








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (8): 91-105.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0208
Previous Articles Next Articles
YE Yun-fang1,2( ), TIAN Qing-yin1,2, SHI Ting-ting1,2, WANG Liang3, YUE Yuan-zheng1,2, YANG Xiu-lian1,2, WANG Liang-gui1,2(
), TIAN Qing-yin1,2, SHI Ting-ting1,2, WANG Liang3, YUE Yuan-zheng1,2, YANG Xiu-lian1,2, WANG Liang-gui1,2( )
)
Received:2023-03-09
Online:2023-08-26
Published:2023-09-05
Contact:
WANG Liang-gui
E-mail:yeyunfang2021@163.com;wlg@njfu.com.cn
YE Yun-fang, TIAN Qing-yin, SHI Ting-ting, WANG Liang, YUE Yuan-zheng, YANG Xiu-lian, WANG Liang-gui. Research Progress in the Biosynthesis and Regulation of β-ionone in Plants[J]. Biotechnology Bulletin, 2023, 39(8): 91-105.
| 名称 Name | 化学结构 Chemical structure | 气味 Scent | 参考文献 Reference |
|---|---|---|---|
| α-紫罗兰酮 α-ionone | 甜腻莓果香、近似覆盆子果酱,有很强大的气味冲击性,存在于桂花、玫瑰等植物中 | [ | |
| β-紫罗兰酮 β-ionone | 具有暖香、木香、紫罗兰花香香气,存在于桂花、雪松(Cedrus deodara)等植物中 | [ | |
| 二氢-β-紫罗兰酮 Dihydro-β-ionone | 具有醇厚、甜美和清新的木香、果香和花香气味 | [ | |
| 甲基-β-紫罗兰酮 Methyl-β- ionone | 紫罗兰、鸢尾花(Iris tectorum)的干燥气味,带有一点甜 | [ | |
| 异甲基-β-紫罗兰酮 Iso-methyl-β-ionone | 具有细致的紫罗兰香气 | [ | |
| 3-羟基-β-紫罗兰酮 3-Hydroxy-β-Ionone | 具有独特气味,存在于苔藓植物(Bryophyta)、水芹(Oenanthe javanica)等植物中 | [ | |
| 3-羟基-5,6-环氧-β-紫罗兰酮 3-Hydroxy-5,6-epoxy-β-ionone | 具有独特香味,存在于烟草(Nicotiana tabacum)或烟气中 | [ |
Table 1 General information of α-ionone, β-ionone and their derivatives
| 名称 Name | 化学结构 Chemical structure | 气味 Scent | 参考文献 Reference |
|---|---|---|---|
| α-紫罗兰酮 α-ionone | 甜腻莓果香、近似覆盆子果酱,有很强大的气味冲击性,存在于桂花、玫瑰等植物中 | [ | |
| β-紫罗兰酮 β-ionone | 具有暖香、木香、紫罗兰花香香气,存在于桂花、雪松(Cedrus deodara)等植物中 | [ | |
| 二氢-β-紫罗兰酮 Dihydro-β-ionone | 具有醇厚、甜美和清新的木香、果香和花香气味 | [ | |
| 甲基-β-紫罗兰酮 Methyl-β- ionone | 紫罗兰、鸢尾花(Iris tectorum)的干燥气味,带有一点甜 | [ | |
| 异甲基-β-紫罗兰酮 Iso-methyl-β-ionone | 具有细致的紫罗兰香气 | [ | |
| 3-羟基-β-紫罗兰酮 3-Hydroxy-β-Ionone | 具有独特气味,存在于苔藓植物(Bryophyta)、水芹(Oenanthe javanica)等植物中 | [ | |
| 3-羟基-5,6-环氧-β-紫罗兰酮 3-Hydroxy-5,6-epoxy-β-ionone | 具有独特香味,存在于烟草(Nicotiana tabacum)或烟气中 | [ |
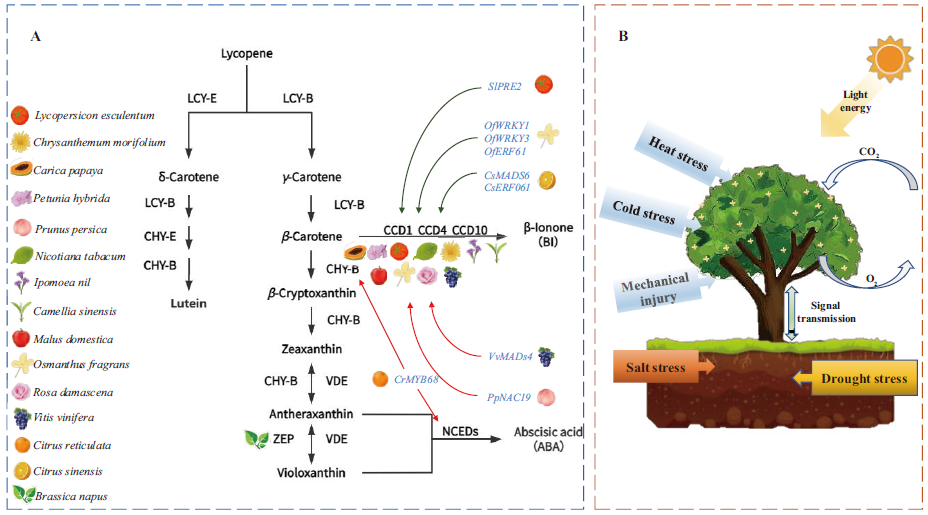
Fig. 2 Regulation map of β-ionone synthesis in plants A: Structural genes related and partial transcription factors associated with β-ionone synthesis in some plants; green arrows indicate positive regulation, red arrows indicate negative regulation. B: Environmental factors of the β-ionone synthetic pathway
| 基因工程技术 Genetic engineering technology | 物种名称 Species | 基因 Gene | 结果 Result | 参考文献 Reference |
|---|---|---|---|---|
| 基因过表达 Gene overexpression | 木瓜Carica papaya | CpCCD1 | β-胡萝卜素和番茄红素降解,进而形成6-甲基-5-庚烯-2-酮和β-紫罗兰酮等特异性挥发物 | [ |
| 桂花Osmanthus fragran | OfCCD4 | 转基因株系花瓣颜色变淡,β-紫罗兰酮含量增加 | [ | |
| 基因沉默 Gene silencing | 菊花 Chrysanthemum morifolium | CmCCD4a | 类胡萝卜素含量 增加,菊花白色花瓣变为黄色花瓣 | [ |
| 甜橙Citrus sinensis | CsCCD1 | 紫黄质、9-顺式-紫黄质的含量皆有显著增加,柑橘颜色轻微变黄 | [ | |
| 欧洲油菜Brassica napus | BnaA09.ZE BnaC09.ZEP | 叶黄素含量显著提高,花色变深 | [ | |
| 牵牛花Ipomoea nil | InCCD4 | 牵牛花花色从白色变为淡黄色,β-紫罗兰酮含量下降 | [ | |
| 番茄 Lycopersicon esculentum | LeCCD1A LeCCD1B | 番茄果实中番茄红素的含量显著提高 | [ | |
| 番茄Solanum lycopersicum | SlNCED | 番茄果实较对照的粉红均呈现深红色,番茄红素和β-胡萝卜素含量较高 | [ | |
| 基因异源表达 Gene heterologous expression | 烟草Nicotiana tabacum | NtCCD10 | 对称切割八氢番茄红素和β-胡萝卜素分别产生香叶基丙酮和β-紫罗兰酮 | [ |
| 茶Camellia sinensis | CsCCD1 | 在大肠杆菌中异源合成β-紫罗兰酮,β-紫罗兰酮含量增加 | [ | |
| 桂花Osmanthus fragrans | OfCCD4 | 在大肠杆菌异源合成β-紫罗兰酮,β-紫罗兰酮含量增加 | [ | |
| 桂花Osmanthus fragrans | OfCCD1 | 异源转入大肠杆菌,β-胡萝卜素的转化率仅低于1%,β-紫罗兰酮含量未明显增加 | [ | |
| 拟南芥Arabidopsis thaliana | AtCCD1 | 异源转入大肠杆菌,β-紫罗兰酮滴度为0.1 mg/L | [ | |
| 葡萄Vitis vinifera | VvCCD1 | 异源转入大肠杆菌,β-紫罗兰酮滴度为13 mg/L | [ | |
| 矮牵牛Petunia hybrida | PhCCD1 | 迄今为止产生β-紫罗兰酮的效率最高的酵母中异源表达,滴度为184 mg/L | [ |
Table 2 Application of β-ionone in plants improvement by genetic engineering technology
| 基因工程技术 Genetic engineering technology | 物种名称 Species | 基因 Gene | 结果 Result | 参考文献 Reference |
|---|---|---|---|---|
| 基因过表达 Gene overexpression | 木瓜Carica papaya | CpCCD1 | β-胡萝卜素和番茄红素降解,进而形成6-甲基-5-庚烯-2-酮和β-紫罗兰酮等特异性挥发物 | [ |
| 桂花Osmanthus fragran | OfCCD4 | 转基因株系花瓣颜色变淡,β-紫罗兰酮含量增加 | [ | |
| 基因沉默 Gene silencing | 菊花 Chrysanthemum morifolium | CmCCD4a | 类胡萝卜素含量 增加,菊花白色花瓣变为黄色花瓣 | [ |
| 甜橙Citrus sinensis | CsCCD1 | 紫黄质、9-顺式-紫黄质的含量皆有显著增加,柑橘颜色轻微变黄 | [ | |
| 欧洲油菜Brassica napus | BnaA09.ZE BnaC09.ZEP | 叶黄素含量显著提高,花色变深 | [ | |
| 牵牛花Ipomoea nil | InCCD4 | 牵牛花花色从白色变为淡黄色,β-紫罗兰酮含量下降 | [ | |
| 番茄 Lycopersicon esculentum | LeCCD1A LeCCD1B | 番茄果实中番茄红素的含量显著提高 | [ | |
| 番茄Solanum lycopersicum | SlNCED | 番茄果实较对照的粉红均呈现深红色,番茄红素和β-胡萝卜素含量较高 | [ | |
| 基因异源表达 Gene heterologous expression | 烟草Nicotiana tabacum | NtCCD10 | 对称切割八氢番茄红素和β-胡萝卜素分别产生香叶基丙酮和β-紫罗兰酮 | [ |
| 茶Camellia sinensis | CsCCD1 | 在大肠杆菌中异源合成β-紫罗兰酮,β-紫罗兰酮含量增加 | [ | |
| 桂花Osmanthus fragrans | OfCCD4 | 在大肠杆菌异源合成β-紫罗兰酮,β-紫罗兰酮含量增加 | [ | |
| 桂花Osmanthus fragrans | OfCCD1 | 异源转入大肠杆菌,β-胡萝卜素的转化率仅低于1%,β-紫罗兰酮含量未明显增加 | [ | |
| 拟南芥Arabidopsis thaliana | AtCCD1 | 异源转入大肠杆菌,β-紫罗兰酮滴度为0.1 mg/L | [ | |
| 葡萄Vitis vinifera | VvCCD1 | 异源转入大肠杆菌,β-紫罗兰酮滴度为13 mg/L | [ | |
| 矮牵牛Petunia hybrida | PhCCD1 | 迄今为止产生β-紫罗兰酮的效率最高的酵母中异源表达,滴度为184 mg/L | [ |
| [1] |
Dudareva N, Negre F, Nagegowda DA, et al. Plant volatiles: recent advances and future perspectives[J]. Crit Rev Plant Sci, 2006, 25(5): 417-440.
doi: 10.1080/07352680600899973 URL |
| [2] | 王艳娜, 孟学成, 赵荻, 等. 四个香榧品种种仁炒制加工后香气物质分析[J]. 南京林业大学学报: 自然科学版, 2022, 46(3): 169-176. |
| Wang YN, Meng XC, Zhao D, et al. Analysis of aroma profiles in kernels of four Torreya grandis cultivars after roasting process[J]. J Nanjing For Univ Nat Sci Ed, 2022, 46(3): 169-176. | |
| [3] |
Dudareva N, Pichersky E, Gershenzon J. Biochemistry of plant volatiles[J]. Plant Physiol, 2004, 135(4): 1893-1902.
doi: 10.1104/pp.104.049981 pmid: 15326281 |
| [4] | Baldermann S, Yamamoto M, Yang ZY, et al. C13-apocarotenoids: more than flavor compounds?[M]//ACS Symposium Series. Washington, DC: American Chemical Society, 2013: 73-80. |
| [5] |
Walter MH, Strack D. Carotenoids and their cleavage products: Biosynthesis and functions[J]. Nat Prod Rep, 2011, 28(4): 663-692.
doi: 10.1039/c0np00036a pmid: 21321752 |
| [6] | 石远洋, 刘家仁. β-紫罗兰酮抗癌活性研究进展[J]. 实用肿瘤学杂志, 2021, 35(3): 268-272. |
| Shi YY, Liu JR. Research progress on anticancer activity of β-ionone[J]. Pract Oncol J, 2021, 35(3): 268-272. | |
| [7] | 颜秀花, 王正武, 王仲妮. β-胡萝卜素的应用及研究进展[J]. 食品与药品, 2007, 9(6): 58-61. |
| Yan XH, Wang ZW, Wang ZN. Application and advancement of β-carotene[J]. Food Drug, 2007, 9(6): 58-61. | |
| [8] |
Lalko J, Lapczynski A, McGinty D, et al. Fragrance material review on ionone[J]. Food Chem Toxicol, 2007, 45(Suppl 1): S251-S257.
doi: 10.1016/j.fct.2007.09.065 URL |
| [9] |
Liu Q, Dong HW, Sun WG, et al. Apoptosis initiation of β-ionone in SGC-7901 gastric carcinoma cancer cells via a PI3K-AKT pathway[J]. Arch Toxicol, 2013, 87(3): 481-490.
doi: 10.1007/s00204-012-0962-8 pmid: 23100158 |
| [10] |
Liu JR, Chen BQ, Yang BF, et al. Apoptosis of human gastric adenocarcinoma cells induced by beta-ionone[J]. World J Gastroenterol, 2004, 10(3): 348-351.
doi: 10.3748/wjg.v10.i3.348 URL |
| [11] | 刘家仁, 杨艳梅, 董宏伟, 等. β-紫罗兰酮对人乳腺癌细胞(Er-)MAPK途径的影响[J]. 卫生研究, 2005, 34(6): 706-709. |
| Liu JR, Yang YM, Dong HW, et al. Effect of β-ionone in human mammary cancer cells(Er-)by MAPK pathway[J]. J Hyg Res, 2005, 34(6): 706-709. | |
| [12] | 孙向荣, 刘家仁, 陈炳卿. β-紫罗兰酮的生物活性研究进展[J]. 毒理学杂志, 2008, 22(6): 477-480. |
| Sun XR, Liu JR, Chen BQ. Research progress on biological activity of β-ionone[J]. J Toxicol, 2008, 22(6): 477-480. | |
| [13] |
de Moura Espíndola R, Mazzantini RP, Ong TP, et al. Geranylgeraniol and beta-ionone inhibit hepatic preneoplastic lesions, cell proliferation, total plasma cholesterol and DNA damage during the initial phases of hepatocarcinogenesis, but only the former inhibits NF-kappaB activation[J]. Carcinogenesis, 2005, 26(6): 1091-1099.
pmid: 15718255 |
| [14] |
Sharma V, Singh G, Kaur H, et al. Synthesis of β-ionone derived chalcones as potent antimicrobial agents[J]. Bioorg Med Chem Lett, 2012, 22(20): 6343-6346.
doi: 10.1016/j.bmcl.2012.08.084 pmid: 22999415 |
| [15] |
Simkin AJ, Schwartz SH, Auldridge M, et al. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone[J]. Plant J, 2004, 40(6): 882-892.
doi: 10.1111/tpj.2004.40.issue-6 URL |
| [16] |
Tandon KS, Baldwin EA, Shewfelt RL. Aroma perception of individual volatile compounds in fresh tomatoes(Lycopersicon esculentum, Mill.)as affected by the medium of evaluation[J]. Postharvest Biol Technol, 2000, 20(3): 261-268.
doi: 10.1016/S0925-5214(00)00143-5 URL |
| [17] |
Sewenig S, Bullinger D, Hener U, et al. Comprehensive authentication of(E)-α(β) -ionone from raspberries, using constant flow MDGC-C/P-IRMS and enantio-MDGC-MS[J]. J Agric Food Chem, 2005, 53(4): 838-844.
doi: 10.1021/jf040356k URL |
| [18] |
Pino JA, Quijano CE. Study of the volatile compounds from plum(Prunus domestica L. cv. Horvin)and estimation of their contribution to the fruit aroma[J]. Food Sci Technol, 2012, 32(1): 76-83.
doi: 10.1590/S0101-20612012005000006 URL |
| [19] |
Nawade B, Shaltiel-Harpaz L, Yahyaa M, et al. Analysis of apocarotenoid volatiles during the development of Ficus carica fruits and characterization of carotenoid cleavage dioxygenase genes[J]. Plant Sci, 2020, 290: 110292.
doi: 10.1016/j.plantsci.2019.110292 URL |
| [20] |
Simkin AJ, Underwood BA, Auldridge M, et al. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers[J]. Plant Physiol, 2004, 136(3): 3504-3514.
pmid: 15516502 |
| [21] |
Yahyaa M, Bar E, Dubey NK, et al. Formation of norisoprenoid flavor compounds in carrot(Daucus carota L.) roots: characterization of a cyclic-specific carotenoid cleavage dioxygenase 1 gene[J]. J Agric Food Chem, 2013, 61(50): 12244-12252.
doi: 10.1021/jf404085k URL |
| [22] |
Gonçalves AC, Campos G, Pinto E, et al. Essential and non-essential elements, and volatile organic compounds for the discrimination of twenty-three sweet cherry cultivars from Fundão, Portugal[J]. Food Chem, 2022, 367: 130503.
doi: 10.1016/j.foodchem.2021.130503 URL |
| [23] |
Lang S, Ozcelik M, Kulozik U, et al. Processing of raspberries to dried fruit foam: impact on major odorants[J]. Eur Food Res Technol, 2020, 246(12): 2537-2548.
doi: 10.1007/s00217-020-03595-9 |
| [24] |
Jayaram CS, Chauhan N, Dolma SK, et al. Chemical composition and insecticidal activities of essential oils against the pulse beetle[J]. Molecules, 2022, 27(2): 568.
doi: 10.3390/molecules27020568 URL |
| [25] |
Fu JX, Hou D, Wang YG, et al. Identification of floral aromatic volatile compounds in 29 cultivars from four groups of Osmanthus fragrans by gas chromatography-mass spectrometry[J]. Hortic Environ Biotechnol, 2019, 60(4): 611-623.
doi: 10.1007/s13580-019-00153-5 |
| [26] |
Scholtes C, Nizet S, Massart H, et al. Occurrence of theaspirane and its odorant degradation products in hop and beer[J]. J Agric Food Chem, 2015, 63(37): 8247-8253.
doi: 10.1021/acs.jafc.5b03195 URL |
| [27] | Camargo KC, Duarte LP, Vidal DM, et al. Chemodiversity of essential oils from nine species of Celastraceae[J]. Chem Biodivers, 2020, 17(5): e2000107. |
| [28] |
Liu QX, Li DW, Wang W, et al. Chemical composition and antioxidant activity of essential oils and methanol extracts of different parts from Juniperus rigida siebold & zucc[J]. Chem Biodivers, 2016, 13(9): 1240-1250.
doi: 10.1002/cbdv.v13.9 URL |
| [29] |
Abd-ElGawad A, El Gendy AEN, El-Amier Y, et al. Essential oil of Bassia muricata: chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale[J]. Saudi J Biol Sci, 2020, 27(7): 1900-1906.
doi: 10.1016/j.sjbs.2020.04.018 pmid: 32565712 |
| [30] |
Eyres GT, Marriott PJ, Dufour JP. Comparison of odor-active compounds in the spicy fraction of hop(Humulus lupulus L.) essential oil from four different varieties[J]. J Agric Food Chem, 2007, 55(15): 6252-6261.
doi: 10.1021/jf070739t URL |
| [31] |
Lawal OA, Ogunwande IA, Gbetoyon FS, et al. Chemical composition and insecticidal activity of essential oils of four varieties of Codiaeum variegatum(L.) from Nigeria[J]. J Essent Oil Bear Plants, 2018, 21(3): 840-847.
doi: 10.1080/0972060X.2017.1422440 URL |
| [32] | 辛秀兰, 张强, 赵新颖, 等. 主成分分析法评价树莓中挥发性香气成分气味活度值[J]. 食品安全质量检测学报, 2022, 13(2): 395-403. |
| Xin XL, Zhang Q, Zhao XY, et al. Evaluation of the relative odor activity value of volatile aroma components in Rubus corchorifolius fruit by principal component analysis[J]. J Food Saf Qual, 2022, 13(2): 395-403. | |
| [33] |
Shi YL, Wang MQ, Dong ZB, et al. Volatile components and key odorants of Chinese yellow tea(Camellia sinensis)[J]. LWT, 2021, 146: 111512.
doi: 10.1016/j.lwt.2021.111512 URL |
| [34] |
Lalko J, Lapczynski A, Politano VT, et al. Fragrance material review on alpha-ionone[J]. Food Chem Toxicol, 2007, 45(Suppl 1): S235-S240.
doi: 10.1016/j.fct.2007.09.046 URL |
| [35] | Budavari S, O'Neill M, Smith A, et al. The merck index:an encyclopedia of chemicals, drugs, and biologicals[M]. 12th ed. New York: Chapman & Hall, 1997. |
| [36] |
Aloum L, Alefishat E, Adem A, et al. Ionone is more than a violet's fragrance: a review[J]. Molecules, 2020, 25(24): 5822.
doi: 10.3390/molecules25245822 URL |
| [37] |
Lapczynski A, Lalko J, McGinty D, et al. Fragrance material review on methyl-beta-ionone[J]. Food Chem Toxicol, 2007, 45(Suppl 1): S290-S293.
doi: 10.1016/j.fct.2007.09.019 URL |
| [38] |
Lalko J, Lapczynski A, McGinty D, et al. Fragrance material review on beta-ionone[J]. Food Chem Toxicol, 2007, 45(Suppl 1): S241-S247.
doi: 10.1016/j.fct.2007.09.052 URL |
| [39] |
Lalko J, Lapczynski A, McGinty D, et al. Fragrance material review on iso-methyl-beta-ionone[J]. Food Chem Toxicol, 2007, 45(Suppl 1): S297-S299.
doi: 10.1016/j.fct.2007.09.025 URL |
| [40] | Knowles WS. Asymmetric hydrogenations(Nobel lecture)[J]. Angew Chem Int Ed Engl, 2002, 41(12): 1999-2007. |
| [41] |
Noyori R. Asymmetric catalysis: science and opportunities(Nobel lecture 2001)[J]. Adv Synth Catal, 2003, 345(1/2): 15-32.
doi: 10.1002/adsc.v345:1/2 URL |
| [42] |
Zhang XS, Liao SY, Cao FL, et al. Cloning and characterization of enoate reductase with high β-ionone to dihydro-β-ionone bioconversion productivity[J]. BMC Biotechnol, 2018, 18(1): 26.
doi: 10.1186/s12896-018-0438-x pmid: 29743047 |
| [43] |
Baldermann S, Kato M, Kurosawa M, et al. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour[J]. J Exp Bot, 2010, 61(11): 2967-2977.
doi: 10.1093/jxb/erq123 pmid: 20478967 |
| [44] |
Luetragoon T, Pankla Sranujit R, Noysang C, et al. Anti-cancer effect of 3-hydroxy-β-ionone identified from Moringa oleifera lam. leaf on human squamous cell carcinoma 15 cell line[J]. Molecules, 2020, 25(16): 3563.
doi: 10.3390/molecules25163563 URL |
| [45] |
Kato-Noguchi H, Seki T. Allelopathy of the moss Rhynchostegium pallidifolium and 3-hydroxy-β-ionone[J]. Plant Signal Behav, 2010, 5(6): 702-704.
doi: 10.4161/psb.5.6.11642 pmid: 20400848 |
| [46] |
Asanka Sanjeewa KK, Kim HS, Lee HG, et al. 3-hydroxy-5, 6-epoxy-β-ionone isolated from invasive harmful brown seaweed Sargassum horneri protects MH-S mouse lung cells from urban particulate matter-induced inflammation[J]. Appl Sci, 2021, 11(22): 10929.
doi: 10.3390/app112210929 URL |
| [47] |
Liu JR, Sun XR, Dong HW, et al. β-Ionone suppresses mammary carcinogenesis, proliferative activity and induces apoptosis in the mammary gland of the Sprague-Dawley rat[J]. Int J Cancer, 2008, 122(12): 2689-2698.
doi: 10.1002/(ISSN)1097-0215 URL |
| [48] |
Cardozo MT, de Conti A, Ong TP, et al. Chemopreventive effects of β-ionone and geraniol during rat hepatocarcinogenesis promotion: distinct actions on cell proliferation, apoptosis, HMGCoA reductase, and RhoA[J]. J Nutr Biochem, 2011, 22(2): 130-135.
doi: 10.1016/j.jnutbio.2009.12.007 pmid: 20435455 |
| [49] |
Asokkumar S, Naveenkumar C, Raghunandhakumar S, et al. Antiproliferative and antioxidant potential of beta-ionone against benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice[J]. Mol Cell Biochem, 2012, 363(1): 335-345.
doi: 10.1007/s11010-011-1186-6 URL |
| [50] | Mihailovic V, Vukovic N, Niciforovic N, et al. Studies on the antimicrobial activity and chemical composition of the essential oils and alcoholic extracts of Gentiana asclepiadea L.[J]. J Med Plant Res, 2011, 5(7): 1164-1174. |
| [51] |
Blažević I, Radonić A, Mastelić J, et al. Hedge mustard(Sisymb-rium officinale): chemical diversity of volatiles and their antimicrobial activity[J]. Chem Biodivers, 2010, 7(8): 2023-2034.
doi: 10.1002/cbdv.200900234 URL |
| [52] |
Hanbali EF, Mellouki F, Akssira M, et al. Composition and antimicrobial activity of essential oil of Anthemis tenuisecta ball[J]. J Essent Oil Bear Plants, 2007, 10(6): 499-503.
doi: 10.1080/0972060X.2007.10643586 URL |
| [53] |
Radulović N, Stojanović G, Palić R. Composition and antimicrobial activity of Equisetum arvense L. essential oil[J]. Phytother Res, 2006, 20(1): 85-88.
doi: 10.1002/ptr.1815 pmid: 16397851 |
| [54] |
Cáceres LA, Lakshminarayan S, Yeung KKC, et al. Repellent and attractive effects of α-, β-, and dihydro-β- ionone to generalist and specialist herbivores[J]. J Chem Ecol, 2016, 42(2): 107-117.
doi: 10.1007/s10886-016-0669-z pmid: 26852133 |
| [55] |
Wei S, Hannoufa A, Soroka J, et al. Enhanced β-ionone emission in Arabidopsis over-expressing AtCCD1 reduces feeding damage in vivo by the crucifer flea beetle[J]. Environ Entomol, 2011, 40(6): 1622-1630.
doi: 10.1603/EN11088 URL |
| [56] |
Faria LRR, Zanella FCV. Beta-ionone attracts Euglossa mandibularis(Hymenoptera, Apidae)males in western Paraná forests[J]. Revista Brasileira De Entomol, 2015, 59(3): 260-264.
doi: 10.1016/j.rbe.2015.05.003 URL |
| [57] |
Zhang CQ, Chen XX, Lindley ND, et al. A “plug-n-play” modular metabolic system for the production of apocarotenoids[J]. Biotechnol Bioeng, 2018, 115(1): 174-183.
doi: 10.1002/bit.26462 URL |
| [58] |
Jia LD, Wang JS, Wang R, et al. Comparative transcriptomic and metabolomic analyses of carotenoid biosynthesis reveal the basis of white petal color in Brassica napus[J]. Planta, 2021, 253(1): 8.
doi: 10.1007/s00425-020-03536-6 |
| [59] |
Sun TH, Li L. Toward the ‘golden’ era: the status in uncovering the regulatory control of carotenoid accumulation in plants[J]. Plant Sci, 2020, 290: 110331.
doi: 10.1016/j.plantsci.2019.110331 URL |
| [60] | Shi JN, Cao C, Xu JY, et al. Research advances on biosynthesis, regulation, and biological activities of apocarotenoid aroma in horticultural plants[J]. J Chem, 2020, 2020: 1-11. |
| [61] |
Walter MH, Floss DS, Strack D. Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles[J]. Planta, 2010, 232(1): 1-17.
doi: 10.1007/s00425-010-1156-3 pmid: 20396903 |
| [62] |
Chen HF, Zuo XY, Shao HX, et al. Genome-wide analysis of carotenoid cleavage oxygenase genes and their responses to various phytohormones and abiotic stresses in apple(Malus domestica)[J]. Plant Physiol Biochem, 2018, 123: 81-93.
doi: 10.1016/j.plaphy.2017.12.001 URL |
| [63] | Daruwalla A, Kiser PD. Structural and mechanistic aspects of carotenoid cleavage dioxygenases(CCDs)[J]. Biochim Biophys Acta Mol Cell Biol Lipids, 2020, 1865(11): 158590. |
| [64] |
Ohmiya A. Carotenoid cleavage dioxygenases and their apocarotenoid products in plants[J]. Plant Biotechnol, 2009, 26(4): 351-358.
doi: 10.5511/plantbiotechnology.26.351 URL |
| [65] |
Li F, Gong XW, Liang YP, et al. Characteristics of a new carotenoid cleavage dioxygenase NtCCD10 derived from Nicotiana tabacum[J]. Planta, 2022, 256(5): 100.
doi: 10.1007/s00425-022-04013-y |
| [66] |
Brandi F, Bar E, Mourgues F, et al. Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism[J]. BMC Plant Biol, 2011, 11: 24.
doi: 10.1186/1471-2229-11-24 |
| [67] |
Zhang XS, Pei JJ, Zhao LG, et al. RNA-Seq analysis and comparison of the enzymes involved in ionone synthesis of three cultivars of Osmanthus[J]. J Asian Nat Prod Res, 2018, 20(7): 649-661.
doi: 10.1080/10286020.2018.1453503 URL |
| [68] |
Ye P, Liang SC, Wang XM, et al. Transcriptome analysis and targeted metabolic profiling for pathway elucidation and identification of a geraniol synthase involved in iridoid biosynthesis from Gardenia jasminoides[J]. Ind Crops Prod, 2019, 132: 48-58.
doi: 10.1016/j.indcrop.2019.02.002 URL |
| [69] |
Vogel JT, Tan BC, McCarty DR, et al. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions[J]. J Biol Chem, 2008, 283(17): 11364-11373.
doi: 10.1074/jbc.M710106200 pmid: 18285342 |
| [70] |
Huang FC, Molnár P, Schwab W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes[J]. J Exp Bot, 2009, 60(11): 3011-3022.
doi: 10.1093/jxb/erp137 URL |
| [71] | Wang JM, Zhao MY, Gao T, et al. Promoter and coding sequence diversity of CsCCD1 may contribute to the differential accumulation of floral β-ionone in fresh tea(Camellia sinensis)leaves[J]. Hortic Plant J, 2022 |
| [72] |
Lashbrooke JG, Young PR, Dockrall SJ, et al. Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family[J]. BMC Plant Biol, 2013, 13: 156.
doi: 10.1186/1471-2229-13-156 pmid: 24106789 |
| [73] |
Meng N, Wei Y, Gao Y, et al. Characterization of transcriptional expression and regulation of carotenoid cleavage dioxygenase 4b in grapes[J]. Front Plant Sci, 2020, 11: 483.
doi: 10.3389/fpls.2020.00483 pmid: 32457771 |
| [74] |
Han YJ, Lu MM, Yue SM, et al. Comparative methylomics and chromatin accessibility analysis in Osmanthus fragrans uncovers regulation of genic transcription and mechanisms of key floral scent production[J]. Hortic Res, 2022, 9: uhac096.
doi: 10.1093/hr/uhac096 URL |
| [75] |
Han YJ, Wu M, Cao LY, et al. Characterization of OfWRKY3, a transcription factor that positively regulates the carotenoid cleavage dioxygenase gene OfCCD4 in Osmanthus fragrans[J]. Plant Mol Biol, 2016, 91(4): 485-496.
doi: 10.1007/s11103-016-0483-6 URL |
| [76] |
Han YJ, Wang HY, Wang XD, et al. Mechanism of floral scent production in Osmanthus fragrans and the production and regulation of its key floral constituents, β-ionone and linalool[J]. Hortic Res, 2019, 6: 106.
doi: 10.1038/s41438-019-0189-4 |
| [77] |
秦娟, 余凡, 刘璐, 等. 桃PpNAC19的克隆及其对PpCCD4启动子活性的调节分析[J]. 核农学报, 2021, 35(6): 1273-1280.
doi: 10.11869/j.issn.100-8551.2021.06.1273 |
| Qin J, Yu F, Liu L, et al. Cloning of peach PpNAC19 and its regulation on PpCCD4 promoter activity[J]. J Nucl Agric Sci, 2021, 35(6): 1273-1280. | |
| [78] |
Zhu F, Luo T, Liu CY, et al. An R2R3-MYB transcription factor represses the transformation of α- and β-branch carotenoids by negatively regulating expression of CrBCH2 and CrNCED5 in flavedo of Citrus reticulate[J]. New Phytol, 2017, 216(1): 178-192.
doi: 10.1111/nph.2017.216.issue-1 URL |
| [79] |
Lu SW, Zhang Y, Zhu KJ, et al. The Citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes[J]. Plant Physiol, 2018, 176(4): 2657-2676.
doi: 10.1104/pp.17.01830 URL |
| [80] |
Zhu KJ, Sun Q, Chen HY, et al. Ethylene activation of carotenoid biosynthesis by a novel transcription factor CsERF061[J]. J Exp Bot, 2021, 72(8): 3137-3154.
doi: 10.1093/jxb/erab047 pmid: 33543285 |
| [81] |
Zhu ZG, Chen GP, Guo XH, et al. Overexpression of SlPRE2, an atypical bHLH transcription factor, affects plant morphology and fruit pigment accumulation in tomato[J]. Sci Rep, 2017, 7(1): 5786.
doi: 10.1038/s41598-017-04092-y |
| [82] |
Schaub P, Wüst F, Koschmieder J, et al. Nonenzymatic β-carotene degradation in provitamin A-biofortified crop plants[J]. J Agric Food Chem, 2017, 65(31): 6588-6598.
doi: 10.1021/acs.jafc.7b01693 URL |
| [83] |
Zhang SL, Guo YT, Zhang YQ, et al. Genome-wide identification, characterization and expression profiles of the CCD gene family in Gossypium species[J]. 3 Biotech, 2021, 11(5): 249.
doi: 10.1007/s13205-021-02805-9 |
| [84] |
Scherzinger D, Al-Babili S. In vitro characterization of a carotenoid cleavage dioxygenase from Nostoc sp. PCC 7120 reveals a novel cleavage pattern, cytosolic localization and induction by highlight[J]. Mol Microbiol, 2008, 69(1): 231-244.
doi: 10.1111/j.1365-2958.2008.06282.x pmid: 18485074 |
| [85] |
Liu HR, Cao XM, Azam M, et al. Metabolism of carotenoids and β-ionone are mediated by carotenogenic genes and PpCCD4 under ultraviolet B irradiation and during fruit ripening[J]. Front Plant Sci, 2022, 13: 814677.
doi: 10.3389/fpls.2022.814677 URL |
| [86] |
Wang JM, Wu B, Zhang N, et al. Dehydration-induced carotenoid cleavage dioxygenase 1 reveals a novel route for β-ionone formation during tea(Camellia sinensis)withering[J]. J Agric Food Chem, 2020, 68(39): 10815-10821.
doi: 10.1021/acs.jafc.0c04208 URL |
| [87] |
Liang MH, Wu FC, Liang ZC, et al. Induction of carotenoid cleavage by salt stress and the effect of their products on cell growth and pigment accumulation in Dunaliella sp. FACHB-847[J]. Algal Res, 2020, 48: 101901.
doi: 10.1016/j.algal.2020.101901 URL |
| [88] |
Havaux M. Carotenoid oxidation products as stress signals in plants[J]. Plant J, 2014, 79(4): 597-606.
doi: 10.1111/tpj.2014.79.issue-4 URL |
| [89] |
Ying SW, Khoo HE, Kong KW, et al. Carotenoids and their geometry isomers in selected tropical fruits[J]. Int J Food Prop, 2013, 16(4): 826-837.
doi: 10.1080/10942912.2011.567431 URL |
| [90] | Winterhalter P, Rouseff R. Carotenoid-derived aroma compounds: an introduction[M]//ACS Symposium Series. Washington, DC: American Chemical Society, 2001: 1-17. |
| [91] |
Ningrum A, Minh NN, Schreiner M. Carotenoids and norisoprenoids as carotenoid degradation products in pandan leaves(Pandanus amaryllifolius Roxb.)[J]. Int J Food Prop, 2015, 18(9): 1905-1914.
doi: 10.1080/10942912.2014.971186 URL |
| [92] | Du F, Hu ZY, Qin LL, et al. Suppression of carotenoid cleavage dioxygenase 1(NtCCD1)increases carotenoid contents and attenuates reactive oxygen species(ROS)in tobacco leaves[J]. Plant Growth Regul, 2023: 1-13. |
| [93] |
Li J, Zhu GF, Wang ZH. Chemical variation in essential oil of Cymbidium sinense flowers from six cultivars[J]. J Essent Oil Bear Plants, 2017, 20(2): 385-394.
doi: 10.1080/0972060X.2017.1311236 URL |
| [94] |
Huang FC, Horváth G, Molnár P, et al. Substrate promiscuity of RdCCD1, a carotenoid cleavage oxygenase from Rosa damascena[J]. Phytochemistry, 2009, 70(4): 457-464.
doi: 10.1016/j.phytochem.2009.01.020 URL |
| [95] |
Ohmiya A, Kishimoto S, Aida R, et al. Carotenoid cleavage dioxygenase(CmCCD4a)contributes to white color formation in chrysanthemum petals[J]. Plant Physiol, 2006, 142(3): 1193-1201.
pmid: 16980560 |
| [96] |
Oms-Oliu G, Hertog MLATM, Van de Poel B, et al. Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life[J]. Postharvest Biol Technol, 2011, 62(1): 7-16.
doi: 10.1016/j.postharvbio.2011.04.010 URL |
| [97] |
Borsani J, Budde CO, Porrini L, et al. Carbon metabolism of peach fruit after harvest: changes in enzymes involved in organic acid and sugar level modifications[J]. J Exp Bot, 2009, 60(6): 1823-1837.
doi: 10.1093/jxb/erp055 pmid: 19264753 |
| [98] |
Lombardo VA, Osorio S, Borsani J, et al. Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage[J]. Plant Physiol, 2011, 157(4): 1696-1710.
doi: 10.1104/pp.111.186064 pmid: 22021422 |
| [99] |
Singh SP, Singh Z, Swinny EE. Sugars and organic acids in Japanese plums(Prunus salicinaLindell)as influenced by maturation, harvest date, storage temperature and period[J]. Int J Food Sci Technol, 2009, 44(10): 1973-1982.
doi: 10.1111/ifs.2009.44.issue-10 URL |
| [100] |
Zhang JJ, Wang X, Yu O, et al. Metabolic profiling of strawberry(Fragaria × ananassa Duch.) during fruit development and maturation[J]. J Exp Bot, 2011, 62(3): 1103-1118.
doi: 10.1093/jxb/erq343 URL |
| [101] |
Basson CE, Groenewald JH, Kossmann J, et al. Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: Invertase is the main sucrose hydrolysing enzyme[J]. Food Chem, 2010, 121(4): 1156-1162.
doi: 10.1016/j.foodchem.2010.01.064 URL |
| [102] |
Yu KQ, Xu Q, Da XL, et al. Transcriptome changes during fruit development and ripening of sweet orange(Citrus sinensis)[J]. BMC Genomics, 2012, 13: 10.
doi: 10.1186/1471-2164-13-10 |
| [103] |
Tang M, Bie ZL, Wu MZ, et al. Changes in organic acids and acid metabolism enzymes in melon fruit during development[J]. Sci Hortic, 2010, 123(3): 360-365.
doi: 10.1016/j.scienta.2009.11.001 URL |
| [104] |
Saradhuldhat P, Paull RE. Pineapple organic acid metabolism and accumulation during fruit development[J]. Sci Hortic, 2007, 112(3): 297-303.
doi: 10.1016/j.scienta.2006.12.031 URL |
| [105] |
Kalua CM, Boss PK. Evolution of volatile compounds during the development of cabernet sauvignon grapes(Vitis vinifera L.)[J]. J Agric Food Chem, 2009, 57(9): 3818-3830.
doi: 10.1021/jf803471n URL |
| [106] |
Baldermann S, Naim M, Fleischmann P. Enzymatic carotenoid degradation and aroma formation in nectarines(Prunus persica)[J]. Food Res Int, 2005, 38(8/9): 833-836.
doi: 10.1016/j.foodres.2005.02.009 URL |
| [107] |
Wang Y, Hao YJ, Zhou DD, et al. Differences in commercial quality and carotenoids profile of yellow- and white-fleshed nectarine fruit during low temperature storage and the regulation of carotenoids by sugar[J]. Postharvest Biol Technol, 2023, 197: 112206.
doi: 10.1016/j.postharvbio.2022.112206 URL |
| [108] |
Adami M, Franceschi P, Brandi F, et al. Identifying a carotenoid cleavage dioxygenase(CCD4)gene controlling yellow/white fruit flesh color of peach[J]. Plant Mol Biol Report, 2013, 31(5): 1166-1175.
doi: 10.1007/s11105-013-0628-6 URL |
| [109] |
Sun L, Yuan B, Zhang M, et al. Fruit-specific RNAi-mediated suppression of SlNCED1 increases both lycopene and β-carotene contents in tomato fruit[J]. J Exp Bot, 2012, 63(8): 3097-3108.
doi: 10.1093/jxb/ers026 pmid: 22345638 |
| [110] | 张印, 万勇, 张婷, 等. 柑橘愈伤组织RNAi沉默CCD1基因对其类胡萝卜素积累的影响[J]. 园艺学报, 2020, 47(10): 1982-1990. |
| Zhang Y, Wan Y, Zhang T, et al. RNAi-mediated suppression of CCD1 gene impacts carotenoid accumulation in Citrus calli[J]. Acta Hortic Sin, 2020, 47(10): 1982-1990. | |
| [111] | 王端, 景晨娟, 陈雪峰, 等. 华北杏不同品种(品系)果实发育阶段香气成分分析[J]. 江苏农业学报, 2021, 37(4): 974-981. |
| Wang D, Jing CJ, Chen XF, et al. Analysis on aroma components in fruits of different cultivars(lines)of North China apricot during developmental stages[J]. Jiangsu J Agric Sci, 2021, 37(4): 974-981. | |
| [112] |
Xi WP, Zheng HW, Zhang QY, et al. Profiling taste and aroma compound metabolism during apricot fruit development and ripening[J]. Int J Mol Sci, 2016, 17(7): 998.
doi: 10.3390/ijms17070998 URL |
| [113] |
Jing GX, Li TT, Qu HX, et al. Carotenoids and volatile profiles of yellow- and red-fleshed papaya fruit in relation to the expression of carotenoid cleavage dioxygenase genes[J]. Postharvest Biol Technol, 2015, 109: 114-119.
doi: 10.1016/j.postharvbio.2015.06.006 URL |
| [114] |
Liu YJ, Ye SH, Yuan GG, et al. Gene silencing of BnaA09.ZEP and BnaC09.ZEP confers orange color in Brassica napus flowers[J]. Plant J, 2020, 104(4): 932-949.
doi: 10.1111/tpj.v104.4 URL |
| [115] |
Watanabe K, Oda-Yamamizo C, Sage-Ono K, et al. Alteration of flower colour in Ipomoea nil through CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase 4[J]. Transgenic Res, 2018, 27(1): 25-38.
doi: 10.1007/s11248-017-0051-0 pmid: 29247330 |
| [116] |
Zhang XS, Pei JJ, Zhao LG, et al. Overexpression and characterization of CCD4 from Osmanthus fragrans and β-ionone biosynthesis from β-carotene in vitro[J]. J Mol Catal B Enzym, 2016, 134: 105-114.
doi: 10.1016/j.molcatb.2016.10.003 URL |
| [117] |
Cataldo VF, López J, Cárcamo M, et al. Chemical vs. biotechnological synthesis of C13-apocarotenoids: current methods, applications and perspectives[J]. Appl Microbiol Biotechnol, 2016, 100(13): 5703-5718.
doi: 10.1007/s00253-016-7583-8 URL |
| [118] |
Beekwilder J, van Rossum HM, Koopman F, et al. Polycistronic expression of a β-carotene biosynthetic pathway in Saccharomyces cerevisiae coupled to β-ionone production[J]. J Biotechnol, 2014, 192 Pt B: 383-392.
doi: 10.1016/j.jbiotec.2013.12.016 pmid: 24486029 |
| [119] |
López J, Essus K, Kim IK, et al. Production of β-ionone by combined expression of carotenogenic and plant CCD1 genes in Saccharomyces cerevisiae[J]. Microb Cell Fact, 2015, 14: 84.
doi: 10.1186/s12934-015-0273-x URL |
| [120] |
Czajka JJ, Nathenson JA, Benites VT, et al. Engineering the oleaginous yeast Yarrowia lipolytica to produce the aroma compound β-ionone[J]. Microb Cell Fact, 2018, 17(1): 136.
doi: 10.1186/s12934-018-0984-x |
| [121] |
Werner N, Ramirez-Sarmiento CA, Agosin E. Protein engineering of carotenoid cleavage dioxygenases to optimize β-ionone biosynthesis in yeast cell factories[J]. Food Chem, 2019, 299: 125089.
doi: 10.1016/j.foodchem.2019.125089 URL |
| [122] | 储陈辰, 孙明升, 吴雨涵, 等. 杨树泛基因组构建与基因组变异分析[J]. 南京林业大学学报: 自然科学版, 2022, 46(6): 251-260. |
| Chu CC, Sun MS, Wu YH, et al. Construction of poplar pan-genome and analysis of genome variation[J]. J Nanjing For Univ Nat Sci Ed, 2022, 46(6): 251-260. | |
| [123] | 郝兆东, 施季森, 陈金慧. 类胡萝卜素介导的植物花色调控机制研究进展[J]. 南京林业大学学报: 自然科学版, 2022, 46(6): 73-82. |
| Hao ZD, Shi JS, Chen JH. Research progress on carotenoid-mediated plant color regulation mechanism[J]. J Nanjing For Univ Nat Sci Ed, 2022, 46(6): 73-82. | |
| [124] |
Rivers JY, Truong TT, Pogson BJ, et al. Volatile apocarotenoid discovery and quantification in Arabidopsis thaliana: optimized sensitive analysis via HS-SPME-GC/MS[J]. Metabolomics, 2019, 15(5): 79.
doi: 10.1007/s11306-019-1529-y pmid: 31087204 |
| [125] |
Ytterberg AJ, Peltier JB, van Wijk KJ. Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes[J]. Plant Physiol, 2006, 140(3): 984-997.
doi: 10.1104/pp.105.076083 pmid: 16461379 |
| [1] | WU Qiao-yin, SHI You-zhi, LI Lin-lin, PENG Zheng, TAN Zai-yu, LIU Li-ping, ZHANG Juan, PAN Yong. In Situ Screening of Carotenoid Degrading Strains and the Application in Improving Quality and Aroma of Cigar [J]. Biotechnology Bulletin, 2023, 39(9): 192-201. |
| [2] | WANG Ling, ZHUO Shen, FU Xue-sen, LIU Zi-xuan, LIU Xiao-rong, WANG Zhi-hui, ZHOU Ri-bao, LIU Xiang-dan. Advances in the Biosynthetic Pathways and Related Genes of Lotus Alkaloids [J]. Biotechnology Bulletin, 2023, 39(7): 56-66. |
| [3] | JIANG Qing-chun, DU Jie, WANG Jia-cheng, YU Zhi-he, WANG Yun, LIU Zhong-yu. Expression and Function Analysis of Transcription Factor PcMYB2 from Polygonum cuspidatum [J]. Biotechnology Bulletin, 2023, 39(5): 217-223. |
| [4] | ZHANG He-chen, YUAN Xin, GAO Jie, WANG Xiao-chen, WANG Hui-juan, LI Yan-min, WANG Li-min, FU Zhen-zhu, LI Bao-yin. Mechanism of Flower Petal Coloration and Molecular Breeding [J]. Biotechnology Bulletin, 2023, 39(5): 23-31. |
| [5] | ZHOU Ding-ding, LI Hui-hu, TANG Xing-yong, YU Fa-xin, KONG Dan-yu, LIU Yi. Research Progress in the Biosynthesis and Regulation of Glycyrrhizic Acid and Liquiritin [J]. Biotechnology Bulletin, 2023, 39(5): 44-53. |
| [6] | YU Hui-li, LI Ai-tao. Application of Cytochrome P450 in the Biosynthesis of Flavors and Fragrances [J]. Biotechnology Bulletin, 2023, 39(4): 24-37. |
| [7] | ZHANG Zhi-xia, LI Tian-pei, ZENG Hong, ZHU Xi-xian, YANG Tian-xiong, MA Si-nan, HUANG Lei. Genome Sequencing and Bioinformatics Analysis of Gelidibacter sp. PG-2 [J]. Biotechnology Bulletin, 2023, 39(3): 290-300. |
| [8] | YAO Xiao-wen, LIANG Xiao, CHEN Qing, WU Chun-ling, LIU Ying, LIU Xiao-qiang, SHUI Jun, QIAO Yang, MAO Yi-ming, CHEN Yin-hua, ZHANG Yin-dong. Study on the Expression Pattern of Genes in Lignin Biosynthesis Pathway of Cassava Resisting to Tetranychus urticae [J]. Biotechnology Bulletin, 2023, 39(2): 161-171. |
| [9] | MIAO Shu-nan, GAO Yu, LI Xin-ru, CAI Gui-ping, ZHANG Fei, XUE Jin-ai, JI Chun-li, LI Run-zhi. Functional Analysis of Soybean GmPDAT1 Genes in the Oil Biosynthesis and Response to Abiotic Stresses [J]. Biotechnology Bulletin, 2023, 39(2): 96-106. |
| [10] | ZHOU Lin, LIANG Xuan-ming, ZHAO Lei. Biosynthesis of Natural Carotenoids:Progress and Perspective [J]. Biotechnology Bulletin, 2022, 38(7): 119-127. |
| [11] | LI Yi-dan, SHAN Xiao-hui. Gibberellin Metabolism Regulation and Green Revolution [J]. Biotechnology Bulletin, 2022, 38(2): 195-204. |
| [12] | YANG Rui-xian, LIU Ping, WANG Zu-hua, RUAN Bao-shuo, WANG Zhi-da. Analysis of Antimicrobial Active Metabolites from Antagonistic Strains Against Fusarium solani [J]. Biotechnology Bulletin, 2022, 38(2): 57-66. |
| [13] | LI Xiao-fan, GENG Dan-dan, BI Yu-lin, JIANG Yong, WANG Zhi-xiu, CHANG Guo-bin, CHEN Guo-hong, BAI Hao. Research Progress in Unconventional miRNA Functions [J]. Biotechnology Bulletin, 2022, 38(12): 1-10. |
| [14] | TIAN Qing-yin, YUE Yuan-zheng, SHEN Hui-min, PAN Duo, YANG Xiu-lian, WANG Liang-gui. Research Progress in the Regulation of Carotenoid Metabolism in Plant Ornamental Organs [J]. Biotechnology Bulletin, 2022, 38(12): 35-46. |
| [15] | YAO Yu, GU Jia-jun, SUN Chao, SHEN Guo-an, GUO Bao-lin. Advances in Plant Flavonoids UDP-glycosyltransferase [J]. Biotechnology Bulletin, 2022, 38(12): 47-57. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||