








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (1): 1-11.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0510
Received:2023-05-30
Online:2024-01-26
Published:2024-02-06
Contact:
SUN Chao
E-mail:g18535776268@163.com;csun@implad.ac.cn
GUAN Zhi-jing, SUN Chao. Research Progress in the Compartmentalization of Plant Specialized Metabolism[J]. Biotechnology Bulletin, 2024, 40(1): 1-11.
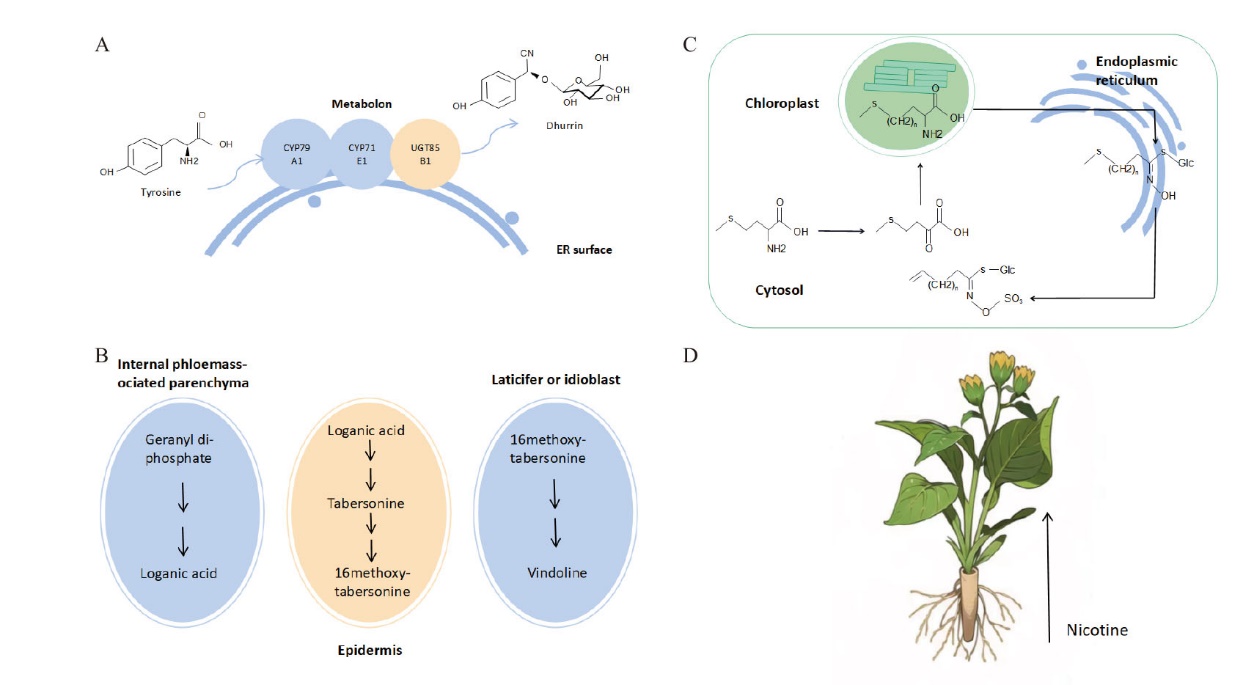
Fig. 1 Plant specialized metabolic compartmentalization on different levels A: L-tyrosine undergoes a metabolon composed of three enzymes(CYP79A1, CYP71E1 and UGT85B1)to finally synthesize dhurrin. B: The biosynthesis of glucosinolates is involved three subcellular compartments, which are cytosol, chloroplast and endoplasmic reticulum. C: The biosynthesis of vindoline is involved four cell types, which are internal phloemassociated parenchyma, epidermal, laticifer and idioblast. D: Nicotine is synthesized in roots and transported to leaves for accumulation
| 转运蛋白家族 Transporter family | 次生代谢产物 Specialized metabolites | 转运蛋白 Transport protein | 转运蛋白序列号 GenBank | 基源植物 Original species | 细胞定位 Cell localization | 转运方向 Transport direction | 功能验证方式 Functional verification method | 参考文献 Reference |
|---|---|---|---|---|---|---|---|---|
| ABC | 小檗碱 | CjMDR1(ABC B) | AB043999.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ |
| CjABCB2(ABC B) | AB674325.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | ||
| CjABCB3(ABC B) | AB674326.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | ||
| 石蒜碱 | LaABCB11(ABC B) | YP_009745292.1 | 忽地笑Lycoris aurea | 细胞膜 | 向细胞外 | 酵母异源表达 | [ | |
| 花青素 | ZmMRP3(ABC C) | AAT37905.1 | 玉米Zea mays | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | VvABCC1(ABC C) | AGC23330.1 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC2(ABC C) | AEC09006.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC1(ABC C) | AEE31213.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC14(ABC C) | AEE80381.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 藏红花素 | CsABCC4a(ABC C) | QEY08349.1 | 番红花Crocus sativus | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| Sclareolide | NpPDR1(ABC G) | FE898762.1 | 皱叶烟草 Nicotiana plumbaginifolia | 细胞膜 | 向细胞外 | 诱导蛋白表达 检测底物浓度 | [ | |
| 西松烯 香紫苏醇 椒二醇 | NtPDR1(ABC G) | AB109388.1 | 烟草Nicotiana tabacum | 细胞膜 | — | 基因过表达 | [ | |
| 椒二醇 | NbABCG1(ABC G) | LC015759.1 | 本氏烟草Nicotiana benthamiana | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| NbABCG2(ABC G) | LC015761 | 本氏烟草Nicotiana benthamiana | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | ||
| β-石竹烯 | AaPDR3(ABC G) | KR153482.1 | 黄花蒿Artemisia annua | 细胞膜 | 向细胞内 | 基因过表达、酵母异源表达 | [ | |
| 单萜 | PbABCG1(ABC G) | JQ088099.1 | 荧光蝴蝶兰Phalaenopsis bellina | 细胞膜 | 向细胞外 | RNA干扰基因沉默、 病毒诱导的基因沉默 | [ | |
| 挥发性有机 化合物 | PhABCG1(ABC G) | AFC36404.1 | 碧冬茄Petunia×hybrida | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | |
| 长春质碱 | CrTPT2(ABC G) | KC511771.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| 植保素Camalexin | AtABCG34(ABC G) | AEC09246.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 香豆素 | AtABCG37(ABC G) | AEE79095.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 香豆素 | NtABCG3(ABC G) | CAJ19055.1 | 烟草Nicotiana tabacum | 细胞膜 | — | 病毒诱导的基因沉默、 基因过表达 | [ | |
| 苜蓿素Medicarpin | MtABCG10(ABC G) | XP_003597819 | 蒺藜苜蓿Medicago truncatula | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 木质素 | PgrABCG14(ABC G) | Pgr018151.1 | 石榴Punica granatum | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 角质层 | AtABCG32(ABC G) | AEC07906.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因敲除 | [ | |
| 角质层 | SIABCG42(ABC G) | XP_006353655.1 | 番茄Solanum lycopersicum | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | |
| SlABCG36(ABC G) | XP_006338166.1 | 番茄Solanum lycopersicum | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | ||
| 蜡质 | ZxABCG11(ABC G) | YP_009990684.1 | 霸王Zygophyllum xanthoxylum | — | — | 基因过表达 | [ | |
| 脱落酸 | AtABCG40(ABC G) | AEE29332.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达、酵母异源表达 | [ | |
| MATE | 尼古丁 | NtMATE1 | AB286963.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 基因过表达、酵母异源表达 | [ |
| NtMATE2 | AB286962.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 基因过表达、酵母异源表达 | [ | ||
| NtJAT1 | BAG68655.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| NtJAT2 | BAG68656.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 小檗碱 | CjMATE1 | BAX73926.1 | 日本黄连Coptis japonica | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 黄酮醇 | AtFFT | OAP00740.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| 原花青素 | AtTT12 | OAP05921.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 原花青素 | MtMATE1 | ACX37118.1 | 蒺藜苜蓿 Medicago truncatula | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 类黄酮 | MtMATE2 | ADV04045.1 | 蒺藜苜蓿 Medicago truncatula | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 原花青素 | MdMATE1 | GU064954 | 栽培苹果Malus x domestica Borkh. | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| MdMATE2 | GU064956 | 栽培苹果Malus x domestica Borkh. | 液泡膜 | 向液泡内 | 突变体验证 | [ | ||
| 原花青素 | GhTT12 | AGW32085.1 | 陆地棉Gossypium hirsutum | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| 原花青素 | FaTT12-1 | AUA60209.1 | 草莓Fragaria×ananassa | 液泡膜 | 向液泡内 | 病毒诱导的基因沉默 | [ | |
| 花青素 | VvAM1 | FJ264202 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| VvAM3 | FJ264203 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 异黄酮 | GmMATE1 | KRG94946.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| GmMATE2 | KAG4396127.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 异黄酮 | GmMATE4 | KRH64938.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| CCoumaroylagmatine | AtDTX18 | AEE76776.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 突变体验证 | [ | |
| 黄酮醇 | NtMATE21 | XP_016475205.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞外 | 突变体验证 | [ | |
| NtMATE22 | XP_016477351.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞外 | 突变体验证 | [ | ||
| 染料木素 | LaMATE2 | KY464927 | 白羽扇豆Lupinus albus | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| 苦味素 | CsMATE1 | AXN55888.1 | 黄瓜Cucumis sativus | 液泡膜 | 向液泡内 | 基因过表达 | [ | |
| 水杨酸 | EDS5 | ABZ03276.1 | 拟南芥Arabidopsis thaliana | 叶绿体被膜 | 向叶绿体外 | 酵母异源表达 | [ | |
| PUP | 尼古丁 | NUP1 | ADP30799.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞内 | 酵母异源表达 | [ |
| 苄基异喹啉生物碱 | BUP1 | QBG64391.1 | 罂粟Papaver somniferum | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | |
| 咖啡因 | CsPUP1 | TEA003596 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | |
| CsPUP3.1 | TEA029223 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | ||
| CsPUP10.1 | TEA023430 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | ||
| NPF | 硫代葡萄糖苷 | AtNPF2.9(AtGTR3) | Q9M9V7.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ |
| 硫代葡萄糖苷 | AtNPF2.10(AtGTR1) | Q944G5.3 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 硫代葡萄糖苷 | AtNPF2.11(AtGTR2) | BAH19623.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 黄酮醇苷 | AtFST1(NPF2.8) | Q3E8X3.2 | 拟南芥Arabidopsis thaliana | 细胞膜 | — | 大肠杆菌异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF2.9 | AQM73449.1 | 长春花Catharanthus roseus | 液泡膜 | 向液泡外 | 病毒诱导的基因沉默 | [ | |
| 长春碱和长春新碱 | CrNPF2.4 | ALE20039.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF 2.5 | ALE20040.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF 2.6 | ALE20041.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 番茄碱 | SlNPF1.5 | OP765903.1 | 番茄Solanum lycopersicum | 液泡膜 | 向液泡外 | 爪蟾卵母细胞异源表达 | [ |
Table 1 Transport proteins that transport plant specialized metabolites
| 转运蛋白家族 Transporter family | 次生代谢产物 Specialized metabolites | 转运蛋白 Transport protein | 转运蛋白序列号 GenBank | 基源植物 Original species | 细胞定位 Cell localization | 转运方向 Transport direction | 功能验证方式 Functional verification method | 参考文献 Reference |
|---|---|---|---|---|---|---|---|---|
| ABC | 小檗碱 | CjMDR1(ABC B) | AB043999.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ |
| CjABCB2(ABC B) | AB674325.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | ||
| CjABCB3(ABC B) | AB674326.1 | 日本黄连Coptis japonica | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | ||
| 石蒜碱 | LaABCB11(ABC B) | YP_009745292.1 | 忽地笑Lycoris aurea | 细胞膜 | 向细胞外 | 酵母异源表达 | [ | |
| 花青素 | ZmMRP3(ABC C) | AAT37905.1 | 玉米Zea mays | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | VvABCC1(ABC C) | AGC23330.1 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC2(ABC C) | AEC09006.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC1(ABC C) | AEE31213.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 花青素 | AtABCC14(ABC C) | AEE80381.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 藏红花素 | CsABCC4a(ABC C) | QEY08349.1 | 番红花Crocus sativus | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| Sclareolide | NpPDR1(ABC G) | FE898762.1 | 皱叶烟草 Nicotiana plumbaginifolia | 细胞膜 | 向细胞外 | 诱导蛋白表达 检测底物浓度 | [ | |
| 西松烯 香紫苏醇 椒二醇 | NtPDR1(ABC G) | AB109388.1 | 烟草Nicotiana tabacum | 细胞膜 | — | 基因过表达 | [ | |
| 椒二醇 | NbABCG1(ABC G) | LC015759.1 | 本氏烟草Nicotiana benthamiana | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| NbABCG2(ABC G) | LC015761 | 本氏烟草Nicotiana benthamiana | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | ||
| β-石竹烯 | AaPDR3(ABC G) | KR153482.1 | 黄花蒿Artemisia annua | 细胞膜 | 向细胞内 | 基因过表达、酵母异源表达 | [ | |
| 单萜 | PbABCG1(ABC G) | JQ088099.1 | 荧光蝴蝶兰Phalaenopsis bellina | 细胞膜 | 向细胞外 | RNA干扰基因沉默、 病毒诱导的基因沉默 | [ | |
| 挥发性有机 化合物 | PhABCG1(ABC G) | AFC36404.1 | 碧冬茄Petunia×hybrida | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | |
| 长春质碱 | CrTPT2(ABC G) | KC511771.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| 植保素Camalexin | AtABCG34(ABC G) | AEC09246.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 香豆素 | AtABCG37(ABC G) | AEE79095.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 香豆素 | NtABCG3(ABC G) | CAJ19055.1 | 烟草Nicotiana tabacum | 细胞膜 | — | 病毒诱导的基因沉默、 基因过表达 | [ | |
| 苜蓿素Medicarpin | MtABCG10(ABC G) | XP_003597819 | 蒺藜苜蓿Medicago truncatula | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 木质素 | PgrABCG14(ABC G) | Pgr018151.1 | 石榴Punica granatum | 细胞膜 | 向细胞外 | 基因过表达 | [ | |
| 角质层 | AtABCG32(ABC G) | AEC07906.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因敲除 | [ | |
| 角质层 | SIABCG42(ABC G) | XP_006353655.1 | 番茄Solanum lycopersicum | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | |
| SlABCG36(ABC G) | XP_006338166.1 | 番茄Solanum lycopersicum | 细胞膜 | 向细胞外 | RNA干扰基因沉默 | [ | ||
| 蜡质 | ZxABCG11(ABC G) | YP_009990684.1 | 霸王Zygophyllum xanthoxylum | — | — | 基因过表达 | [ | |
| 脱落酸 | AtABCG40(ABC G) | AEE29332.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 基因过表达、酵母异源表达 | [ | |
| MATE | 尼古丁 | NtMATE1 | AB286963.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 基因过表达、酵母异源表达 | [ |
| NtMATE2 | AB286962.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 基因过表达、酵母异源表达 | [ | ||
| NtJAT1 | BAG68655.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| NtJAT2 | BAG68656.1 | 烟草Nicotiana tabacum | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 小檗碱 | CjMATE1 | BAX73926.1 | 日本黄连Coptis japonica | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 黄酮醇 | AtFFT | OAP00740.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| 原花青素 | AtTT12 | OAP05921.1 | 拟南芥Arabidopsis thaliana | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 原花青素 | MtMATE1 | ACX37118.1 | 蒺藜苜蓿 Medicago truncatula | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 类黄酮 | MtMATE2 | ADV04045.1 | 蒺藜苜蓿 Medicago truncatula | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| 原花青素 | MdMATE1 | GU064954 | 栽培苹果Malus x domestica Borkh. | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| MdMATE2 | GU064956 | 栽培苹果Malus x domestica Borkh. | 液泡膜 | 向液泡内 | 突变体验证 | [ | ||
| 原花青素 | GhTT12 | AGW32085.1 | 陆地棉Gossypium hirsutum | 液泡膜 | 向液泡内 | 突变体验证 | [ | |
| 原花青素 | FaTT12-1 | AUA60209.1 | 草莓Fragaria×ananassa | 液泡膜 | 向液泡内 | 病毒诱导的基因沉默 | [ | |
| 花青素 | VvAM1 | FJ264202 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| VvAM3 | FJ264203 | 葡萄Vitis vinifera | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 异黄酮 | GmMATE1 | KRG94946.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| GmMATE2 | KAG4396127.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | ||
| 异黄酮 | GmMATE4 | KRH64938.1 | 大豆Glycine max | 液泡膜 | 向液泡内 | 酵母异源表达 | [ | |
| CCoumaroylagmatine | AtDTX18 | AEE76776.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞外 | 突变体验证 | [ | |
| 黄酮醇 | NtMATE21 | XP_016475205.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞外 | 突变体验证 | [ | |
| NtMATE22 | XP_016477351.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞外 | 突变体验证 | [ | ||
| 染料木素 | LaMATE2 | KY464927 | 白羽扇豆Lupinus albus | 细胞膜 | 向细胞外 | 病毒诱导的基因沉默 | [ | |
| 苦味素 | CsMATE1 | AXN55888.1 | 黄瓜Cucumis sativus | 液泡膜 | 向液泡内 | 基因过表达 | [ | |
| 水杨酸 | EDS5 | ABZ03276.1 | 拟南芥Arabidopsis thaliana | 叶绿体被膜 | 向叶绿体外 | 酵母异源表达 | [ | |
| PUP | 尼古丁 | NUP1 | ADP30799.1 | 烟草Nicotiana tabacum | 细胞膜 | 向细胞内 | 酵母异源表达 | [ |
| 苄基异喹啉生物碱 | BUP1 | QBG64391.1 | 罂粟Papaver somniferum | 细胞膜 | 向细胞内 | 酵母异源表达 | [ | |
| 咖啡因 | CsPUP1 | TEA003596 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | |
| CsPUP3.1 | TEA029223 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | ||
| CsPUP10.1 | TEA023430 | 茶树Camellia sinensis | 细胞膜 | 向细胞内 | 基因过表达 | [ | ||
| NPF | 硫代葡萄糖苷 | AtNPF2.9(AtGTR3) | Q9M9V7.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ |
| 硫代葡萄糖苷 | AtNPF2.10(AtGTR1) | Q944G5.3 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 硫代葡萄糖苷 | AtNPF2.11(AtGTR2) | BAH19623.1 | 拟南芥Arabidopsis thaliana | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 黄酮醇苷 | AtFST1(NPF2.8) | Q3E8X3.2 | 拟南芥Arabidopsis thaliana | 细胞膜 | — | 大肠杆菌异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF2.9 | AQM73449.1 | 长春花Catharanthus roseus | 液泡膜 | 向液泡外 | 病毒诱导的基因沉默 | [ | |
| 长春碱和长春新碱 | CrNPF2.4 | ALE20039.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF 2.5 | ALE20040.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 长春碱和长春新碱 | CrNPF 2.6 | ALE20041.1 | 长春花Catharanthus roseus | 细胞膜 | 向细胞内 | 爪蟾卵母细胞异源表达 | [ | |
| 番茄碱 | SlNPF1.5 | OP765903.1 | 番茄Solanum lycopersicum | 液泡膜 | 向液泡外 | 爪蟾卵母细胞异源表达 | [ |
| [1] |
Gani U, Vishwakarma RA, Misra P. Membrane transporters: the key drivers of transport of secondary metabolites in plants[J]. Plant Cell Rep, 2021, 40(1): 1-18.
doi: 10.1007/s00299-020-02599-9 |
| [2] |
O'Connor SE. Engineering of secondary metabolism[J]. Annu Rev Genet, 2015, 49: 71-94.
doi: 10.1146/annurev-genet-120213-092053 pmid: 26393965 |
| [3] |
Shi YS, Wang D, Li RS, et al. Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides[J]. Metab Eng, 2021, 67: 104-111.
doi: 10.1016/j.ymben.2021.06.002 pmid: 34153454 |
| [4] |
Zhang YJ, Fernie AR. Metabolons, enzyme-enzyme assemblies that mediate substrate channeling, and their roles in plant metabolism[J]. Plant Commun, 2020, 2(1): 100081.
doi: 10.1016/j.xplc.2020.100081 URL |
| [5] |
Zhang YH. Substrate channeling and enzyme complexes for biotechnological applications[J]. Biotechnol Adv, 2011, 29(6): 715-725.
doi: 10.1016/j.biotechadv.2011.05.020 pmid: 21672618 |
| [6] |
Laursen T, Borch J, Knudsen C, et al. Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum[J]. Science, 2016, 354(6314): 890-893.
pmid: 27856908 |
| [7] |
Achnine L, Blancaflor EB, Rasmussen S, et al. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis[J]. Plant Cell, 2004, 16(11): 3098-3109.
doi: 10.1105/tpc.104.024406 pmid: 15472080 |
| [8] |
Verma P, Mathur AK, Srivastava A, et al. Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloid pathway in Catharanthus roseus: a literature update[J]. Protoplasma, 2012, 249(2): 255-268.
doi: 10.1007/s00709-011-0291-4 URL |
| [9] |
Li J, Kristiansen KA, Hansen BG, et al. Cellular and subcellular localization of flavin-monooxygenases involved in glucosinolate biosynthesis[J]. J Exp Bot, 2011, 62(3): 1337-1346.
doi: 10.1093/jxb/erq369 pmid: 21078824 |
| [10] |
Heinig U, Gutensohn M, Dudareva N, et al. The challenges of cellular compartmentalization in plant metabolic engineering[J]. Curr Opin Biotechnol, 2013, 24(2): 239-246.
doi: 10.1016/j.copbio.2012.11.006 URL |
| [11] |
Brillouet JM, Verdeil JL, Odoux E, et al. Phenol homeostasis is ensured in vanilla fruit by storage under solid form in a new chloroplast-derived organelle, the phenyloplast[J]. J Exp Bot, 2014, 65(9): 2427-2435.
doi: 10.1093/jxb/eru126 URL |
| [12] |
Brillouet JM, Romieu C, Schoefs B, et al. The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta[J]. Ann Bot, 2013, 112(6): 1003-1014.
doi: 10.1093/aob/mct168 URL |
| [13] |
Jacobowitz JR, Weng JK. Exploring uncharted territories of plant specialized metabolism in the postgenomic era[J]. Annu Rev Plant Biol, 2020, 71: 631-658.
doi: 10.1146/annurev-arplant-081519-035634 pmid: 32176525 |
| [14] |
Sun SJ, Shen XF, Li Y, et al. Single-cell RNA sequencing provides a high-resolution roadmap for understanding the multicellular compartmentation of specialized metabolism[J]. Nat Plants, 2023, 9(1): 179-190.
doi: 10.1038/s41477-022-01291-y |
| [15] |
Ozber N, Facchini PJ. Phloem-specific localization of benzylisoquinoline alkaloid metabolism in opium poppy[J]. J Plant Physiol, 2022, 271: 153641.
doi: 10.1016/j.jplph.2022.153641 URL |
| [16] |
de Brito Francisco R, Martinoia E. The vacuolar transportome of plant specialized metabolites[J]. Plant Cell Physiol, 2018, 59(7): 1326-1336.
doi: 10.1093/pcp/pcy039 pmid: 29452376 |
| [17] |
Nogia P, Pati PK. Plant secondary metabolite transporters: diversity, functionality, and their modulation[J]. Front Plant Sci, 2021, 12: 758202.
doi: 10.3389/fpls.2021.758202 URL |
| [18] |
Dechorgnat J, Nguyen CT, Armengaud P, et al. From the soil to the seeds: the long journey of nitrate in plants[J]. J Exp Bot, 2011, 62(4): 1349-1359.
doi: 10.1093/jxb/erq409 pmid: 21193579 |
| [19] |
Léran S, Varala K, Boyer JC, et al. A unified nomenclature of nitrate transporter 1/peptide transporter family members in plants[J]. Trends Plant Sci, 2014, 19(1): 5-9.
doi: 10.1016/j.tplants.2013.08.008 pmid: 24055139 |
| [20] |
Shitan N, Bazin I, Dan K, et al. Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica[J]. Proc Natl Acad Sci USA, 2003, 100(2): 751-756.
doi: 10.1073/pnas.0134257100 URL |
| [21] |
Shitan N, Dalmas F, Dan K, et al. Characterization of Coptis japonica CjABCB2, an ATP-binding cassette protein involved in alkaloid transport[J]. Phytochemistry, 2013, 91: 109-116.
doi: 10.1016/j.phytochem.2012.02.012 URL |
| [22] |
Wang R, Liu YT, Xu S, et al. An ATP-binding cassette transporter, LaABCB11, contributes to alkaloid transport in Lycoris aurea[J]. Int J Mol Sci, 2021, 22(21): 11458.
doi: 10.3390/ijms222111458 URL |
| [23] |
Goodman CD, Casati P, Walbot V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays[J]. Plant Cell, 2004, 16(7): 1812-1826.
doi: 10.1105/tpc.022574 URL |
| [24] |
Francisco RM, Regalado A, Ageorges A, et al. ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-Glucosides[J]. Plant Cell, 2013, 25(5): 1840-1854.
doi: 10.1105/tpc.112.102152 URL |
| [25] |
Behrens CE, Smith KE, Iancu CV, et al. Transport of anthocyanins and other flavonoids by the Arabidopsis ATP-binding cassette transporter AtABCC2[J]. Sci Rep, 2019, 9(1): 437.
doi: 10.1038/s41598-018-37504-8 pmid: 30679715 |
| [26] |
Dean JV, Willis M, Shaban L. Transport of acylated anthocyanins by the Arabidopsis ATP-binding cassette transporters AtABCC1, AtABCC2, and AtABCC14[J]. Physiol Plant, 2022, 174(5): e13780.
doi: 10.1111/ppl.v174.5 URL |
| [27] | Demurtas OC, de Brito Francisco R, Diretto G, et al. ABCC transporters mediate the vacuolar accumulation of crocins in saffron stigmas[J]. Plant Cell, 2019, 31(11): 2789-2804. |
| [28] |
Jasiński M, Stukkens Y, Degand H, et al. A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion[J]. Plant Cell, 2001, 13(5): 1095-1107.
pmid: 11340184 |
| [29] |
Pierman B, Toussaint F, Bertin A, et al. Activity of the purified plant ABC transporter NtPDR1 is stimulated by diterpenes and sesquiterpenes involved in constitutive and induced defenses[J]. J Biol Chem, 2017, 292(47): 19491-19502.
doi: 10.1074/jbc.M117.811935 pmid: 28972149 |
| [30] |
Shibata Y, Ojika M, Sugiyama A, et al. The full-size ABCG transporters Nb-ABCG1 and Nb-ABCG2 function in pre- and postinvasion defense against Phytophthora infestans in Nicotiana benthamiana[J]. Plant Cell, 2016, 28(5): 1163-1181.
doi: 10.1105/tpc.15.00721 URL |
| [31] |
Fu XQ, Shi P, He Q, et al. AaPDR3, a PDR transporter 3, is involved in sesquiterpene β-caryophyllene transport in Artemisia annua[J]. Front Plant Sci, 2017, 8: 723.
doi: 10.3389/fpls.2017.00723 URL |
| [32] |
Chang YL, Huang LM, Kuo XZ, et al. PbABCG1 and PbABCG2 transporters are required for the emission of floral monoterpenes in Phalaenopsis bellina[J]. Plant J, 2023, 114(2): 279-292.
doi: 10.1111/tpj.v114.2 URL |
| [33] |
Adebesin F, Widhalm JR, Boachon B, et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter[J]. Science, 2017, 356(6345): 1386-1388.
doi: 10.1126/science.aan0826 pmid: 28663500 |
| [34] |
Yu F, De Luca V. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus[J]. Proc Natl Acad Sci USA, 2013, 110(39): 15830-15835.
doi: 10.1073/pnas.1307504110 URL |
| [35] | Khare D, Choi H, Huh SU, et al. Arabidopsis ABCG34 contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin[J]. Proc Natl Acad Sci USA, 2017, 114(28): E5712-E5720. |
| [36] |
Fourcroy P, Sisó-Terraza P, Sudre D, et al. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency[J]. New Phytol, 2014, 201(1): 155-167.
doi: 10.1111/nph.12471 pmid: 24015802 |
| [37] |
Lefèvre F, Fourmeau J, Pottier M, et al. The Nicotiana tabacum ABC transporter NtPDR3 secretes O-methylated coumarins in response to iron deficiency[J]. J Exp Bot, 2018, 69(18): 4419-4431.
doi: 10.1093/jxb/ery221 URL |
| [38] |
Biala W, Banasiak J, Jarzyniak K, et al. Medicago truncatula ABCG10 is a transporter of 4-coumarate and liquiritigenin in the medicarpin biosynthetic pathway[J]. J Exp Bot, 2017, 68(12): 3231-3241.
doi: 10.1093/jxb/erx059 URL |
| [39] |
Yu Q, Li JY, Qin GH, et al. Characterization of the ABC transporter G subfamily in pomegranate and function analysis of PgrABCG14[J]. Int J Mol Sci, 2022, 23(19): 11661.
doi: 10.3390/ijms231911661 URL |
| [40] |
Fabre G, Garroum I, Mazurek S, et al. The ABCG transporter PEC1/ABCG32 is required for the formation of the developing leaf cuticle in Arabidopsis[J]. New Phytol, 2016, 209(1): 192-201.
doi: 10.1111/nph.2016.209.issue-1 URL |
| [41] |
Elejalde-Palmett C, Martinez San Segundo I, Garroum I, et al. ABCG transporters export cutin precursors for the formation of the plant cuticle[J]. Curr Biol, 2021, 31(10): 2111-2123.e9.
doi: 10.1016/j.cub.2021.02.056 URL |
| [42] | Liu LB, Bao AK, Li HJ, et al. Overexpression of ZxABCG11 from Zygophyllum xanthoxylum enhances tolerance to drought and heat in alfalfa by increasing cuticular wax deposition[J]. Crop J, 2022 |
| [43] |
Kang J, Hwang JU, Lee M, et al. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid[J]. Proc Natl Acad Sci USA, 2010, 107(5): 2355-2360.
doi: 10.1073/pnas.0909222107 pmid: 20133880 |
| [44] |
Shoji T, Inai K, Yazaki Y, et al. Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots[J]. Plant Physiol, 2009, 149(2): 708-718.
doi: 10.1104/pp.108.132811 pmid: 19098091 |
| [45] |
Morita M, Shitan N, Sawada K, et al. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion(MATE)transporter in Nicotiana tabacum[J]. Proc Natl Acad Sci USA, 2009, 106(7): 2447-2452.
doi: 10.1073/pnas.0812512106 URL |
| [46] |
Shitan N, Minami S, Morita M, et al. Involvement of the leaf-specific multidrug and toxic compound extrusion(MATE)transporter Nt-JAT2 in vacuolar sequestration of nicotine in Nicotiana tabacum[J]. PLoS One, 2014, 9(9): e108789.
doi: 10.1371/journal.pone.0108789 URL |
| [47] |
Takanashi K, Yamada Y, Sasaki T, et al. A multidrug and toxic compound extrusion transporter mediates berberine accumulation into vacuoles in Coptis japonica[J]. Phytochemistry, 2017, 138: 76-82.
doi: S0031-9422(17)30103-6 pmid: 28318534 |
| [48] |
Thompson EP, Wilkins C, Demidchik V, et al. An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development[J]. J Exp Bot, 2010, 61(2): 439-451.
doi: 10.1093/jxb/erp312 pmid: 19995827 |
| [49] |
Marinova K, Pourcel L, Weder B, et al. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat[J]. Plant Cell, 2007, 19(6): 2023-2038.
doi: 10.1105/tpc.106.046029 URL |
| [50] |
Zhao J, Dixon RA. MATE transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis[J]. Plant Cell, 2009, 21(8): 2323-2340.
doi: 10.1105/tpc.109.067819 URL |
| [51] |
Zhao J, Huhman D, Shadle G, et al. MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula[J]. Plant Cell, 2011, 23(4): 1536-1555.
doi: 10.1105/tpc.110.080804 URL |
| [52] |
Frank S, Keck M, Sagasser M, et al. Two differentially expressed MATE factor genes from apple complement the Arabidopsis transparent testa12 mutant[J]. Plant Biol, 2011, 13(1): 42-50.
doi: 10.1111/plb.2010.13.issue-1 URL |
| [53] |
Gao JS, Wu N, Shen ZL, et al. Molecular cloning, expression analysis and subcellular localization of a Transparent Testa 12 ortholog in brown cotton(Gossypium hirsutum L.)[J]. Gene, 2016, 576(2 Pt 2): 763-769.
doi: 10.1016/j.gene.2015.11.002 URL |
| [54] |
Chen SY, Tang YM, Hu YY, et al. FaTT12-1, a multidrug and toxin extrusion(MATE)member involved in proanthocyanidin transport in strawberry fruits[J]. Sci Hortic, 2018, 231: 158-165.
doi: 10.1016/j.scienta.2017.12.032 URL |
| [55] |
Gomez C, Terrier N, Torregrosa L, et al. Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters[J]. Plant Physiol, 2009, 150(1): 402-415.
doi: 10.1104/pp.109.135624 pmid: 19297587 |
| [56] |
Ng MS, Ku YS, Yung WS, et al. MATE-type proteins are responsible for isoflavone transportation and accumulation in soybean seeds[J]. Int J Mol Sci, 2021, 22(21): 12017.
doi: 10.3390/ijms222112017 URL |
| [57] |
Ku YS, Cheng SS, Cheung MY, et al. The poly-glutamate motif of GmMATE4 regulates its isoflavone transport activity[J]. Membranes, 2022, 12(2): 206.
doi: 10.3390/membranes12020206 URL |
| [58] |
Dobritzsch M, Lübken T, Eschen-Lippold L, et al. MATE transporter-dependent export of hydroxycinnamic acid amides[J]. Plant Cell, 2016, 28(2): 583-596.
doi: 10.1105/tpc.15.00706 URL |
| [59] |
Gani U, Nautiyal AK, Kundan M, et al. Two homeologous MATE transporter genes, NtMATE21 and NtMATE22, are involved in the modulation of plant growth and flavonol transport in Nicotiana tabacum[J]. J Exp Bot, 2022, 73(18): 6186-6206.
doi: 10.1093/jxb/erac249 URL |
| [60] |
Biała-Leonhard W, Zanin L, Gottardi S, et al. Identification of an isoflavonoid transporter required for the nodule establishment of the Rhizobium- Fabaceae symbiotic interaction[J]. Front Plant Sci, 2021, 12: 758213.
doi: 10.3389/fpls.2021.758213 URL |
| [61] |
Ma YS, Li DW, Zhong Y, et al. Vacuolar MATE/DTX protein-mediated cucurbitacin C transport is co-regulated with bitterness biosynthesis in cucumber[J]. New Phytol, 2023, 238(3): 995-1003.
doi: 10.1111/nph.18786 pmid: 36732026 |
| [62] |
Serrano M, Wang BJ, Aryal B, et al. Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5[J]. Plant Physiol, 2013, 162(4): 1815-1821.
doi: 10.1104/pp.113.218156 pmid: 23757404 |
| [63] |
Hildreth SB, Gehman EA, Yang HB, et al. Tobacco nicotine uptake permease(NUP1)affects alkaloid metabolism[J]. Proc Natl Acad Sci USA, 2011, 108(44): 18179-18184.
doi: 10.1073/pnas.1108620108 pmid: 22006310 |
| [64] |
Dastmalchi M, Chang LM, Chen RJ, et al. Purine permease-type benzylisoquinoline alkaloid transporters in opium poppy[J]. Plant Physiol, 2019, 181(3): 916-933.
doi: 10.1104/pp.19.00565 pmid: 31467164 |
| [65] |
Zhang YZ, Wei K, Guo LL, et al. Functional identification of purine permeases reveals their roles in caffeine transport in tea plants(Camellia sinensis)[J]. Front Plant Sci, 2022, 13: 1033316.
doi: 10.3389/fpls.2022.1033316 URL |
| [66] |
Jørgensen ME, Xu DY, Crocoll C, et al. Origin and evolution of transporter substrate specificity within the NPF family[J]. eLife, 2017, 6: e19466.
doi: 10.7554/eLife.19466 URL |
| [67] |
Nour-Eldin HH, Andersen TG, Burow M, et al. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds[J]. Nature, 2012, 488(7412): 531-534.
doi: 10.1038/nature11285 |
| [68] |
Grunewald S, Marillonnet S, Hause G, et al. The tapetal major facilitator NPF2.8 is required for accumulation of flavonol glycosides on the pollen surface in Arabidopsis thaliana[J]. Plant Cell, 2020, 32(5): 1727-1748.
doi: 10.1105/tpc.19.00801 URL |
| [69] |
Payne RME, Xu DY, Foureau E, et al. An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole[J]. Nat Plants, 2017, 3: 16208.
doi: 10.1038/nplants.2016.208 pmid: 28085153 |
| [70] |
Larsen B, Fuller VL, Pollier J, et al. Identification of iridoid glucoside transporters in Catharanthus roseus[J]. Plant Cell Physiol, 2017, 58(9): 1507-1518.
doi: 10.1093/pcp/pcx097 pmid: 28922750 |
| [71] |
Kazachkova Y, Zemach I, Panda S, et al. The GORKY glycoalkaloid transporter is indispensable for preventing tomato bitterness[J]. Nat Plants, 2021, 7(4): 468-480.
doi: 10.1038/s41477-021-00865-6 pmid: 33707737 |
| [72] |
Alam MT, Olin-Sandoval V, Stincone A, et al. The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization[J]. Nat Commun, 2017, 8: 16018.
doi: 10.1038/ncomms16018 pmid: 28691704 |
| [73] |
Sirikantaramas S, Yamazaki M, Saito K. Mechanisms of resistance to self-produced toxic secondary metabolites in plants[J]. Phytochem Rev, 2008, 7(3): 467-477.
doi: 10.1007/s11101-007-9080-2 URL |
| [74] |
Roze LV, Chanda A, Linz JE. Compartmentalization and molecular traffic in secondary metabolism: a new understanding of established cellular processes[J]. Fungal Genet Biol, 2011, 48(1): 35-48.
doi: 10.1016/j.fgb.2010.05.006 pmid: 20519149 |
| [75] |
Knudsen C, Gallage NJ, Hansen CC, et al. Dynamic metabolic solutions to the sessile life style of plants[J]. Nat Prod Rep, 2018, 35(11): 1140-1155.
doi: 10.1039/c8np00037a pmid: 30324199 |
| [76] |
Wu SQ, Schalk M, Clark A, et al. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants[J]. Nat Biotechnol, 2006, 24(11): 1441-1447.
doi: 10.1038/nbt1251 pmid: 17057703 |
| [77] |
Nour-Eldin HH, Madsen SR, Engelen S, et al. Reduction of antinutritional glucosinolates in Brassica oilseeds by mutation of genes encoding transporters[J]. Nat Biotechnol, 2017, 35(4): 377-382.
doi: 10.1038/nbt.3823 pmid: 28288105 |
| [78] |
Yamada Y, Urui M, Oki H, et al. Transport engineering for improving the production and secretion of valuable alkaloids in Escherichia coli[J]. Metab Eng Commun, 2021, 13: e00184.
doi: 10.1016/j.mec.2021.e00184 URL |
| [79] |
Zhou K, Qiao KJ, Edgar S, et al. Distributing a metabolic pathway among a microbial consortium enhances production of natural products[J]. Nat Biotechnol, 2015, 33(4): 377-383.
doi: 10.1038/nbt.3095 pmid: 25558867 |
| [1] | ZHOU Hui-wen, WU Lan-hua, HAN De-peng, ZHENG Wei, YU Pao-lan, WU Yang, XIAO Xiao-jun. Genome-wide Association Study of Seed Glucosinolate Content in Brassica napus [J]. Biotechnology Bulletin, 2024, 40(1): 222-230. |
| [2] | SONG Zhi-zhong, XU Wei-hua, XIAO Hui-lin, TANG Mei-ling, CHEN Jing-hui, GUAN Xue-qiang, LIU Wan-hao. Cloning, Expression and Function of Iron Regulated Transporter VvIRT1 in Wine Grape(Vitis vinifera L.) [J]. Biotechnology Bulletin, 2023, 39(8): 234-240. |
| [3] | MA Fang-fang, LIU Guan-wen, PANG Bing, JIANG Chun-mei, SHI Jun-ling. Strategies of Increasing Flavonoid Production in Engineered Bacteria by Intensifying the Efflux of Flavonoid in Cells [J]. Biotechnology Bulletin, 2023, 39(5): 63-76. |
| [4] | GAO Kai-yue, GUO Yu-ting, DU Yi-mou, ZHENG Xiao-mei, MA Xin-rong, ZHAO Wei, ZHENG Ping, SUN Ji-bin. A Quantitative Detection Approach for Glucose Uptake in Aspergillus niger: A Case Study of Glucose Transporter MstC [J]. Biotechnology Bulletin, 2023, 39(12): 71-80. |
| [5] | LI Xin-yue, ZHOU Ming-hai, FAN Ya-chao, LIAO Sha, ZHANG Feng-li, LIU Chen-guang, SUN Yue, ZHANG Lin, ZHAO Xin-qing. Research Progress in the Improvement of Microbial Strain Tolerance and Efficiency of Biological Manufacturing Based on Transporter Engineering [J]. Biotechnology Bulletin, 2023, 39(11): 123-136. |
| [6] | HONG Tian-shu, HAI Ying, ENHE Ba-ya-er, GAO Feng. Analysis of Expression Characteristics of CmABCG8 Gene in Cucumis melo L. [J]. Biotechnology Bulletin, 2022, 38(7): 178-185. |
| [7] | ZHOU Guo-yan, YIN Shan-shan, GAO Jia-xin, WU Chun-cheng, YAN Li-ying, XIE Yang. Identification of AHP Gene Family in Cucumis sativus and Its Expression Analysis Under Abiotic Stress [J]. Biotechnology Bulletin, 2022, 38(6): 112-119. |
| [8] | XUE Xin-yue, YU Xue-ran, LIU Xiao-gang, MA Jia-xin, TIAN Lei, LI Pei-fu. Research Progress in Absorption,Transportation and Accumulation Mechanism of Zinc in Rice [J]. Biotechnology Bulletin, 2022, 38(4): 29-43. |
| [9] | ZHAO Ting-ting, WANG Jun-gang, WANG Wen-zhi, FENG Cui-lian, FENG Xiao-yan, ZHANG Shu-zhen. Sequence and Tissue Expression Analysis of Monosaccharide Transporter Gene ShSTP7 in Sugarcane [J]. Biotechnology Bulletin, 2022, 38(4): 72-78. |
| [10] | CAO Ying-hui, HU Mei-juan, TONG Yan, ZHANG Yan-ping, ZHAO Kai, PENG Dong-hui, ZHOU Yu-zhen. Identification of the ABC Gene Family and Expression Pattern Analysis During Flower Development in Cymbidium ensifolium [J]. Biotechnology Bulletin, 2022, 38(11): 162-174. |
| [11] | WANG Jie, CAI Yu-meng, ZHANG Nan, ZHANG Ya-li. Regulatory Factors and Molecular Mechanism of Sucrose Transporters’ Expressions in Plant [J]. Biotechnology Bulletin, 2021, 37(3): 115-124. |
| [12] | SU Wen, LIU Jing, WANG Bing, LI Wei, DAI Liang-ying. Research Progress on the HAK Function of Plant High Affinity Potassium Ion Transporter [J]. Biotechnology Bulletin, 2020, 36(8): 144-152. |
| [13] | LI Xiao-ting, YUAN Jian-zhen, WANG Fang-zhen, LI Ren-hui, CUI Yan-nong, MA Qing. Research Progress on the Function of NO3- Transporters in the Adaptation of Plants to Adversity [J]. Biotechnology Bulletin, 2019, 35(2): 156-162. |
| [14] | ZHAO Ting-ting, WANG Jun-gang, YANG Ben-peng, SHEN Lin-bo, FENG Xiao-yan, WANG Wen-zhi, FENG Cui-lian, XIONG Guo-ru, ZHANG Shu-zhen. The Expression Analysis of Sucrose Transporter Genes in Disease Free Sugarcane [J]. Biotechnology Bulletin, 2018, 34(12): 125-131. |
| [15] | LIU Yuan-feng,LI Su-zhen,GUO Jin-jie,CHEN Jing-tang. Research Progress on YSL Transporters Gene Family [J]. Biotechnology Bulletin, 2017, 33(9): 1-9. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
