








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (10): 164-174.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0297
Previous Articles Next Articles
ZHANG Huan-huan1,2( ), MU Xiao-ya2, ZHOU Jing-yi2, LYU Gao-pei2, XIAO Nan2, LI Min3, HAO Yao-shan1, WU Shen-jie1,2(
), MU Xiao-ya2, ZHOU Jing-yi2, LYU Gao-pei2, XIAO Nan2, LI Min3, HAO Yao-shan1, WU Shen-jie1,2( )
)
Received:2025-03-19
Online:2025-10-26
Published:2025-10-28
Contact:
WU Shen-jie
E-mail:frank.red@163.com;sj__wu@126.com
ZHANG Huan-huan, MU Xiao-ya, ZHOU Jing-yi, LYU Gao-pei, XIAO Nan, LI Min, HAO Yao-shan, WU Shen-jie. An Efficient Genetic Transformation System for High-frequency Embryogenic Broomcorn Millet Line[J]. Biotechnology Bulletin, 2025, 41(10): 164-174.
| 编号 No. | 名称 Name | 编号 No. | 名称 Name |
|---|---|---|---|
| M1 | 晋黍5号 | M21 | 赤黍3号 |
| M2 | 晋黍6号 | M22 | 品糜3号 |
| M3 | 冀黍3号 | M23 | 陇糜5号 |
| M4 | 陇糜6号 | M24 | 宁糜16号 |
| M5 | 赤黍1号 | M25 | 晋黍2号 |
| M6 | 赤黍2号 | M26 | 伊糜5号 |
| M7 | 赤黍9号 | M27 | 品糜2号 |
| M8 | 宁糜10号 | M28 | 陇糜5号 |
| M9 | 陇糜8号 | M29 | 宁糜12号 |
| M10 | 固糜20号 | M30 | 宁糜15号 |
| M11 | 陇糜11号 | M31 | 赤黍5号 |
| M12 | 品糜1号 | M32 | 赤糜1号 |
| M13 | 年丰5号 | M33 | 红黍子3号 |
| M14 | 雁7号 | M34 | 黑黍子4号 |
| M15 | 齐黍1号 | M35 | 大黄糜子6号 |
| M16 | 陇糜15号 | M36 | 大黄糜子2号 |
| M17 | 宁糜13号 | M37 | 大黄糜子7号 |
| M18 | 宁糜11号 | M38 | 大黄糜子8号 |
| M19 | 赤黍8号 | M39 | 南燕川穄子2号 |
| M20 | 固糜21号 |
Table 1 39 broomcorn millet varieties in this study
| 编号 No. | 名称 Name | 编号 No. | 名称 Name |
|---|---|---|---|
| M1 | 晋黍5号 | M21 | 赤黍3号 |
| M2 | 晋黍6号 | M22 | 品糜3号 |
| M3 | 冀黍3号 | M23 | 陇糜5号 |
| M4 | 陇糜6号 | M24 | 宁糜16号 |
| M5 | 赤黍1号 | M25 | 晋黍2号 |
| M6 | 赤黍2号 | M26 | 伊糜5号 |
| M7 | 赤黍9号 | M27 | 品糜2号 |
| M8 | 宁糜10号 | M28 | 陇糜5号 |
| M9 | 陇糜8号 | M29 | 宁糜12号 |
| M10 | 固糜20号 | M30 | 宁糜15号 |
| M11 | 陇糜11号 | M31 | 赤黍5号 |
| M12 | 品糜1号 | M32 | 赤糜1号 |
| M13 | 年丰5号 | M33 | 红黍子3号 |
| M14 | 雁7号 | M34 | 黑黍子4号 |
| M15 | 齐黍1号 | M35 | 大黄糜子6号 |
| M16 | 陇糜15号 | M36 | 大黄糜子2号 |
| M17 | 宁糜13号 | M37 | 大黄糜子7号 |
| M18 | 宁糜11号 | M38 | 大黄糜子8号 |
| M19 | 赤黍8号 | M39 | 南燕川穄子2号 |
| M20 | 固糜21号 |
培养基名称 Medium name | 培养基配方 Medium composition |
|---|---|
OS培养基[ OS medium | N6盐2.5 g/L,蔗糖30 g/L,L-脯氨酸3 g/L,2,4-D 2.5 mg/L,植物凝胶2.6 g/L,pH 5.8 N6 salts 2.5 g/L, sucrose 30 g/L, L-proline 3 g/L, 2,4-D 2.5 mg/L, and phytagel 2.6 g/L, pH 5.8 |
TA培养基[ TA medium | MS盐4.4 g/L,蔗糖30 g/L,MES 500 mg/L,麦草畏2 mg/L,植物凝胶2.4 g/L,pH 5.8 MS salts 4.4 g/L, sucrose 30 g/L, MES 500 mg/L, dicamba 2 mg/L, and phytagel 2.6 g/L, pH 5.8 |
SV培养基[ SV medium | MS盐4.4 g/L,蔗糖30 g/L,五水硫酸铜 0.6 mg/L,七水硫酸锌 35 mg/L,2,4-D 2.0 mg/L,KT 0.5 mg/L,植物凝胶 2.6 g/L,pH 5.8 MS salts 4.4 g/L, sucrose 30 g/L, CuSO4·5H2O 0.6 mg/L, ZnSO4·7H2O 35 mg/L, 2,4-D 2.0 mg/L, KT 0.5 mg/L, and phytagel 2.6 g/L, pH 5.8 |
SB培养基[ SB medium | Ms盐 4.4 g/L,蔗糖30 g/L,五水硫酸铜0.16 mg/L,L-脯氨酸1 g/L,磷酸二氢钾 0.16 mg/L,2,4-D 1.0 mg/L,KT 0.5 mg/L,植物凝胶3 g/L,pH 5.8 MS salts 4.4 g/L, sucrose 30 g/L, CuSO4·5H2O 0.16 mg/L, L-proline 1 g/L, KH2PO4 0.16 mg/L, 2,4-D 1.0 mg/L, KT 0.5 mg/L, and phytagel 2.6 g/L, pH 5.8 |
SI培养基[ SI medium | MS盐4.4 g/L,蔗糖30 g/L,酸水解酪蛋白 300 mg/L, D-生物素1 mg/L, 五水硫酸铜0.6 mg/L,2,4-D 2.0 mg/L,KT 0.5 mg/L,硝酸银 2 mg/L,柠檬酸 1 mg/L,植物凝胶2.6 g/L,pH 5.8 MS salts 4.4 g/L, sucrose 30 g/L, casein hydrolysate 300 mg/L, D-biotin 1 mg/L, CuSO4·5H2O 0.6 mg/L, 2,4-D 2.0 mg/L, KT 0.5 mg/L, AgNO3 2 mg/L, citric acid 1 mg/L, and phytagel 2.6 g/L, pH 5.8 |
ZM培养基[ ZM medium | N6盐4.0 g/L,蔗糖30 g/L,2,4-D 2.0 mg/L,6-BA 0.2 mg/L,植物凝胶 2.6 g/L,pH 5.8 N6 salts 4.0g/L, sucrose 30 g/L, 2,4-D 2.0 mg/L, 6-BA 0.2 mg/L, and phytagel 2.6 g/L, pH 5.8 |
MRS培养基 MRS medium | MS盐4.4 g/L,酸水解酪蛋白300 mg/L,蔗糖30 g/L,L-谷氨酰胺100 mg/L,L-天冬酰胺100 mg/L,6-BA 1 mg/L,植物凝胶2.6 g/L,pH 5.8 MS salts 4.4 g/L, casein hydrolysate 300 mg/L, sucrose 30 g/L, L-glutamine 100 mg/L, L-asparagine 100 mg/L, 6-BA 1.0 mg/L, and phytagel 2.6 g/L, pH 5.8 |
RIM培养基 RIM medium | MS盐2.2 g/L,蔗糖30 g/L,硫酸镁0.3 g/L,IBA 1 mg/L,植物凝胶2.6 g/L,pH 5.8 MS salts 2.2 g/L, sucrose 30 g/L, MgSO4·5H2O 0.3 g/L, IBA 1.0 mg/L, and phytagel 2.6 g/L, pH 5.8 |
Table 2 Medium composition for broomcorn millet tissue culture
培养基名称 Medium name | 培养基配方 Medium composition |
|---|---|
OS培养基[ OS medium | N6盐2.5 g/L,蔗糖30 g/L,L-脯氨酸3 g/L,2,4-D 2.5 mg/L,植物凝胶2.6 g/L,pH 5.8 N6 salts 2.5 g/L, sucrose 30 g/L, L-proline 3 g/L, 2,4-D 2.5 mg/L, and phytagel 2.6 g/L, pH 5.8 |
TA培养基[ TA medium | MS盐4.4 g/L,蔗糖30 g/L,MES 500 mg/L,麦草畏2 mg/L,植物凝胶2.4 g/L,pH 5.8 MS salts 4.4 g/L, sucrose 30 g/L, MES 500 mg/L, dicamba 2 mg/L, and phytagel 2.6 g/L, pH 5.8 |
SV培养基[ SV medium | MS盐4.4 g/L,蔗糖30 g/L,五水硫酸铜 0.6 mg/L,七水硫酸锌 35 mg/L,2,4-D 2.0 mg/L,KT 0.5 mg/L,植物凝胶 2.6 g/L,pH 5.8 MS salts 4.4 g/L, sucrose 30 g/L, CuSO4·5H2O 0.6 mg/L, ZnSO4·7H2O 35 mg/L, 2,4-D 2.0 mg/L, KT 0.5 mg/L, and phytagel 2.6 g/L, pH 5.8 |
SB培养基[ SB medium | Ms盐 4.4 g/L,蔗糖30 g/L,五水硫酸铜0.16 mg/L,L-脯氨酸1 g/L,磷酸二氢钾 0.16 mg/L,2,4-D 1.0 mg/L,KT 0.5 mg/L,植物凝胶3 g/L,pH 5.8 MS salts 4.4 g/L, sucrose 30 g/L, CuSO4·5H2O 0.16 mg/L, L-proline 1 g/L, KH2PO4 0.16 mg/L, 2,4-D 1.0 mg/L, KT 0.5 mg/L, and phytagel 2.6 g/L, pH 5.8 |
SI培养基[ SI medium | MS盐4.4 g/L,蔗糖30 g/L,酸水解酪蛋白 300 mg/L, D-生物素1 mg/L, 五水硫酸铜0.6 mg/L,2,4-D 2.0 mg/L,KT 0.5 mg/L,硝酸银 2 mg/L,柠檬酸 1 mg/L,植物凝胶2.6 g/L,pH 5.8 MS salts 4.4 g/L, sucrose 30 g/L, casein hydrolysate 300 mg/L, D-biotin 1 mg/L, CuSO4·5H2O 0.6 mg/L, 2,4-D 2.0 mg/L, KT 0.5 mg/L, AgNO3 2 mg/L, citric acid 1 mg/L, and phytagel 2.6 g/L, pH 5.8 |
ZM培养基[ ZM medium | N6盐4.0 g/L,蔗糖30 g/L,2,4-D 2.0 mg/L,6-BA 0.2 mg/L,植物凝胶 2.6 g/L,pH 5.8 N6 salts 4.0g/L, sucrose 30 g/L, 2,4-D 2.0 mg/L, 6-BA 0.2 mg/L, and phytagel 2.6 g/L, pH 5.8 |
MRS培养基 MRS medium | MS盐4.4 g/L,酸水解酪蛋白300 mg/L,蔗糖30 g/L,L-谷氨酰胺100 mg/L,L-天冬酰胺100 mg/L,6-BA 1 mg/L,植物凝胶2.6 g/L,pH 5.8 MS salts 4.4 g/L, casein hydrolysate 300 mg/L, sucrose 30 g/L, L-glutamine 100 mg/L, L-asparagine 100 mg/L, 6-BA 1.0 mg/L, and phytagel 2.6 g/L, pH 5.8 |
RIM培养基 RIM medium | MS盐2.2 g/L,蔗糖30 g/L,硫酸镁0.3 g/L,IBA 1 mg/L,植物凝胶2.6 g/L,pH 5.8 MS salts 2.2 g/L, sucrose 30 g/L, MgSO4·5H2O 0.3 g/L, IBA 1.0 mg/L, and phytagel 2.6 g/L, pH 5.8 |

Fig. 1 Callus induction of Chishu 2 on 6 different mediaA‒F: Details of Chishu 2 induced for 15 d on different culture media; G‒L: overall status of Chishu 2 embryogenesis induced for 30 d on various culture media. A and G: OS medium; B and H: TA medium; C and I: SI medium; D and J: SB medium; E and K: SI medium; F and L: ZM medium. Scale = 1 cm
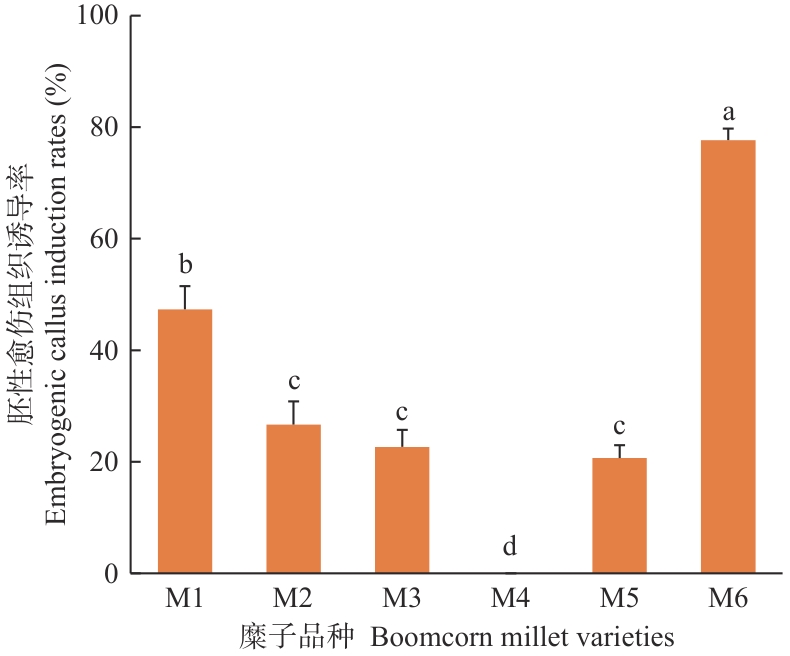
Fig. 2 Embryogenic callus induction rates of different broomcorn millet varieties on the SI mediumM1 to M6 are as follows: Jinshu 5, Jinshu 6, Jishu 3, Longmi 6, Chishu 1, and Chishu 2.The data were analyzed by one-way ANOVA (Turkey). Different lowercase letters indicate significant differences at P<0.05 level. The same below

Fig. 4 Ability of inducing callus in different tissuesA‒C: Millet root growth states on SI medium after 10, 20, and 30 d. D‒F: Millet shoot tips growth states on SI medium after 10, 20, and 30 d. G‒I: Millet mesocotyl growth states on SI medium after 10, 20, and 30 d. Scale = 1 cm

Fig. 5 In vitro regeneration system of broomcorn millet Chishu 2A: Mature seeds. B: Callus after 15 d induction. C: Embryonic callus after 30 d induction. D: Embryonic callus transferred to MRS medium. E: Seedlings on MRS medium. F: New root on RIM medium. Scale = 1 cm
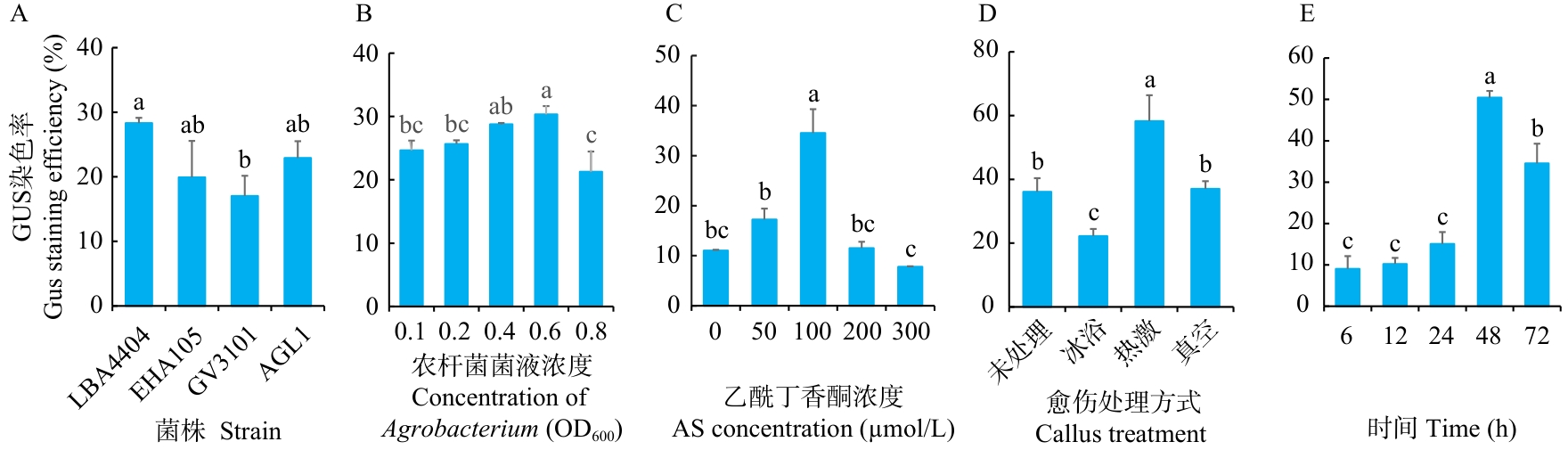
Fig. 6 Effects of different infection conditions on the infection efficiency of broomcorn milletA: Agrobacterium strain. B: Concentration of Agrobacterium liquid. C: Acetosyringone concentration. D: Callus treatment (Untreated, ice bath, heat shock and vacuumize). E: Co-culture time

Fig. 7 PCR detection of T0 transgenic plantsM: Marker DL2000. P: Positive control (pBI121). N: Blank control (ddH2O). CK: Negative control (wild type). 1‒20: T0 transgene plants
筛选方式 Screening method | 总愈伤数 Number of calluses | 抗性愈伤数 Number of survival callus | 抗性苗数 Number of survival seedlings | PCR阳性数 Number of PCR positive plants | PCR阳性率 PCR positive frequency (%) | 转化效率 Transformation frequency (%) |
|---|---|---|---|---|---|---|
| SI + Kan 50 mg/L | 92 | 30 | 24 | 3 | 12.50 | 3.26 |
| SI + Kan 100 mg/L | 100 | 22 | 19 | 5 | 26.30 | 5.00 |
| MRS + Kan 20 mg/L | 110 | 27 | 9 | 6 | 66.70 | 5.50 |
| RIM + Kan 50mg/L | 100 | 28 | 15 | 6 | 40.00 | 6.00 |
Table 3 Genetic transformation results of broomcorn millet
筛选方式 Screening method | 总愈伤数 Number of calluses | 抗性愈伤数 Number of survival callus | 抗性苗数 Number of survival seedlings | PCR阳性数 Number of PCR positive plants | PCR阳性率 PCR positive frequency (%) | 转化效率 Transformation frequency (%) |
|---|---|---|---|---|---|---|
| SI + Kan 50 mg/L | 92 | 30 | 24 | 3 | 12.50 | 3.26 |
| SI + Kan 100 mg/L | 100 | 22 | 19 | 5 | 26.30 | 5.00 |
| MRS + Kan 20 mg/L | 110 | 27 | 9 | 6 | 66.70 | 5.50 |
| RIM + Kan 50mg/L | 100 | 28 | 15 | 6 | 40.00 | 6.00 |

Fig. 8 Protocol of genetic transformation for broomcorn milletA: Embryogenic callus for transformation. B: Callus on co-culture medium. C: GUS transient staining. D: Recovery culture. E: Resistance screening. F: Shoot regeneration from resistant callus. G: Rooting. H: GUS staining of positive seedling. I: Flowchart of broomcorn millet genetic transformation. Scale = 1 cm
| [1] | 王宇卓, 林元香, 薛亚鹏, 等. 山西糜子核心种质分子身份证构建 [J]. 植物学报, 2023, 58(1): 159-168. |
| Wang YZ, Lin YX, Xue YP, et al. Construction of molecular ID card of core germplasm of hog millet (Panicum miliaceum) in Shanxi [J]. Chin Bull Bot, 2023, 58(1): 159-168. | |
| [2] | 杨清华, 王洪露, 冯佰利. 糜子品质研究进展与展望 [J]. 植物学报, 2023, 58(1): 22-33. |
| Yang QH, Wang HL, Feng BL. Progress and prospect of research on the quality of broomcorn millet [J]. Chin Bull Bot, 2023, 58(1): 22-33. | |
| [3] | 杨天育. 糜子分子遗传研究进展与展望 [J]. 寒旱农业科学, 2022(10): 32-36. |
| Yang TY. Research progress and prospect on molecular genetics of millet (Panicum miliaceum L.) [J]. J Cold Arid Agric Sci, 2022(10): 32-36. | |
| [4] | 薛亚鹏, 丁艺冰, 王宇卓, 等. 基于荧光SSR构建中国糜子核心种质DNA分子身份证 [J]. 中国农业科学, 2023, 56(12): 2249-2277. |
| Xue YP, Ding YB, Wang YZ, et al. Construction of DNA molecular identity card of core germplasm of broomcorn millet in China based on fluorescence SSR [J]. Sci Agric Sin, 2023, 56(12): 2249-2277. | |
| [5] | Shi JP, Ma XX, Zhang JH, et al. Chromosome conformation capture resolved near complete genome assembly of broomcorn millet [J]. Nat Commun, 2019, 10(1): 464. |
| [6] | Zou CS, Li LT, Miki D, et al. The genome of broomcorn millet [J]. Nat Commun, 2019, 10(1): 436. |
| [7] | Chen JF, Liu Y, Liu MX, et al. Pangenome analysis reveals genomic variations associated with domestication traits in broomcorn millet [J]. Nat Genet, 2023, 55(12): 2243-2254. |
| [8] | Bai YH, Liu SN, Bai Y, et al. Application of CRISPR/Cas12i.3 for targeted mutagenesis in broomcorn millet (Panicum miliaceum L.) [J]. J Integr Plant Biol, 2024, 66(8): 1544-1547. |
| [9] | Liu Y, Cheng ZX, Chen WY, et al. Establishment of genome-editing system and assembly of a near-complete genome in broomcorn millet [J]. J Integr Plant Biol, 2024, 66(8): 1688-1702. |
| [10] | 王春芳, 刘晶晶, 李伟, 等. 糜子愈伤诱导及再分化最适条件研究 [J]. 中国农学通报, 2020, 36(9): 94-99. |
| Wang CF, Liu JJ, Li W, et al. The optimal condition for callus induction and redifferentiation of Panicum miliaceum L [J]. Chin Agric Sci Bull, 2020, 36(9): 94-99. | |
| [11] | 吴会琴. 糜子不同外植体再生体系建立及优化 [D]. 杨凌: 西北农林科技大学, 2020. |
| Wu HQ. Establishment and optimization of regeneration system of different explants of broomcorn millet [D]. Yangling: Northwest A & F University, 2020. | |
| [12] | Wu J, Chang X, Li C, et al. QTLs related to rice callus regeneration ability: localization and effect verification of qPRR3 [J]. Cells, 2022, 11(24): 4125. |
| [13] | 石磊. 小麦WOX基因挖掘及其对遗传转化效率的影响和作用机制研究 [D]. 北京: 中国农业科学院, 2021. |
| Shi L. Identification of WOX genes and their functions and mechanisms in improving genetic transformation efficiency in Triticum aestivum L. [D]. Beijing: Chinese Academy of Agricultural Sciences, 2021. | |
| [14] | Nguyen DQ, Van Eck J, Eamens AL, et al. Robust and reproducible Agrobacterium-mediated transformation system of the C4 genetic model species Setaria viridis [J]. Front Plant Sci, 2020, 11: 281. |
| [15] | Silva TN, Kelly ME, Vermerris W. Use of Sorghum bicolor leaf whorl explants to expedite regeneration and increase transformation throughput [J]. Plant Cell Tissue Organ Cult, 2020, 141(2): 243-255. |
| [16] | Santos CM, Romeiro D, Silva JP, et al. An improved protocol for efficient transformation and regeneration of Setaria italica [J]. Plant Cell Rep, 2020, 39(4): 501-510. |
| [17] | Du DX, Jin RC, Guo JJ, et al. Infection of embryonic callus with Agrobacterium enables high-speed transformation of maize [J]. Int J Mol Sci, 2019, 20(2): 279. |
| [18] | 贺榆婷, 卫云丰, 张洁, 等. 谷子高效离体再生基因型和培养基的筛选 [J]. 核农学报, 2019, 33(7): 1265-1272. |
| He YT, Wei YF, Zhang J, et al. Screening of high efficient genotypes and medium for in vitro regeneration in foxtail millet [J]. J Nucl Agric Sci, 2019, 33(7): 1265-1272. | |
| [19] | Lowe K, Wu E, Wang N, et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation [J]. Plant Cell, 2016, 28(9): 1998-2015. |
| [20] | Li JP, Pan WB, Zhang S, et al. A rapid and highly efficient sorghum transformation strategy using GRF4-GIF1/ternary vector system [J]. Plant J, 2024, 117(5): 1604-1613. |
| [21] | 李素娟, 樊秀霞, 王华, 等. 水稻不同品种组培再生和转基因频率研究 [J]. 核农学报, 2013, 27(12): 1817-1827. |
| Li SJ, Fan XX, Wang H, et al. Investigation of regeneration and transformation frequencies generated from different genotypes in rice (Oryza sativa L.) [J]. J Nucl Agric Sci, 2013, 27(12): 1817-1827. | |
| [22] | 许洁婷, 刘相国, 金敏亮, 等. 不依赖基因型的高效玉米遗传转化体系的建立 [J]. 作物学报, 2022, 48(12): 2987-2993. |
| Xu JT, Liu XG, Jin ML, et al. Establishment of genotype-independent high-efficiency transformation system in maize [J]. Acta Agron Sin, 2022, 48(12): 2987-2993. | |
| [23] | Wang K, Shi L, Liang XN, et al. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation [J]. Nat Plants, 2022, 8(2): 110-117. |
| [24] | Bull T, Debernardi J, Reeves M, et al. GRF-GIF chimeric proteins enhance in vitro regeneration and Agrobacterium-mediated transformation efficiencies of lettuce (Lactuca spp.) [J]. Plant Cell Rep, 2023, 42(3): 629-643. |
| [25] | Yang WT, Zhai HW, Wu FM, et al. Peptide REF1 is a local wound signal promoting plant regeneration [J]. Cell, 2024, 187(12): 3024-3038.e14. |
| [26] | Purnhauser L, Gyulai G. Effect of copper on shoot and root regeneration in wheat, Triticale, rape and tobacco tissue cultures [J]. Plant Cell Tissue Organ Cult, 1993, 35(2): 131-139. |
| [27] | Kumar Sahrawat A, Chand S. Stimulatory effect of copper on plant regeneration in indica rice (Oryza sativa L.) [J]. J Plant Physiol, 1999, 154(4): 517-522. |
| [28] | Cho M, Banh J, Yu M, et al. Improvement of Agrobacterium-mediated transformation frequency in multiple modern elite commercial maize (Zea mays L.) inbreds by media modifications [J]. Plant Cell Tissue Organ Cult, 2015, 121(3): 519-529. |
| [29] | 赵成章. 锌元素对籼稻成熟胚愈伤组织绿苗分化率的影响 [J]. 作物学报, 2001, 27(3): 404-407. |
| Zhao CZ. Effect of ZnSO4 on redifferentiation frequency of callus from mature embryo of indica rice [J]. Acta Agron Sin, 2001, 27(3): 404-407. | |
| [30] | Kothari SL, Agarwal K, Kumar S. Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millet—Eleusine coracana (L.) Gaertn [J]. Vitro Cell Dev Biol Plant, 2004, 40(5): 515-519. |
| [31] | 殷榕, 颜如玉, 赵丫杰, 等. 玉米成熟胚高效苗再生体系的建立 [J]. 山东农业大学学报: 自然科学版, 2023, 54(1): 25-31. |
| Yin R, Yan RY, Zhao YJ, et al. Establishment of high-efficiency shoots regeneration system from mature embryo of maize (Zea mays L.) [J]. J Shandong Agric Univ Nat Sci Ed, 2023, 54(1): 25-31. | |
| [32] | 张东武, 刘辉, 赵惠贤. 小麦成熟胚组织培养再生体系的优化及高再生率基因型的筛选 [J]. 麦类作物学报, 2011, 31(5): 847-852. |
| Zhang DW, Liu H, Zhao HX. Optimization of regeneration system of tissue culture from mature embryos and screening of wheat genotypes with high regeneration frequency [J]. J Triticeae Crops, 2011, 31(5): 847-852. | |
| [33] | Zhang WJ, Dewey RE, Boss W, et al. Enhanced Agrobacterium-mediated transformation efficiencies in monocot cells is associated with attenuated defense responses [J]. Plant Mol Biol, 2013, 81(3): 273-286. |
| [34] | 陈倩楠, 王轲, 汤沙, 等. 以抗除草剂Bar基因稳定转化谷子技术研究 [J]. 作物学报, 2018, 44(10): 1423-1432. |
| Chen QN, Wang K, Tang S, et al. Use of bar gene for the stable transformation of herbicide-resistant foxtail millet plants [J]. Acta Agron Sin, 2018, 44(10): 1423-1432. | |
| [35] | 杨雅文, 朱东杰, 潘弘, 等. 玉米无基因型限制遗传转化体系建立和应用 [J]. 作物学报, 2024, 50(11): 2674-2683. |
| Yang YW, Zhu DJ, Pan H, et al. Genotype-independent transformation technique development and application in maize [J]. Acta Agron Sin, 2024, 50(11): 2674-2683. |
| [1] | HU Lu, WANG Kai, XU Jing-yi, YE Li-hui, WANG Yong-fei, WANG Li-hua, LI Jie-qin. Research Progress in Genetic Transformation Technologies of Maize and Sorghum [J]. Biotechnology Bulletin, 2025, 41(9): 32-43. |
| [2] | DENG Mei-bi, YAN Lang, ZHAN Zhi-tian, ZHU Min, HE Yu-bing. Efficient CRISPR Gene Editing in Rice Assisted by RUBY [J]. Biotechnology Bulletin, 2025, 41(8): 65-73. |
| [3] | PEI Jing-qi, ZHAO Meng-ran, HUANG Chen-yang, WU Xiang-li. Discovery and Verification of a Functional Gene Influencing the Growth and Development of Pleurotus ostreatus [J]. Biotechnology Bulletin, 2025, 41(6): 327-334. |
| [4] | YANG Chao-jie, ZHANG Lan, CHEN Hong, HUANG Juan, SHI Tao-xiong, ZHU Li-wei, CHEN Qing-fu, LI Hong-you, DENG Jiao. Functional Identification of the Transcription Factor Gene FtbHLH3 in Regulating Flavonoid Biosynthesis in Fagopyrum tataricum [J]. Biotechnology Bulletin, 2025, 41(4): 134-144. |
| [5] | WEN Bo-lin, WAN Min, HU Jian-jun, WANG Ke-xiu, JING Sheng-lin, WANG Xin-yue, ZHU Bo, TANG Ming-xia, LI Bing, HE Wei, ZENG Zi-xian. Establishment of Genetic Transformation and Gene Editing System for a Potato Cultivar Chuanyu 50 [J]. Biotechnology Bulletin, 2025, 41(4): 88-97. |
| [6] | WANG Bi-cheng, JING Hai-qing, WAN Kun, ZHANG Ying-ying, DING Jia-hao, LI Run-zhi, XUE Jin-ai, ZHANG Hai-ping. Identification of Soybean BCAT Gene Family and Functional Analysis of GmBCAT3 in Soybean Responses to Drought Stress [J]. Biotechnology Bulletin, 2025, 41(10): 196-209. |
| [7] | LI Zhan-qian, LI Chen, LI Shu-ting, MA Ju-hua, JING Hai-qing, SUN Yan, ZHOU Ya-li, XUE Jin-ai, LI Run-zhi. Genome-wide Identification of the ME Family in Cyperus esculentusis and Functional Analysis of CeNAD-ME2 [J]. Biotechnology Bulletin, 2025, 41(10): 210-221. |
| [8] | SONG Qian-na, DUAN Yong-hong, FENG Rui-yun. Establishment of CRISPR/Cas9-mediated Highly Efficient Gene Editing System in Microtubers of Potatoes [J]. Biotechnology Bulletin, 2024, 40(9): 33-41. |
| [9] | ZHANG Mei-yu, ZHAO Yu-bin, WANG Ling-yun, SONG Yuan-da, ZHAO Xin-he, REN Xiao-jie. Research Progress in the Production of Functional Fatty Acid DHA by Microalga Thraustochytrids [J]. Biotechnology Bulletin, 2024, 40(6): 81-94. |
| [10] | WANG Di ZHANG Xiao-yu SONG Yu-xin ZHENG Dong-ran TIAN Jing LI Yu-hua WANG Yu WU Hao. Advances in the Molecular Mechanisms of Plant Tissue Culture and Regeneration Regulated by Totipotency-related Transcription Factors [J]. Biotechnology Bulletin, 2024, 40(6): 23-33. |
| [11] | MEI Xian-jun, SONG Hui-yang, LI Jing-hao, MEI Chao, SONG Qian-na, FENG Rui-yun, CHEN Xi-ming. Cloning and Expression Analysis of StDof5 Gene in Potato [J]. Biotechnology Bulletin, 2024, 40(3): 181-192. |
| [12] | HUA Xuan, TIAN Bo-wen, ZHOU Xin-tong, JIANG Zi-han, WANG Shi-qi, HUANG Qian-hui, ZHANG Jian, CHEN Yan-hong. Cloning SmERF B3-45 from Salix matsudana and Functional Analysis on Its Tolerance to Salt [J]. Biotechnology Bulletin, 2024, 40(12): 124-135. |
| [13] | HUANG Wen-li, LI Xiang-xiang, ZHOU Wen-ting, LUO Sha, YAO Wei-jia, MA Jie, ZHANG Fen, SHEN Yu-sen, GU Hong-hui, WANG Jian-sheng, SUN Bo. Targeted Editing of BoZDS in Broccoli by CRISPR/Cas9 Technology [J]. Biotechnology Bulletin, 2023, 39(2): 80-87. |
| [14] | ZHOU Jia-yan, ZOU Jian, CHEN Wei-ying, WU Yi-chao, CHEN Xi-tong, WANG Qian, ZENG Wen-jing, HU Nan, YANG Jun. Construction of Multi-gene Interference System for Plant and Analysis of Its Application Efficiency [J]. Biotechnology Bulletin, 2023, 39(1): 115-126. |
| [15] | SUN Wei, ZHANG Yan, WANG Yu-han, XU Hui, XU Xiao-rong, JU Zhi-gang. Cloning of Rd3GT1 in Rhododendron delavayi and Its Effect on Flower Color Formation of Petunia hybrida [J]. Biotechnology Bulletin, 2022, 38(9): 198-206. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||