J]. Biotechnology Bulletin, 2024, 40(9): 11-19. |
| [2] | MA Bo-tao, WU Guo-qiang, WEI Ming. Roles of bZIP Transcription Factor in the Response to Stresses, and Growth and Development in Plants [J]. Biotechnology Bulletin, 2024, 40(9): 148-160. |
| [3] | TAN Bo-wen, ZHANG Yi, ZHANG Peng, WANG Zhen-yu, MA Qiu-xiang. Identification and Bioinformatics Analysis of Gene in the Magnesium Transporter Family in Cassava [J]. Biotechnology Bulletin, 2024, 40(9): 20-32. |
| [4] | YANG Wei, ZHAO Li-fen, TANG Bing, ZHOU Lin-bi, YANG Juan, MO Chuan-yuan, ZHANG Bao-hui, LI Fei, RUAN Song-lin, DENG Ying. Genome-wide Identification and Expression Analysis of the SRO Gene Family in Brassica juncea L. [J]. Biotechnology Bulletin, 2024, 40(8): 129-141. |
| [5] | HU Ya-dan, WU Guo-qiang, LIU Chen, WEI Ming. Roles of MYB Transcription Factor in Regulating the Responses of Plants to Stress [J]. Biotechnology Bulletin, 2024, 40(6): 5-22. |
| [6] | XIAO Liang, WU Zheng-dan, LU Liu-ying, SHI Ping-li, SHANG Xiao-hong, CAO Sheng, ZENG Wen-dan, YAN Hua-bing. Research Progress of Important Traits Genes in Cassava [J]. Biotechnology Bulletin, 2023, 39(6): 31-48. |
| [7] | YAO Xiao-wen, LIANG Xiao, CHEN Qing, WU Chun-ling, LIU Ying, LIU Xiao-qiang, SHUI Jun, QIAO Yang, MAO Yi-ming, CHEN Yin-hua, ZHANG Yin-dong. Study on the Expression Pattern of Genes in Lignin Biosynthesis Pathway of Cassava Resisting to Tetranychus urticae [J]. Biotechnology Bulletin, 2023, 39(2): 161-171. |
| [8] | WANG Chen-yu, ZHOU Chu-yuan, HE Di, FAN Zi-hao, WANG Meng-meng, YANG Liu-yan. Role and Mechanism of Polyphosphate in the Microbial Response to Environmental Stresses [J]. Biotechnology Bulletin, 2023, 39(11): 168-181. |
| [9] | YU Xiao-ling, LI Wen-bin, LI Zhi-bo, RUAN Meng-bin. Cold Resistance Function Analysis of Cassava MeMYC2.2 [J]. Biotechnology Bulletin, 2023, 39(1): 224-231. |
| [10] | HAN Zhi-ling, CHEN Qing, LIANG Xiao, WU Chun-ling, LIU Ying, WU Mu-feng, XU Xue-lian. Influence on Expression of Jasmonic Acid Signaling Pathway Gene in Tetranychus urticae Fed on Mite-resistant and Mite-susceptible Cassava Cultivars [J]. Biotechnology Bulletin, 2022, 38(6): 211-220. |
| [11] | ZHANG Bin, YANG Xin-xia. Identification of Key Transcription Factors in Response to Salt Stress in Rice [J]. Biotechnology Bulletin, 2022, 38(3): 9-15. |
| [12] | ZOU Liang-ping, GUO Xin, QI Deng-feng, ZHAI Min, LI Zhuang, ZHAO Ping-juan, PENG Ming, NIU Xing-kui. Anthocyanin Accumulation and Its Gene Expression Induced by Low Nitrogen Stress in Cassava Seedlings [J]. Biotechnology Bulletin, 2022, 38(2): 75-82. |
| [13] | LUO Wei, MU Qiong, SHU Jian-hong, WU Jia-hai, WANG Xiao-li. Expression,Protein Interactions and Biological Function Analysis of FaFT in Festuca arundinacea [J]. Biotechnology Bulletin, 2021, 37(4): 8-17. |
| [14] | XU Lin-na, HU Meng-ke, TONG Wen-yan, LI Fen. Effects of T1084d and T1084A Point Mutations in the NtTkr Tail of Nicotiana tabacum on Coiled-helix Structure and Interaction with Target Proteins [J]. Biotechnology Bulletin, 2019, 35(5): 64-69. |
| [15] | JIA Jian-lei, CHEN Qian, JIN Ji-peng, YUAN Zan, ZHANG Li-ping. Eukaryotic Expression of BMPR1B in Sheep and Identification of Its Interaction Proteins [J]. Biotechnology Bulletin, 2019, 35(12): 94-104. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||