








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (10): 72-86.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0620
Previous Articles Next Articles
FAN Yan-fei1,2,3( ), YE Lu-huan1,2, LI Yu-tong1,2,3, WANG Chuan-luo1,2,3, ZHANG Rui1,2, LUO Jian-hua1,2,3, WANG Peng1,2(
), YE Lu-huan1,2, LI Yu-tong1,2,3, WANG Chuan-luo1,2,3, ZHANG Rui1,2, LUO Jian-hua1,2,3, WANG Peng1,2( )
)
Received:2025-06-13
Online:2025-10-26
Published:2025-07-30
Contact:
WANG Peng
E-mail:fanyanfei@cemps.ac.cn;wangpeng@cemps.ac.cn
FAN Yan-fei, YE Lu-huan, LI Yu-tong, WANG Chuan-luo, ZHANG Rui, LUO Jian-hua, WANG Peng. Utilizing Wheat Hybrid Lines to Mine Genes Regulating Cyclic Electron Flow and Applying Them in Improving Photosynthetic Efficiency in Crops[J]. Biotechnology Bulletin, 2025, 41(10): 72-86.

Fig. 1 Planting of wheat recombinant inbred population, and measurements of initial P700+ dark-reduction rates and ETR(Ⅰ)- ETR(Ⅱ) in representative varietiesA: The above is a photo of one batch of wheat materials during the first round of P700+ reduction rate measurement; the bottom left shows five representative wheat varieties; the bottom right displays the wheat population growing in the field. B: Left, P700+reduction curves of five representative wheat varieties; right, initial slope of curve decline (bar chart). C: Line chart of ETR(Ⅰ)-ETR(Ⅱ) for five representative wheat varieties under increasing light intensity gradients
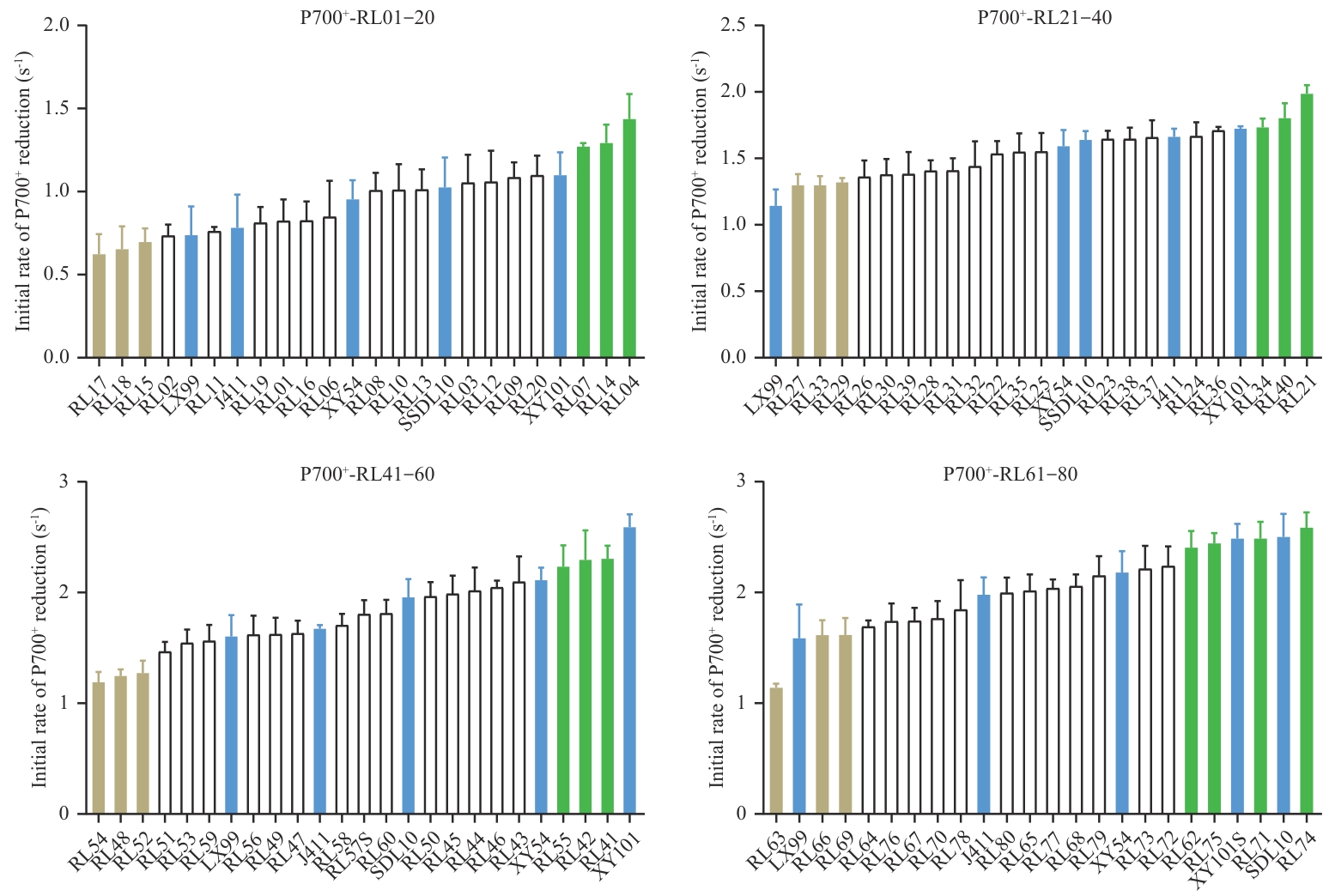
Fig. 2 Distribution examples of initial P700+ dark-reduction rates of wheat population in the first-round measurementThe first-round screening of initial P700+ dark-reduction rates covered the entire wheat recombinant inbred line population. Here, the measurements of the first 80 lines (RL01-RL80, “RL” is short for “recombinant inbred line”) are displayed, with every consecutive 20 lines plus 5 representative cultivars measured and plotted as a group. The representative varieties are displayed in blue, while the lines with high and low cyclic electron transport activity selected from both ends of the data distribution are shown in green and light brown, respectively

Fig. 3 Distribution of initial P700+ dark reduction rates of wheat population in the second-round measurementThe second-round initial P700+ dark-reduction rate screening focused on lines with either high or low cyclic electron transport activity from the. They were randomly grouped into 4 batches alongside representative cultivars and measured and graphed usingthe same protocol as the first-round screening. Representative varieties are still shown in blue, while the lines with high and low cyclic electron transport activity selected from the first-round screening remain displayed in green and light brown, respectively. “RL” is short for “recombinant inbred line”
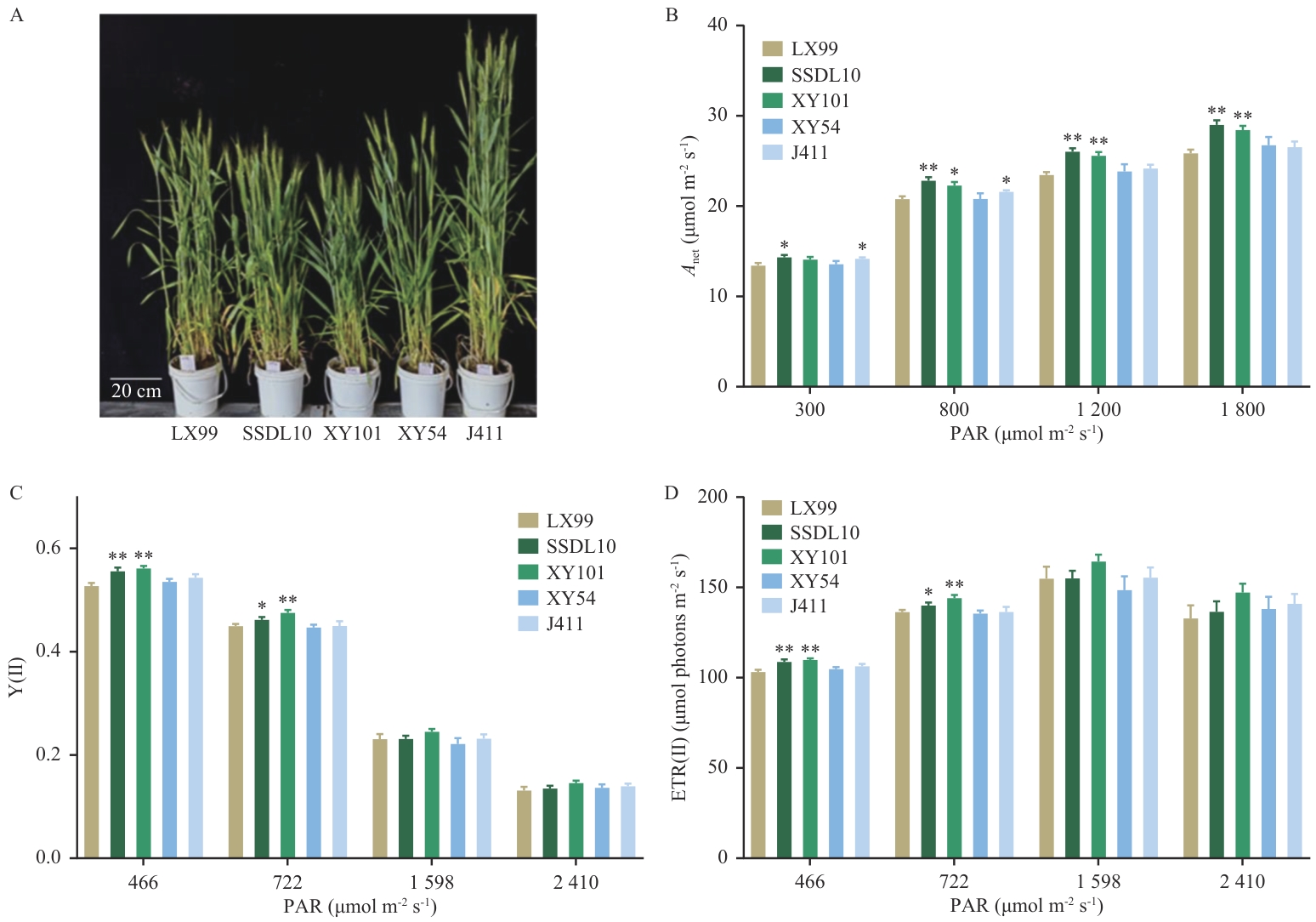
Fig. 4 Growth phenotypes of representative wheat cultivars and light-intensity-dependent photosynthetic parametersA: Five representative wheat varieties after heading. B-D: Net photosynthetic rate (Anet), Y(Ⅱ) and ETR(Ⅱ) of representative varieties under light-intensity gradients. Field measurements of photosynthetic rates and electron transport activities under varying light intensities were conducted on flag leaves of wheat representative cultivars (LX99,SSDL10,XY101, XY54, and J411) using Li-6400 photosynthesis system and PAM-2000 portable chlorophyll fluorometer. The measurements were performed on clear mornings during the post-heading stage, with 4-6 biological replicates per cultivar
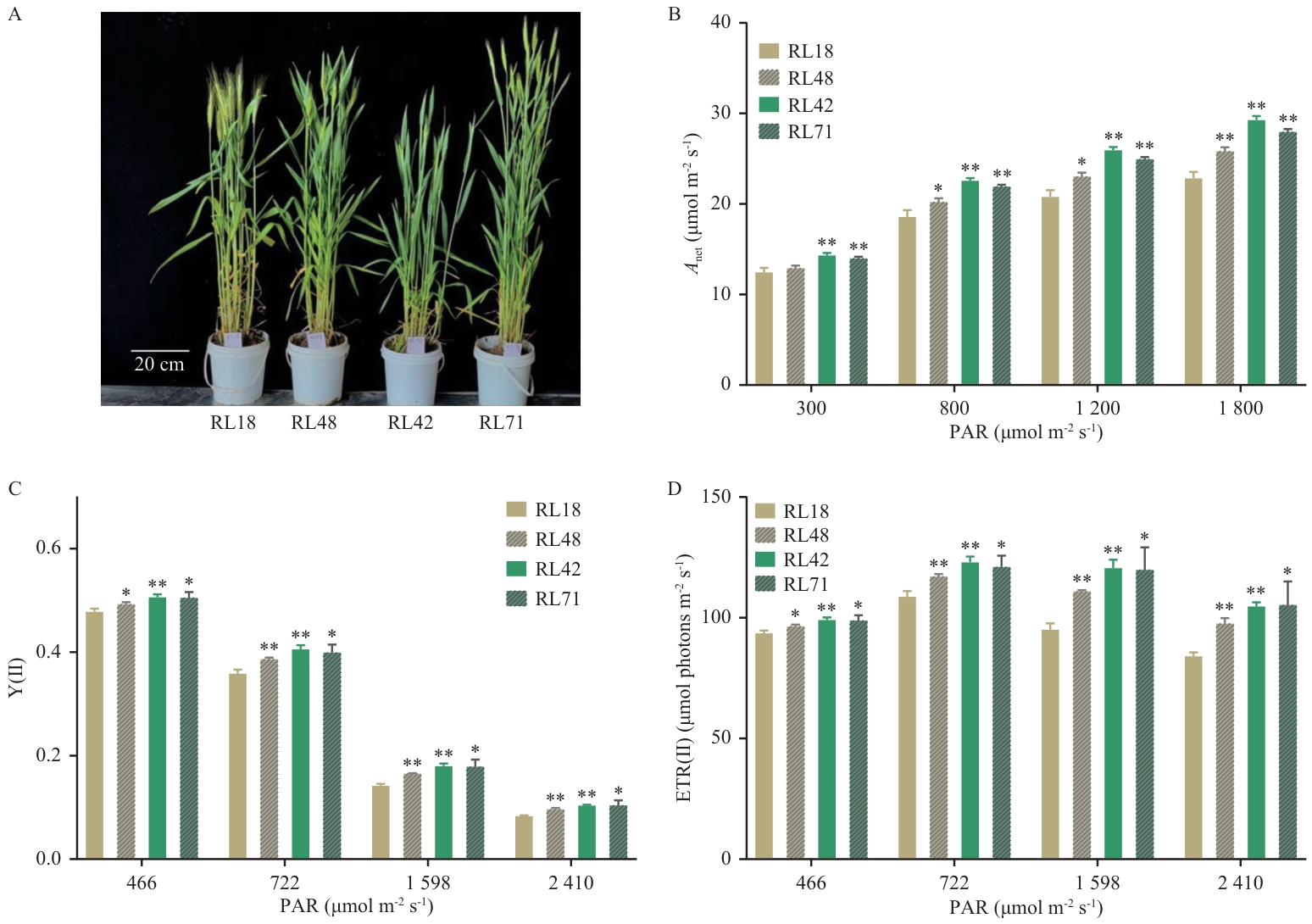
Fig. 5 Growth phenotypes of wheat recombinant inbred lines and light-intensity-dependent photosynthetic parametersA: Four wheat recombinant inbred lines after heading. B-D: Net photosynthetic rate (Anet), Y(Ⅱ) and ETR(Ⅱ) of wheat recombinant inbred lines under light-intensity gradients, respectively. Field measurements of photosynthetic rates and electron transport activities under varying light intensities were conducted on flag leaves of wheat recombinant inbred lines (RL18, RL48, RL42 and RL71) using Li-6400 photosynthesis system and PAM-2000 portable chlorophyll fluorometer. The measurements were performed on clear mornings during the post-heading stage, with 5-6 biological replicates per cultivar

Fig. 6 Determination of NDH subunit content in recombinant inbred line populationA: Determination of NDH-H subunit content in recombinant inbred line population (first round). B: Second-round quantification of NDH complex content in the recombinant inbred line population. The Western blot results are comparable within each blot, but the signal intensities may vary between different blots. Each immunoblotting image contains XY101 (high NDH-H content) and LX99 (low NDH-H content) as references

Fig. 7 Analysis example of transcriptome sequencing resultsA-D: Differential expressions of genes related to intersystem electron carriers, cyclic photosynthetic electron transport, PSI and transcription factors respectively. The heatmap demonstrates the logarithmic values |Log2(FC)| of the fold change of FPKM values for each variety relative to the average of “LX99+J411”

Fig. 8 Photosynthetic rate analysis of T1 and T2 generation rice lines overexpressing wheat PnsL2 and NAC genesA-C: Net photosynthetic rate (Anet) of wheat PnsL2 gene-overexpressing rice lines under different measuring light intensities in Hainan and Shanghai. D-F: Net photosynthetic rate (Anet) of wheat transcription factor gene NAC-overexpressing rice lines under different measuring light intensities in Hainan and Shanghai
| [1] | McGuire S. FAO, IFAD, and WFP. the state of food insecurity in the world 2015: meeting the 2015 international hunger targets: taking stock of uneven progress. Rome: FAO, 2015 [J]. Adv Nutr, 2015, 6(5): 623-624. |
| [2] | Mazza JJ. Climate change and agriculture: future implications [J]. WMJ, 2017, 116(4): 191. |
| [3] | Nakamura N, Iwano M, Havaux M, et al. Promotion of cyclic electron transport around photosystem I during the evolution of NADP-malic enzyme-type C4 photosynthesis in the genus Flaveria [J]. New Phytol, 2013, 199(3): 832-842. |
| [4] | Zhu X-G, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield [J]. Annu Rev Plant Biol, 2010, 61: 235-261. |
| [5] | Bailey-Serres J, Parker JE, Ainsworth EA, et al. Genetic strategies for improving crop yields [J]. Nature, 2019, 575(7781): 109-118. |
| [6] | Ma MZ, Liu YF, Bai CM, et al. The significance of chloroplast NAD(P)H dehydrogenase complex and its dependent cyclic electron transport in photosynthesis [J]. Front Plant Sci, 2021, 12: 661863. |
| [7] | Yamori W, Makino A, Shikanai T. A physiological role of cyclic electron transport around photosystem Ⅰ in sustaining photosynthesis under fluctuating light in rice [J]. Sci Rep, 2016, 6: 20147. |
| [8] | Shen LL, Tang KL, Wang WD, et al. Architecture of the chloroplast PSI-NDH super complex in Hordeum vulgare . [J]. Nature, 2022, 601(7894): 649-654. |
| [9] | Lu JZ, Yin ZP, Lu T, et al. Cyclic electron flow modulate the linear electron flow and reactive oxygen species in tomato leaves under high temperature [J]. Plant Sci, 2020, 292: 110387. |
| [10] | Sun YJ, Geng QW, Du YP, et al. Induction of cyclic electron flow around photosystem I during heat stress in grape leaves [J]. Plant Sci, 2017, 256: 65-71. |
| [11] | Yamori W, Sakata N, Suzuki Y, et al. Cyclic electron flow around photosystem Ⅰ via chloroplast NAD(P)H dehydrogenase (NDH) complex performs a significant physiological role during photosynthesis and plant growth at low temperature in rice [J]. Plant J, 2011, 68(6): 966-976. |
| [12] | Yamori W, Shikanai T, Makino A. Photosystem I cyclic electron flow via chloroplast NADH dehydrogenase-like complex performs a physiological role for photosynthesis at low light [J]. Sci Rep, 2015, 5: 13908. |
| [13] | Yamori W, Shikanai T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth [J]. Annu Rev Plant Biol, 2016, 67: 81-106. |
| [14] | Jia HS, Oguchi R, Hope AB, et al. Differential effects of severe water stress on linear and cyclic electron fluxes through Photosystem Ⅰ in spinach leaf discs in CO2-enriched air [J]. Planta, 2008, 228(5): 803-812. |
| [15] | Kohzuma K, Cruz JA, Akashi K, et al. The long-term responses of the photosynthetic proton circuit to drought [J]. Plant Cell Environ, 2009, 32(3): 209-219. |
| [16] | Strand DD, Livingston AK, Satoh-Cruz M, et al. Activation of cyclic electron flow by hydrogen peroxide in vivo [J]. Proc Natl Acad Sci U S A, 2015, 112(17): 5539-5544. |
| [17] | Joliot P, Joliot A. Cyclic electron transfer in plant leaf [J]. Proc Natl Acad Sci U S A, 2002, 99(15): 10209-10214. |
| [18] | Breyton C, Nandha B, Johnson GN, et al. Redox modulation of cyclic electron flow around photosystem Ⅰ in C3 plants [J]. Biochemistry, 2006, 45(45): 13465-13475. |
| [19] | Livingston AK, Kanazawa A, Cruz JA, et al. Regulation of cyclic electron flow in C3 plants: differential effects of limiting photosynthesis at ribulose-1, 5-bisphosphate carboxylase/oxygenase and glyceraldehyde-3-phosphate dehydrogenase [J]. Plant Cell Environ, 2010, 33(11): 1779-1788. |
| [20] | Wang HW, Su JH, Shen YG. Difference in response of photosynthesis to bisulfite between two wheat genotypes [J]. J Plant Physiol Mol Biol, 2003, 29(1): 27-32. |
| [21] | 程建峰, 马为民, 陈根云, 等. 小偃54和京411及其杂交后代稳定优选株系光合特性的动态变化 [J]. 作物学报, 2009, 35(6): 1051-1058. |
| Cheng JF, Ma WM, Chen GY, et al. Dynamic changes of photosynthetic characteristics in Xiaoyan 54, Jing 411, and the stable selected superior strains of their hybrid progenies [J]. Acta Agron Sin, 2009, 35(6): 1051-1058. | |
| [22] | He ZH, Li HW, Shen YK, et al. Comparative analysis of the chloroplast proteomes of a wheat (Triticum aestivum L.) single seed descent line and its parents [J]. J Plant Physiol, 2013, 170(13): 1139-1147. |
| [23] | 魏家绵, 沈允钢, 李德耀, 等. 亚硫酸氢钠在低光强下对叶绿体循环光合磷酸化的促进作用 [J]. 植物生理学报, 1989, 15(1): 101-104. |
| Wei JM, Shen YG, Li DY, et al. Stimulatory effect of sodium bisulfite on cyclic-photophosphorylation of chloroplasts under low light intensity [J]. Plant Physiol J, 1989, 15(1): 101-104. | |
| [24] | 王宏炜, 魏家绵, 沈允钢, 等. 低浓度NaHSO3促进田间水稻的光合磷酸化和光合作用(英文) [J]. 植物学报, 2000, 12: 1295-1299. |
| Wang HW, Wei JM, Shen RG, et al. Enhancement of photophosphorylation and photosynthesis in rice by low concentrations of NaHSO3: Under field conditions [J]. J Integr Plant Biol, 2000, (12): 1295-1299. | |
| [25] | Wu YX, Zheng FF, Ma WM, et al. Regulation of NAD(P)H dehydrogenase-dependent cyclic electron transport around PSI by NaHSO3 at low concentrations in tobacco chloroplasts [J]. Plant Cell Physiol, 2011, 52(10): 1734-1743. |
| [26] | 李娜, 韦嘉励, 李庆华, 等. 低浓度NaHSO3促进拟南芥PGR5/PGRL1介导的循环电子传递途径的运转 [J]. 植物生理学报, 2016, 52(11): 1745-1751. |
| Li N, Wei JL, Li QH, et al. Low concentration of NaHSO3 enhances the cyclic electron transport pathway mediated by PGR5/PGRL1 in Arabidopsis thaliana [J]. Plant Physiol J, 2016, 52(11): 1745-1751. | |
| [27] | Hashimoto M, Endo T, Peltier G, et al. A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis [J]. Plant J, 2003, 36(4): 541-549. |
| [28] | Kotera E, Tasaka M, Shikanai T. A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts [J]. Nature, 2005, 433(7023): 326-330. |
| [29] | Peng LW, Cai WH, Shikanai T. Retracted: Chloroplast stromal proteins, CRR6 and CRR7, are required for assembly of the NAD(P)H dehydrogenase sub complex A in Arabidopsis [J]. Plant J, 2010, 63(2): 203-211. |
| [30] | Long TA, Okegawa Y, Shikanai T, et al. Conserved role of proton gradient regulation 5 in the regulation of psi cyclic electron transport [J]. Planta, 2008, 228(6): 907-918. |
| [31] | Okegawa Y, Long TA, Iwano M, et al. A balanced PGR5 level is required for chloroplast development and optimum operation of cyclic electron transport around photosystem I [J]. Plant Cell Physiol, 2007, 48(10): 1462-1471. |
| [32] | Tazoe Y, Ishikawa N, Shikanai T, et al. Overproduction of PGR5 enhances the electron sink downstream of photosystem I in a C4 plant, Flaveria bidentis . [J]. Plant J, 2020, 103(2): 814-823. |
| [33] | Ishikawa N, Yokoe Y, Nishimura T, et al. PsbQ-like protein 3 functions as an assembly factor for the chloroplast NADH dehydrogenase-like complex in Arabidopsis [J]. Plant Cell Physiol, 2020, 61(7): 1252-1261. |
| [34] | Xiong HY, He HD, Chang Y, et al. Multiple roles of NAC transcription factors in plant development and stress responses [J]. J Integr Plant Biol, 2025, 67(3): 510-538. |
| [35] | Ma B, Zhang Y, Fan YF, et al. Genetic improvement of phosphate-limited photosynthesis for high yield in rice [J]. Proc Natl Acad Sci U S A, 2024, 121(34): e2404199121. |
| [1] | DENG Mei-bi, YAN Lang, ZHAN Zhi-tian, ZHU Min, HE Yu-bing. Efficient CRISPR Gene Editing in Rice Assisted by RUBY [J]. Biotechnology Bulletin, 2025, 41(8): 65-73. |
| [2] | CHENG Xue, FU Ying, CHAI Xiao-jiao, WANG Hong-yan, DENG Xin. Identification of LHC Gene Family in Setaria italica and Expression Analysis under Abiotic Stresses [J]. Biotechnology Bulletin, 2025, 41(8): 102-114. |
| [3] | DOU Fei-fei, REN Yu-zhao, WANG Shi-lei, LIU Chun-ying, WANG Xiao-dong, WANG Zhao-yi, LIU Cai-xia, LIU Feng-lou, WANG Zhang-jun, LI Qing-feng. Construction and Phenotypic Variation Analysis of Ningchun No. 4 Wheat EMS Mutant Library [J]. Biotechnology Bulletin, 2025, 41(8): 92-101. |
| [4] | LI Cheng-hua, DOU Fei-fei, REN Yu-zhao, LIU Cai-xia, LIU Feng-lou, WANG Zhang-jun, LI Qing-feng. Effect of Exogenous Salicylic Acid on Wheat Infested with Blumeria graminis f. sp. tritici and Its Transcriptome Analysis [J]. Biotechnology Bulletin, 2025, 41(7): 272-280. |
| [5] | HOU Ying-xiang, FEI Si-tian, LI Ni, LI Lan, SONG Song-quan, WANG Wei-ping, ZHANG Chao. Research Progress in Response of Rice miRNAs to Biotic Stress [J]. Biotechnology Bulletin, 2025, 41(7): 69-80. |
| [6] | WU Hao, DONG Wei-feng, HE Zi-tian, LI Yan-xiao, XIE Hui, SUN Ming-zhe, SHEN Yang, SUN Xiao-li. Genome-wide Identification and Expression Analysis of the Rice BXL Gene Family [J]. Biotechnology Bulletin, 2025, 41(6): 87-98. |
| [7] | WANG Yi-min, LI Ying, DONG Hai-tao, ZHANG Heng-rui, CHANG Lu, GAO Tian-tian, HAN De-jun, WU Jian-hui. Evolutionary Patterns of SRO Family Proteins in the Polyploidization Process of Wheat [J]. Biotechnology Bulletin, 2025, 41(5): 70-81. |
| [8] | DU Liang-heng, TANG Huang-lei, ZHANG Zhi-guo. Map-based Cloning of Light-responsive Gene ELM1 in Rice [J]. Biotechnology Bulletin, 2025, 41(5): 82-89. |
| [9] | WANG Wei-wei, ZHAO Zhen-jie, WANG Zhi, ZOU Jing-wei, LUO Zheng-hui, ZHANG Yu-jie, NIU Li-ya, YU Liang, YANG Xue-ju. Research Progress in Salt-tolerant Genes Related to Physiological Response of Wheat to Salt Stress [J]. Biotechnology Bulletin, 2025, 41(5): 14-22. |
| [10] | LIU Yuan-yuan, CHEN Xi-feng, QIAN Qian, GAO Zhen-yu. Advances in Molecular Mechanisms Regulating Panicle Development in Rice [J]. Biotechnology Bulletin, 2025, 41(5): 1-13. |
| [11] | CHEN Xiao-jun, HUI Jian, MA Hong-wen, BAI Hai-Bo, ZHONG Nan, LI Jia-run, FAN Yun-fang. Creating Rice Gerplasm Resources OsALS Rsistant to Herbicide through Single Base Gene Editing Technology [J]. Biotechnology Bulletin, 2025, 41(4): 106-114. |
| [12] | YANG Chao-jie, ZHANG Lan, CHEN Hong, HUANG Juan, SHI Tao-xiong, ZHU Li-wei, CHEN Qing-fu, LI Hong-you, DENG Jiao. Functional Identification of the Transcription Factor Gene FtbHLH3 in Regulating Flavonoid Biosynthesis in Fagopyrum tataricum [J]. Biotechnology Bulletin, 2025, 41(4): 134-144. |
| [13] | WU Xia-ming, YANG Min, ZHOU Chen-ping, KUANG Rui-bin, LIU Chuan-he, HE Han, XU Ze, WEI Yue-rong. Effects of Different Concentrations of Melatonin on the Physiological Characteristics of Strawberry Seedlings under High-temperature Stress [J]. Biotechnology Bulletin, 2025, 41(3): 181-189. |
| [14] | WANG Bin, WANG Yu-kun, XIAO Yan-hui. Comparative Transcriptomic Analysis of Clove Basil (Ocimum gratissimum) Leaves in Response to Cadmium Stress [J]. Biotechnology Bulletin, 2025, 41(3): 255-270. |
| [15] | MA Yao-wu, ZHANG Qi-yu, YANG Miao, JIANG Cheng, ZHANG Zhen-yu, ZHANG Yi-lin, LI Meng-sha, XU Jia-yang, ZHANG Bin, CUI Guang-zhou, JIANG Ying. Screening, Indentification and Promotion Performance Investigation of Tobacco Growth-promoting Rhizobacteria [J]. Biotechnology Bulletin, 2025, 41(3): 271-281. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||