








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (1): 62-73.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0554
Previous Articles Next Articles
GAO Xin-ru1( ), XU Wen-tao1,2(
), XU Wen-tao1,2( )
)
Received:2024-06-11
Online:2025-01-26
Published:2025-01-22
Contact:
XU Wen-tao
E-mail:gao1587697334@qq.com;xuwentao@cau.edu.cn
GAO Xin-ru, XU Wen-tao. Sulforaphane Nanoparticles and Biological Applications[J]. Biotechnology Bulletin, 2025, 41(1): 62-73.
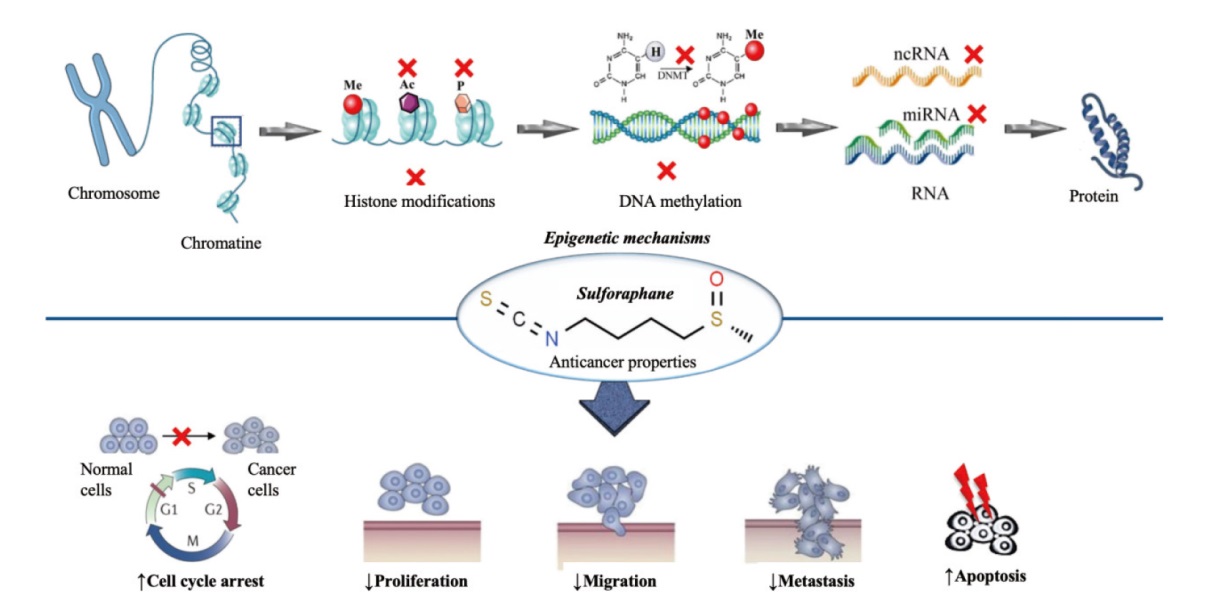
Fig. 2 Summarized scheme regarding epigenetic modifications of sulforaphane in cancer X: Iinhibition; Ac: acetylation; P: phosphorylation; Me: methylation; DNMT: DNA methyltransferase
| [1] | Sikdar S, Papadopoulou M, Dubois J. What do we know about sulforaphane protection against photoaging?[J]. J Cosmet Dermatol, 2016, 15(1): 72-77. |
| [2] | Greaney AJ, Maier NK, Leppla SH, et al. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism[J]. J Leukoc Biol, 2016, 99(1): 189-199. |
| [3] |
Johansson NL, Pavia CS, Chiao JW. Growth inhibition of a spectrum of bacterial and fungal pathogens by sulforaphane, an isothiocyanate product found in broccoli and other cruciferous vegetables[J]. Planta Med, 2008, 74(7): 747-750.
doi: 10.1055/s-2008-1074520 pmid: 18484523 |
| [4] | Kaiser AE, Baniasadi M, Giansiracusa D, et al. Sulforaphane: a broccoli bioactive phytocompound with cancer preventive potential[J]. Cancers, 2021, 13(19): 4796. |
| [5] |
Giacoppo S, Galuppo M, Montaut S, et al. An overview on neuroprotective effects of isothiocyanates for the treatment of neurodegenerative diseases[J]. Fitoterapia, 2015, 106: 12-21.
doi: 10.1016/j.fitote.2015.08.001 pmid: 26254971 |
| [6] | Wu YF, Mao JW, Mei LH, et al. Kinetic studies of the thermal degradation of sulforaphane and its hydroxypropyl-β-cyclodextrin inclusion complex[J]. Food Res Int, 2013, 53(1): 529-533. |
| [7] | Oravczová V, Garaiová Z, Hianik T. Nanoparticles and nanomotors modified by nucleic acids aptamers for targeted drug delivery[J]. Russ J Bioorg Chem, 2021, 47(2): 344-366. |
| [8] |
Krissanaprasit A, Key CM, Pontula S, et al. Self-assembling nucleic acid nanostructures functionalized with aptamers[J]. Chem Rev, 2021, 121(22): 13797-13868.
doi: 10.1021/acs.chemrev.0c01332 pmid: 34157230 |
| [9] | Marques AC, Costa PJ, Velho S, et al. Functionalizing nanoparticles with cancer-targeting antibodies: a comparison of strategies[J]. J Control Release, 2020, 320: 180-200. |
| [10] | Chen L, Zhang WL, Xie DQ, et al. Sulforaphane alleviates hepatic ischemia-reperfusion injury through promoting the activation of Nrf-2/HO-1 signaling[J]. Transpl Immunol, 2021, 68: 101439. |
| [11] |
Peng N, Jin LP, He AZ, et al. Effect of sulphoraphane on newborn mouse cardiomyocytes undergoing ischaemia/reperfusion injury[J]. Pharm Biol, 2019, 57(1): 753-759.
doi: 10.1080/13880209.2019.1680705 pmid: 31686558 |
| [12] | Wu DM, Zheng ZH, Fan SH, et al. Sulforaphane administration alleviates diffuse axonal injury(DAI)via regulation signaling pathway of NRF2 and HO-1[J]. J Cell Biochem, 2020, 121(1): 430-442. |
| [13] | Yang SH, Li P, Yu LH, et al. Sulforaphane protect against cadmium-induced oxidative damage in mouse leydigs cells by activating Nrf2/ARE signaling pathway[J]. Int J Mol Sci, 2019, 20(3): 630. |
| [14] |
Yanaka A. Role of NRF2 in protection of the gastrointestinal tract against oxidative stress[J]. J Clin Biochem Nutr, 2018, 63(1): 18-25.
doi: 10.3164/jcbn.17-139 pmid: 30087539 |
| [15] |
Guerrero-Beltrán CE, Calderón-Oliver M, Martínez-Abundis E, et al. Protective effect of sulforaphane against cisplatin-induced mitochondrial alterations and impairment in the activity of NAD(P)H: quinone oxidoreductase 1 and γ glutamyl cysteine ligase: studies in mitochondria isolated from rat kidney and in LLC-PK1 cells[J]. Toxicol Lett, 2010, 199(1): 80-92.
doi: 10.1016/j.toxlet.2010.08.009 pmid: 20732396 |
| [16] |
Kubo ER, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress[J]. Sci Rep, 2017, 7(1): 14130.
doi: 10.1038/s41598-017-14520-8 pmid: 29074861 |
| [17] | Patel B, Mann GE, Chapple SJ. Concerted redox modulation by sulforaphane alleviates diabetes and cardiometabolic syndrome[J]. Free Radic Biol Med, 2018, 122: 150-160. |
| [18] | Ahmed SMU, Luo L, Namani A, et al. Nrf2 signaling pathway: Pivotal roles in inflammation[J]. Biochim Biophys Acta Mol Basis Dis, 2017, 1863(2): 585-597. |
| [19] |
Heiss E, Herhaus C, Klimo K, et al. Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms[J]. J Biol Chem, 2001, 276(34): 32008-32015.
doi: 10.1074/jbc.M104794200 pmid: 11410599 |
| [20] | Liu YC, Hsieh CW, Weng YC, et al. Sulforaphane inhibition of monocyte adhesion via the suppression of ICAM-1 and NF-kappaB is dependent upon glutathione depletion in endothelial cells[J]. Vascul Pharmacol, 2008, 48(1): 54-61. |
| [21] |
Xu CJ, Shen GX, Chen C, et al. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells[J]. Oncogene, 2005, 24(28): 4486-4495.
doi: 10.1038/sj.onc.1208656 pmid: 15856023 |
| [22] | Hung CN, Huang HP, Wang CJ, et al. Sulforaphane inhibits TNF-α-induced adhesion molecule expression through the Rho A/ROCK/NF-κB signaling pathway[J]. J Med Food, 2014, 17(10): 1095-1102. |
| [23] | Jhang KA, Park JS, Kim HS, et al. Sulforaphane rescues amyloid-β peptide-mediated decrease in MerTK expression through its anti-inflammatory effect in human THP-1 macrophages[J]. J Neuroinflammation, 2018, 15(1): 75. |
| [24] | Heiss E, Gerhäuser C. Time-dependent modulation of thioredoxin reductase activity might contribute to sulforaphane-mediated inhibition of NF-kappaB binding to DNA[J]. Antioxid Redox Signal, 2005, 7(11-12): 1601-1611. |
| [25] |
Reddy SA, Shelar SB, Dang TM, et al. Sulforaphane and its methylcarbonyl analogs inhibit the LPS-stimulated inflammatory response in human monocytes through modulating cytokine production, suppressing chemotactic migration and phagocytosis in a NF-κB- and MAPK-dependent manner[J]. Int Immunopharmacol, 2015, 24(2): 440-450.
doi: S1567-5769(15)00003-X pmid: 25585231 |
| [26] |
Murata W, Yamaguchi Y, Fujita KI, et al. Enhancement of paraben-fungicidal activity by sulforaphane, a cruciferous vegetable-derived isothiocyanate, via membrane structural damage in Saccha-romyces cerevisiae[J]. Lett Appl Microbiol, 2019, 69(6): 403-410.
doi: 10.1111/lam.13230 pmid: 31596500 |
| [27] |
Ordonez AA, Korin Bullen C, Villabona-Rueda AF, et al. Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice[J]. Commun Biol, 2022, 5(1): 242.
doi: 10.1038/s42003-022-03189-z pmid: 35304580 |
| [28] | Janczewski Ł. Sulforaphane and its bifunctional analogs: synthesis and biological activity[J]. Molecules, 2022, 27(5): 1750. |
| [29] | Moon JK, Kim JR, Ahn YJ, et al. Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli(Brassica oleracea L.)sprouts[J]. J Agric Food Chem, 2010, 58(11): 6672-6677. |
| [30] |
Fahey JW, Haristoy X, Dolan PM, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Hel-icobacter pylori and prevents benzo[a]Pyrene-induced stomach tumors[J]. Proc Natl Acad Sci USA, 2002, 99(11): 7610-7615.
doi: 10.1073/pnas.112203099 pmid: 12032331 |
| [31] |
Deramaudt TB, Ali M, Vinit S, et al. Sulforaphane reduces intracellular survival of Staphylococcus aureus in macrophages through inhibition of JNK and p38 MAPK-induced inflammation[J]. Int J Mol Med, 2020, 45(6): 1927-1941.
doi: 10.3892/ijmm.2020.4563 pmid: 32323751 |
| [32] | Bendary MM, Ali MAM, Abdel Halim AS, et al. Investigating Sulforaphane's anti-virulence and anti-quorum sensing properties against Pseudomonas aeruginosa[J]. Front Pharmacol, 2024, 15: 1406653. |
| [33] |
Lenzi M, Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer[J]. Cancer Treat Res, 2014, 159: 207-223.
doi: 10.1007/978-3-642-38007-5_12 pmid: 24114482 |
| [34] | Su XL, Jiang X, Meng LB, et al. Anticancer activity of sulforaphane: the epigenetic mechanisms and the Nrf2 signaling pathway[J]. Oxid Med Cell Longev, 2018, 2018: 5438179. |
| [35] | Pradhan N, Kar S, Parbin S, et al. Epigenetic dietary interventions for prevention of cancer[M]// Epigenetics of Cancer Prevention. Amsterdam: Elsevier, 2019: 23-48. |
| [36] | Asif Ali M, Khan N, Kaleem N, et al. Anticancer properties of sulforaphane: current insights at the molecular level[J]. Front Oncol, 2023, 13: 1168321. |
| [37] |
Ho E, Clarke JD, Dashwood RH. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention[J]. J Nutr, 2009, 139(12): 2393-2396.
doi: 10.3945/jn.109.113332 pmid: 19812222 |
| [38] | Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs[J]. Am J Transl Res, 2011, 3(2): 166-179. |
| [39] |
Atwell LL, Beaver LM, Shannon J, et al. Epigenetic regulation by sulforaphane: opportunities for breast and prostate cancer chemoprevention[J]. Curr Pharmacol Rep, 2015, 1(2): 102-111.
pmid: 26042194 |
| [40] | Jiang LL, Zhou SJ, Zhang XM, et al. Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity[J]. Biomed Pharmacother, 2016, 78: 74-80. |
| [41] |
da Silva dos Santos PW, Machado ART, De Grandis RA, et al. Transcriptome and DNA methylation changes modulated by sulforaphane induce cell cycle arrest, apoptosis, DNA damage, and suppression of proliferation in human liver cancer cells[J]. Food Chem Toxicol, 2020, 136: 111047.
doi: 10.1016/j.fct.2019.111047 pmid: 31838189 |
| [42] |
Abbaoui B, Telu KH, Lucas CR, et al. The impact of cruciferous vegetable isothiocyanates on histone acetylation and histone phosphorylation in bladder cancer[J]. J Proteomics, 2017, 156: 94-103.
doi: S1874-3919(17)30026-X pmid: 28132875 |
| [43] |
Denis H, Ndlovu MN, Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms[J]. EMBO Rep, 2011, 12(7): 647-656.
doi: 10.1038/embor.2011.110 pmid: 21660058 |
| [44] |
Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy[J]. Cell, 2012, 150(1): 12-27.
doi: 10.1016/j.cell.2012.06.013 pmid: 22770212 |
| [45] | Ali Khan M, Kedhari Sundaram M, Hamza A, et al. Sulforaphane reverses the expression of various tumor suppressor genes by targeting DNMT3B and HDAC1 in human cervical cancer cells[J]. Evid Based Complement Alternat Med, 2015, 2015: 412149. |
| [46] | Hsu A, Wong CP, Yu Z, et al. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells[J]. Clin Epigenetics, 2011, 3(1): 3. |
| [47] |
Hyun TK. A recent overview on sulforaphane as a dietary epigenetic modulator[J]. EXCLI J, 2020, 19: 131-134.
doi: 10.17179/excli2019-2039 pmid: 32194360 |
| [48] | Mattick JS, Makunin IV. Non-coding RNA[J]. Hum Mol Genet, 2006, 15(suppl_1): R17-R29. |
| [49] |
Rafiei H, Ashrafizadeh M, Ahmadi Z. microRNAs as novel targets of sulforaphane in cancer therapy: The beginning of a new tale?[J]. Phytother Res, 2020, 34(4): 721-728.
doi: 10.1002/ptr.6572 pmid: 31972874 |
| [50] | Kalhori MR, Khodayari H, Khodayari S, et al. Regulation of long non-coding RNAs by plant secondary metabolites: a novel anticancer therapeutic approach[J]. Cancers, 2021, 13(6): 1274. |
| [51] | Liu CM, Peng CY, Liao YW, et al. Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction[J]. J Formos Med Assoc, 2017, 116(1): 41-48. |
| [52] | Martin SL, Kala R, Tollefsbol TO. Mechanisms for the inhibition of colon cancer cells by sulforaphane through epigenetic modulation of microRNA-21 and human telomerase reverse transcriptase(hTERT)down-regulation[J]. Curr Cancer Drug Targets, 2018, 18(1): 97-106. |
| [53] | Wang DX, Zou YJ, Zhuang XB, et al. Sulforaphane suppresses EMT and metastasis in human lung cancer through miR-616-5p-mediated GSK3β/β-catenin signaling pathways[J]. Acta Pharmacol Sin, 2017, 38(2): 241-251. |
| [54] |
Beaver LM, Kuintzle R, Buchanan A, et al. Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer[J]. J Nutr Biochem, 2017, 42: 72-83.
doi: S0955-2863(16)30565-4 pmid: 28131897 |
| [55] |
Bhat SA, Kamal MA, Yarla NS, et al. Synopsis on managment strategies for neurodegenerative disorders: challenges from bench to bedside in successful drug discovery and development[J]. Curr Top Med Chem, 2017, 17(12): 1371-1378.
doi: 10.2174/1568026616666161222121229 pmid: 28017151 |
| [56] | Ladak Z, Garcia E, Yoon J, et al. Sulforaphane(SFA)protects neuronal cells from oxygen & glucose deprivation(OGD)[J]. PLoS One, 2021, 16(3): e0248777. |
| [57] |
Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult[J]. J Neurosci, 2004, 24(5): 1101-1112.
doi: 10.1523/JNEUROSCI.3817-03.2004 pmid: 14762128 |
| [58] |
Mizuno K, Kume T, Muto C, et al. Glutathione biosynthesis via activation of the nuclear factor E2-related factor 2(Nrf2)—antioxidant-response element(ARE)pathway is essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl)hexyl isothiocyanate[J]. J Pharmacol Sci, 2011, 115(3): 320-328.
pmid: 21358121 |
| [59] |
Hong Y, Yan W, Chen S, et al. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice[J]. Acta Pharmacol Sin, 2010, 31(11): 1421-1430.
doi: 10.1038/aps.2010.101 pmid: 20953205 |
| [60] | Flint Beal M. Therapeutic approaches to mitochondrial dysfunction in Parkinson's disease[J]. Parkinsonism Relat Disord, 2009, 15(Suppl 3): S189-S194. |
| [61] |
Denzer I, Münch G, Friedland K. Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds[J]. Pharmacol Res, 2016, 103: 80-94.
doi: 10.1016/j.phrs.2015.11.019 pmid: 26626189 |
| [62] | Luis-García ER, Limón-Pacheco JH, Serrano-García N, et al. Sulforaphane prevents quinolinic acid-induced mitochondrial dysfunction in rat striatum[J]. J Biochem Mol Toxicol, 2017, 31(2). DOI: 10.1002/jbt.21837. |
| [63] | Hernandez-Rabaza V, Cabrera-Pastor A, Taoro-Gonzalez L, et al. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia[J]. J Neuroinflammation, 2016, 13(1): 83. |
| [64] |
Qin SS, Yang CH, Huang WH, et al. Sulforaphane attenuates microglia-mediated neuronal necroptosis through down-regulation of MAPK/NF-κB signaling pathways in LPS-activated BV-2 microglia[J]. Pharmacol Res, 2018, 133: 218-235.
doi: S1043-6618(17)31137-4 pmid: 29391237 |
| [65] |
Zhao FF, Zhang JL, Chang N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer's disease[J]. Eur J Pharmacol, 2018, 824: 1-10.
doi: S0014-2999(18)30059-1 pmid: 29382536 |
| [66] | Kim J, Lee S, Choi BR, et al. Sulforaphane epigenetically enhances neuronal BDNF expression and TrkB signaling pathways[J]. Mol Nutr Food Res, 2017, 61(2). DOI: 10.1002/mnfr.201600194. |
| [67] | Wu HH, Liang H, Yuan QP, et al. Preparation and stability investigation of the inclusion complex of sulforaphane with hydroxypropyl-β-cyclodextrin[J]. Carbohydr Polym, 2010, 82(3): 613-617. |
| [68] | Wu YF, Zou LG, Mao JW, et al. Stability and encapsulation efficiency of sulforaphane microencapsulated by spray drying[J]. Carbohydr Polym, 2014, 102: 497-503. |
| [69] | Lee S, Lee G, Jeon G, et al. Anti-aging and lightening effects of Au-decorated zeolite-based biocompatible nanocomposites in epidermal delivery systems[J]. J Funct Biomater, 2023, 14(2): 66. |
| [70] | Zhang J, Dong YY, Liu X, et al. Effective myocardial infarction treatment by targeted accumulation of Sulforaphane using porous magnetic silica nanoparticles[J]. Int J Pharm, 2023, 645: 123389. |
| [71] |
Ibrahim Fouad G, El-Sayed SAM, Mabrouk M, et al. Neuroprotective potential of intranasally delivered sulforaphane-loaded iron oxide nanoparticles against cisplatin-induced neurotoxicity[J]. Neurotox Res, 2022, 40(5): 1479-1498.
doi: 10.1007/s12640-022-00555-x pmid: 35969308 |
| [72] | Wu SQ, Li L, Liang QQ, et al. A DFT study of sulforaphane adsorbed on M12O12(M=Be, Mg and Ca)nanocages[J]. Mater Today Commun, 2024, 38: 107687. |
| [73] |
Danafar H, Sharafi A, Kheiri Manjili H, et al. Sulforaphane delivery using mPEG-PCL co-polymer nanoparticles to breast cancer cells[J]. Pharm Dev Technol, 2017, 22(5): 642-651.
doi: 10.3109/10837450.2016.1146296 pmid: 26916923 |
| [74] | Cristiano MC, Froiio F, Spaccapelo R, et al. Sulforaphane-loaded ultradeformable vesicles as a potential natural nanomedicine for the treatment of skin cancer diseases[J]. Pharmaceutics, 2019, 12(1): 6. |
| [75] | Azarashkan Z, Motamedzadegan A, Ghorbani-HasanSaraei A, et al. Improvement of the stability and release of sulforaphane-enriched broccoli sprout extract nanoliposomes by co-encapsulation into basil seed gum[J]. Food Bioprocess Technol, 2022, 15(7): 1573-1587. |
| [76] | Li RH, Hu XC, Shan SQ, et al. Study on the controlled release properties of modified multi-walled carbon nanotubes on sulforaphane[J]. Carbon Lett, 2024, 34(2): 757-765. |
| [77] |
Manjili HK, Ma'mani L, Naderi-Manesh H. Monodisperse rattle-structured gold nanorod-mesoporous silica nanoparticles core-shell as sulforaphane carrier and its sustained-release property[J]. Drug Res, 2018, 68(9): 504-513.
doi: 10.1055/a-0573-8966 pmid: 29660748 |
| [78] | Lu WJ, Du FF, Zhao XW, et al. Sulforaphane-conjugated carbon dots: a versatile nanosystem for targeted imaging and inhibition of EGFR-overexpressing cancer cells[J]. ACS Biomater Sci Eng, 2019, 5(9): 4692-4699. |
| [79] | Ibrahim Fouad G, Mabrouk M, El-Sayed SAM, et al. Neurotherapeutic efficacy of loaded sulforaphane on iron oxide nanoparticles against cuprizone-induced neurotoxicity: role of MMP-9 and S100β[J]. Toxicol Mech Methods, 2023, 33(6): 463-479. |
| [80] | Zhang P, Li TT, Liu C, et al. Nano-sulforaphane attenuates PhIP-induced early abnormal embryonic neuro-development[J]. Ann Anat, 2021, 233: 151617. |
| [81] | Danafar H, Shara\ufb 01 A, Kheiri S, et al. Co-delivery of sulforaphane and curcumin with PEGylated iron oxide-gold core shell nanoparticles for delivery to breast cancer cell line[J]. Iran J Pharm Res, 2018, 17(2): 480-494. |
| [82] | Gu HF, Ren FZ, Mao XY, et al. Mineralized and GSH-responsive hyaluronic acid based nano-carriers for potentiating repressive effects of sulforaphane on breast cancer stem cells-like properties[J]. Carbohydr Polym, 2021, 269: 118294. |
| [83] |
Xu Y, Han XX, Li YY, et al. Sulforaphane mediates glutathione depletion via polymeric nanoparticles to restore cisplatin chemosensitivity[J]. ACS Nano, 2019, 13(11): 13445-13455.
doi: 10.1021/acsnano.9b07032 pmid: 31670945 |
| [84] | Dutta D, Siddiqui L, Shah S, et al. Synergistic anticancer activity of Sulphoraphane and Teriflunomide co loaded lignin nanoparticles against triple negative breast cancer: Targeted nanoparticle delivery and drug repurposing[J]. Med Hypotheses, 2024, 189: 111404. |
| [85] | Zhang X, Peng X, Hui JQ, et al. Sulforaphane functionalized chitosan nanoparticles serve as an effective nano complex system for the treatment of human cervical cancer[J]. J Clust Sci, 2023, 34(6): 2963-2975. |
| [86] | Li SM, Xu ZK, Alrobaian M, et al. EGF-functionalized lipid-polymer hybrid nanoparticles of 5-fluorouracil and sulforaphane with enhanced bioavailability and anticancer activity against colon carcinoma[J]. Biotechnol Appl Biochem, 2022, 69(5): 2205-2221. |
| [87] |
Soni K, Mujtaba A, Akhter MH, et al. Optimisation of ethosomal nanogel for topical nano-CUR and sulphoraphane delivery in effective skin cancer therapy[J]. J Microencapsul, 2020, 37(2): 91-108.
doi: 10.1080/02652048.2019.1701114 pmid: 31810417 |
| [88] | Yepes-Molina L, Pérez-Jiménez MI, Martínez-Esparza M, et al. Membrane vesicles for nanoencapsulated sulforaphane increased their anti-inflammatory role on an in vitro human macrophage model[J]. Int J Mol Sci, 2022, 23(4): 1940. |
| [89] | Ramírez-Pavez T, García-Peñaranda A, Garcia-Ibañez P, et al. Potential of sulforaphane and broccoli membrane vesicles as regulators of M1/M2 human macrophage activity[J]. Int J Mol Sci, 2022, 23(19): 11141. |
| [90] | Essien EN, Revi N, Khatri V, et al. Methotrexate and sulforaphane loaded PBA-G5-PAMAM dendrimers as a combination therapy for anti-inflammatory response in an intra-articular joint arthritic animal model[J]. Int J Pharm, 2023, 642: 123150. |
| [91] |
黄心童, 耿宇昊, 刘恒源, 等. 微流控制备新型功能纳米粒子研究进展[J]. 化工学报, 2023, 74(1): 355-364.
doi: 10.11949/0438-1157.20220935 |
|
Huang XT, Geng YH, Liu HY, et al. Research progress on new functional nanoparticles prepared by microfluidic technology[J]. CIESC J, 2023, 74(1): 355-364.
doi: 10.11949/0438-1157.20220935 |
|
| [92] | 董翠芳, 刘树恒, 刘长霞. 环境响应壳聚糖纳米材料的制备及其应用研究进展[J]. 沧州师范学院学报, 2017, 33(2): 49-52, 57. |
| Dong CF, Liu SH, Liu CX. Research progress in preparation and application of environment-responsive chitosan nanomaterial[J]. J Cangzhou Norm Univ, 2017, 33(2): 49-52, 57. |
| [1] | DU Zhong-yang, YANG Ze, LIANG Meng-jing, LIU Yi-zhen, CUI Hong-li, SHI Da-ming, XUE Jin-ai, SUN Yan, ZHANG Chun-hui, JI Chun-li, LI Run-zhi. Effect of Nano-selenium(SeNPs)in Alleviating Lead Stress and Promoting Growth of Tobacco Seedlings [J]. Biotechnology Bulletin, 2024, 40(7): 183-196. |
| [2] | LU Xin-hua, SUN De-quan, ZHANG Xiu-mei. Genetic Transformation of Plant Cells Mediated by Mesoporous Silica Nanoparticles [J]. Biotechnology Bulletin, 2022, 38(7): 194-204. |
| [3] | SUN De-quan, LU Xin-hua, LI Wei-ming, HU Yu-lin, DUAN Ya-jie, PANG Zhen-cai, HU Hui-gang. Application of Mesoporous Silica Nanoparticles in Agriculture [J]. Biotechnology Bulletin, 2022, 38(5): 228-239. |
| [4] | WANG Qiao-ju, HU Yu-meng, WEN Ya-ya, SONG Li, MENG Chuang, PAN Zhi-ming, JIAO Xin-an. Expression and Activity Identification of SARS-CoV-2 S1 Protein [J]. Biotechnology Bulletin, 2022, 38(3): 157-163. |
| [5] | ZHANG Man-man, HE Teng-xia, DING Chen-yu, CHEN Meng-ping, WU Qi-feng. Research Progress of the Toxic Effects and Detoxification Measures of Engineered Nanoparticles in Biological Nitrogen-removing Process [J]. Biotechnology Bulletin, 2022, 38(2): 227-236. |
| [6] | GAO Qi-yu, XU Guang-cui, CUI Cai-xia, ZHANG Wen-bo. Research Progress in Microbial Ferritin [J]. Biotechnology Bulletin, 2022, 38(1): 269-277. |
| [7] | ZHANG Xiu-min, MA Shao-ying, YANG Jie, BAO Jin-yu, ZHANG Xiao-ling, TIAN Peng, LU Ya-qi, LI Sheng. Optimization of Hairy Roots Culture System of Broccoli Aiming at the Yield of Secondary Metabolites [J]. Biotechnology Bulletin, 2021, 37(8): 75-84. |
| [8] | QIAO Zi-peng, WANG Qi-zhi, YANG Dao-mao, RUAN Li-ping. Research Progress in Fungi-mediated Biosynthesis of Sliver Nanoparticles [J]. Biotechnology Bulletin, 2021, 37(3): 185-197. |
| [9] | WANG Lei-lei, DONG Lian-hua, YANG Jing-ya, WANG Xia. Research Progress of Gene Mutation Detection Methods Based on Nanoparticles [J]. Biotechnology Bulletin, 2021, 37(3): 241-251. |
| [10] | SUN Jing-shuang, HU Rui-yang, ZHENG Guang-shun, MA Wen-jun, XU Yan, WANG Jun-hui. Research Progress and Prospect of Plant Genetic Transformation Mediated by Nano-gene Vector [J]. Biotechnology Bulletin, 2021, 37(2): 162-173. |
| [11] | ZHANG Wei-ye, SONG Hao-zhi, LIU Xing-jian, LI Yi-nü, ZHANG Zhi-fang. Fusion Expression of Ferritin and Foot-and-Mouth Disease Virus VP1 in Escherichia coli and Self-assembly of Nanoparticles [J]. Biotechnology Bulletin, 2021, 37(2): 96-102. |
| [12] | LI Xue, LI Jun-min, ZHANG Lei, LI Shan. Expression and Purification of Cell-penetrating Peptide M918 Conjugate Antibody and Study on Its Uptake Efficiency [J]. Biotechnology Bulletin, 2021, 37(12): 198-204. |
| [13] | HANG Xin-ru, GENG Rong-qing, QIAN Lin. Preparation of Recombinant Milk Allergen β-Lactoglobulin and Establishment of Magnetic Particle Chemiluminescence Detection Method [J]. Biotechnology Bulletin, 2020, 36(3): 193-198. |
| [14] | YAO Lin-tong, LIU Ya-ting, LIU Ya-jing, CHEN Zhen-zhen. Research Progress on Mesoporous Silica in Cancer Therapy [J]. Biotechnology Bulletin, 2019, 35(2): 182-191. |
| [15] | ZHANG Wen-jun, WU Meng-ting, LÜ Chun-yan, WANG Qing, CHEN Yong-lin. Research Progress on Mesoporous Silica in Drug Delivery System and in vitro/in vivo [J]. Biotechnology Bulletin, 2019, 35(12): 159-168. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||