








生物技术通报 ›› 2021, Vol. 37 ›› Issue (6): 97-107.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1353
廖兆民1( ), 蔡俊1,2,3(
), 蔡俊1,2,3( ), 林建国1, 杜馨1, 王常高1
), 林建国1, 杜馨1, 王常高1
收稿日期:2020-11-03
出版日期:2021-06-26
发布日期:2021-07-08
作者简介:廖兆民,男,硕士,研究方向:酶工程;E-mail: 基金资助:
LIAO Zhao-min1( ), CAI Jun1,2,3(
), CAI Jun1,2,3( ), LIN Jian-guo1, DU Xin1, WANG Chang-gao1
), LIN Jian-guo1, DU Xin1, WANG Chang-gao1
Received:2020-11-03
Published:2021-06-26
Online:2021-07-08
摘要:
从黑曲霉中克隆葡萄糖氧化酶(glucose oxidase,E.C.1.1.3.4,GOD)基因,使其在毕赤酵母GS115中高效表达,为葡萄糖氧化酶的工业化生产提供理论依据。通过设计简并引物,扩增GOD,将该基因连接到质粒pPICZαA上并在毕赤酵母中表达,依据摇瓶水平优化最佳诱导产酶条件,在30 L发酵罐中进行发酵放大试验,共表达His4,采用DO-STAT的甲醇流加策略进行高密度发酵。结果表明,重组毕赤酵母GS115/pPICZαA-GOD在摇瓶水平经0.5%甲醇诱导96 h,发酵液上清GOD酶活达到32.25 U/mL。经优化得到最佳发酵条件为:在30℃、pH 6.0、250 r/min条件下,发酵液体积1%的甲醇诱导96 h,GOD酶活达到50.1 U/mL,相比初始发酵,酶活提高了55.3%。经30 L发酵罐放大试验,GOD酶活达到307.52 U/mL,提高了5.1倍。共表达His4使细胞湿重提高了72.5%,GOD酶活达到了461 U/mL,相比初始重组酵母提高了50.2%。SDS-PAGE分析发现GOD分子量约90 kD。重组GOD能在毕赤酵母中高效表达,且表达产物纯度高,杂蛋白少,有利于纯化。
廖兆民, 蔡俊, 林建国, 杜馨, 王常高. 黑曲霉葡萄糖氧化酶基因在毕赤酵母中的表达及产酶条件的优化[J]. 生物技术通报, 2021, 37(6): 97-107.
LIAO Zhao-min, CAI Jun, LIN Jian-guo, DU Xin, WANG Chang-gao. Expression of Glucose Oxidase Gene from Aspergillus niger in Pichia pastoris and Optimization of Enzyme Production Conditions[J]. Biotechnology Bulletin, 2021, 37(6): 97-107.
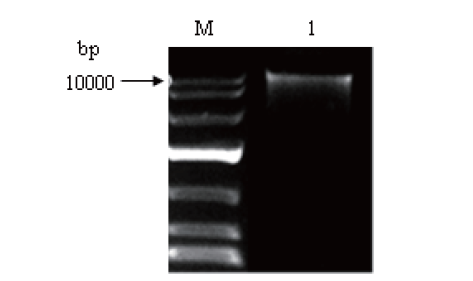
图2 黑曲霉全基因组的琼脂糖凝胶电泳 M:DNA marker. 1:黑曲霉基因组DNA
Fig.2 Agarose gel electrophoresis of whole genome extraction from Aspergillus niger M: DNA marker. 1: A. niger genomic DNA
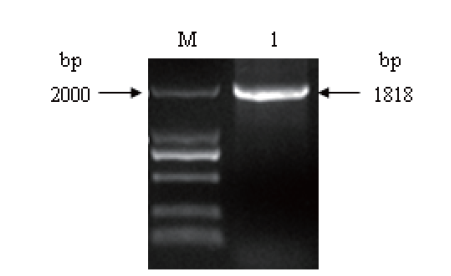
图3 简并引物Z1、Z2的PCR扩增产物的琼脂糖凝胶电泳 M:DNA marker. 1:简并引物Z1、Z2的PCR扩增产物
Fig. 3 Agarose gel electrophoresis of PCR products of degenerate primers Z1 and Z2 M: DNA marker. 1: PCR products of degenerate primer Z1 and Z2

图5 重组质粒pPICZαA-GOD构建及双酶切鉴定 A:重组表达载体的质粒图谱;B:双酶切鉴定结果;M:DNA marker.1:pPICZαA-GOD的双酶切产物
Fig.5 Construction of recombinant plasmid pPICZαA-GOD and identification by double restriction endonuclease digestion A: The plasmid map of the recombinant expression vector. B: The results of double restriction endonuclease digestion. M: DNA marker. 1: The product of double restriction endonuclease digestion of pPICZαA-GOD
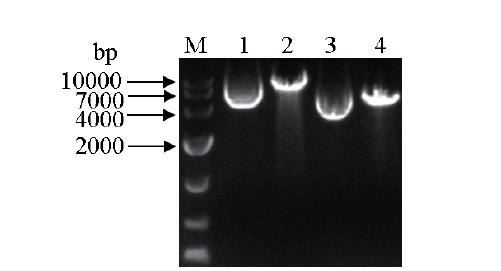
图6 pPICZαA-GOD和pPICZαA线性化产物的琼脂糖凝胶电泳 M:DNA marker. 1:pPICZαA-GOD;2:pPICZαA-GOD线性化产物;3:pPICZαA;4:pPICZαA线性化产物
Fig.6 Agarose gel electrophoresis of the linearized products of pPICZαA-GOD and pPICZαA M: DNA marker. 1: pPICZαA-GOD; 2: pPICZαA-GOD linearization product;3: pPICZαA; 4: pPICZαA linearization product
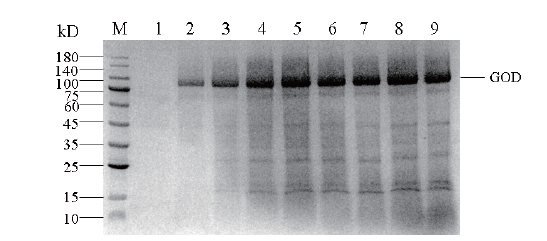
图12 30 L发酵罐中重组毕赤酵母表达葡萄糖氧化酶蛋白的SDS-PAGE M:蛋白Maker;1-9:经甲醇诱导0、12、24、36、48、60、72、84和96 h葡萄糖氧化酶表达量
Fig.12 SDS-PAGE of glucose oxidase protein expressed in recombinant P. pastoris in 30 L bioreactor M: Protein Maker; 1-9: the expression of glucose oxidase was induced by methanol at 0, 12, 24, 36, 48, 60, 72, 84 and 96 h
| [1] |
Ferri S, Kojima K, Sode K. Review of glucose oxidases and glucose dehydrogenases:a bird’s eye view of glucose sensing enzymes[J]. J Diabetes Sci Technol, 2011, 5(5):1068-1076.
doi: 10.1177/193229681100500507 URL |
| [2] |
Wang HC, Lee AR. Recent developments in blood glucose sensors[J]. J Food Drug Anal, 2015, 23(2):191-200.
doi: 10.1016/j.jfda.2014.12.001 URL |
| [3] | Cichello SA. Oxygen absorbers in food preservation:a review[J]. Journal of Food Science & Technology, 2015, 52(4):1889-1895. |
| [4] |
Kriaa M, Ouhibi R, Graba H, et al. Synergistic effect of Aspergillus tubingensis CTM 507 glucose oxidase in presence of ascorbic acid and alpha amylase on dough properties, baking quality and shelf life of bread[J]. J Food Sci Technol, 2016, 53(2):1259-1268.
doi: 10.1007/s13197-015-2092-9 URL |
| [5] | 汤海鸥, 高秀华, 李学军, 等. 葡萄糖氧化酶对仔猪生长性能、粪便菌群和血清指标的影响[J]. 动物营养学报, 2014, 26(12):3781-3786. |
| Tang HO, Gao XH, Li XJ, et al. Effects of glucose oxidase on growth performance, faecal microflora and serum parameters of piglets[J]. Chinese Journal of Animal Nutrition, 2014, 26(12):3781-3786. | |
| [6] | 廖兆民, 蔡俊, 林建国. 微生物葡萄糖氧化酶的研究进展[J]. 食品与发酵工业, 2018, 44(7):308-315. |
| Liao ZM, Cai J, Lin JG, Research progress of microbial glucose oxidase[J]. Food and Fermentation Industries, 2018, 44(7):308-315. | |
| [7] | 范新蕾, 肖成建, 顾秋亚, 等. ARTP诱变选育葡萄糖氧化酶高产菌株及发酵条件优化[J]. 工业微生物, 2015(1):15-19. |
| Fan XL, Xiao CJ, Gu QY, et al. ARTP mutation breeding of glucose oxidase-producing strains and optimization of fermentation conditions[J]. Industrial Microbiology, 2015(1):15-19. | |
| [8] | Zehra A, Dubey MK, Ttwari A, et al. Fungal biomolecules and their implications[M]. John Wiley & Sons, Ltd, 2015. |
| [9] | Damasceno LM, Huang CJ, Batt CA. Protein secretion in Pichia pastoris and advances in protein production[J]. Applied Microbiology & Biotechnology, 2012, 93(1):31-39. |
| [10] | 高庆华, 胡美荣, 吴芳彤, 等. 点青霉葡萄糖氧化酶基因的克隆及其酶学性质研究[J]. 生物技术通报, 2016, 32(7):152-159. |
| Gao QH, Hu MR, Wu FT, et al. Cloning of gene for a glucose oxidase from Penicillium notatum and its enzymatic properties[J]. Biotechnology Bulletin, 2016, 32(7):152-159. | |
| [11] | 闵兆升, 郭会明, 颜旭, 等. 巴斯德毕赤酵母(P. pastoris)高密度发酵研究进展[J]. 生物技术通报, 2014(3):42-49. |
| Min ZS, Guo HM, Yan X, et al. Progress of Pichia pastoris engineering bacteria on high-density fermentation[J]. Biotechnology Bulletin, 2014, 30(3):42-49. | |
| [12] | 高立云. 一种快速葡萄糖氧化酶活性测定方法与应用效果研究[D]. 济南:齐鲁工业大学, 2017. |
| Gao LY. Study of rapid activity analytic assay and application performance evaluation of glucose oxidase[D]. Ji’nan:Qilu University of Technology, 2017. | |
| [13] | 陈楠, 肖成建, 范新蕾, 等. 黑曲霉葡萄糖氧化酶基因在毕赤酵母SMD1168中的表达[J]. 食品与生物技术学报, 2017, 36(9):975-981. |
| Chen N, Xiao CJ, Fan XL, et al. Expression of Aspergillus niger glucose oxidase gene in Pichia pastoris SMD1168[J]. Journal of Food Science and Biotechnology, 2017, 36(9):975-981. | |
| [14] | 顾磊. Aspergillus niger葡萄糖氧化酶的异源分泌表达、分子改造和发酵生产[D]. 无锡:江南大学, 2014. |
| Gu L. Heterologous expression, molecular molecular modification, and fermentation of glucose oxidase from Aspergillus niger[D]. Wuxi:Jiangnan University, 2014. | |
| [15] | 郝杰清, 王帅坤, 师慧, 等. 重组毕赤酵母葡萄糖氧化酶的纯化和性质[J]. 食品科学, 2013, 34(9):159-163. |
| Hao JQ, Wang SK, Shi H, et al. Purification and characterization of recombinant glucose oxidase from Pichia pastoris[J]. Food Science, 2013, 34(9):159-163. | |
| [16] | 闻一凡, 顾磊, 张娟, 等. 定点突变提高毕赤酵母产葡萄糖氧化酶的氧化稳定性[J]. 食品与生物技术学报, 2016, 35(12):1260-1267. |
| Wen YF, Gu L, Zhang J, et al. Enhancing oxidative stability of glucose oxidase from Aspergillus niger by site-directed mutagenesis[J]. Journal of Food Science and Biotechnology, 2016, 35(12):1260-1267. | |
| [17] |
Cregg JM. Recombinant protein expression in Pichia pastoris[J]. Molecular Biotechnology, 2000, 16(1):23-52.
doi: 10.1385/MB:16:1 URL |
| [18] | Veenhuis M, Dijken JPV, Harder W. The significance of peroxisomes in the metabolism of one-carbon compounds in yeasts[J]. Advances in Microbial Physiology, 1983, 24:1-82. |
| [19] |
Wegner G. Emerging applications of the methylotrophic yeasts[J]. FEMS Microbiol Rev, 1990, 7(3/4):279-283.
doi: 10.1111/fml.1980.7.issue-4 URL |
| [20] |
Cregg JM, Vedvick TS, Raschke WC. Recent advances in the expression of foreign genes in Pichia pastoris[J]. Nature Biotechnology, 1993, 11(8):905-910.
doi: 10.1038/nbt0893-905 URL |
| [21] | 关波. 改良人血清白蛋白融合蛋白在毕赤酵母中分泌表达的研究[D]. 无锡:江南大学, 2014. |
| Guan B. Improvement of secretory expression of human serum albumin fusion protein in Pichia pastoris[D]. Wuxi:Jiangnan University, 2014. | |
| [22] | 刘瑜. 高效表达黑曲霉葡萄糖氧化酶基因工程菌的构建[D]. 济南:齐鲁工业大学, 2013. |
| Liu Y. Cloning and high efficiency expression of glucose oxidase from Aspergillus niger[D]. Ji’nan:Qilu University of Technology, 2013. | |
| [23] | 周亚凤, 张先恩, 刘虹, 等. 黑曲霉葡萄糖氧化酶基因的克隆及其在酵母中的高效表达[J]. 生物工程学报, 2001, 17(4):400-405. |
| Zhou YF, Zhang XE, Liu H, et al. Cloning and expression of Aspergillus niger glucose oxidase gene in methylotrophic yeast[J]. Chinese Journal of Biotechnology, 2001, 17(4):400-405. | |
| [24] | Crognale S, Petruccioli M, Fenice M, et al. Fed-batch gluconic acid production from Penicillium variabile P16 under different feeding strategies[J]. Enzyme & Microbial Technology, 2008, 42(5):445-449. |
| [25] | 郭瑶. Aspergillus niger Z-25葡萄糖氧化酶基因在毕赤酵母中的表达[D]. 南京:南京农业大学, 2010. |
| Guo Y. Expression of glucose oxidase gene from Aspergillus niger Z-25 in Pichia pastoris[D]. Nanjing:Nanjing Agricultural University, 2010. | |
| [26] | Crognale S, Pulci V, Brozzoli V, et al. Expression of Penicillium variabile P16 glucose oxidase gene in Pichia pastoris and characterization of the recombinant enzyme[J]. Enzyme & Microbial Technology, 2006, 39(6):1230-1235. |
| [1] | 赵思佳, 王晓璐, 孙纪录, 田健, 张杰. 代谢工程改造毕赤酵母生产赤藓糖醇[J]. 生物技术通报, 2023, 39(8): 137-147. |
| [2] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [3] | 董聪, 高庆华, 王玥, 罗同阳, 王庆庆. 基于联合策略提高FAD依赖的葡萄糖脱氢酶的酵母表达[J]. 生物技术通报, 2023, 39(6): 316-324. |
| [4] | 马玉倩, 孙东辉, 岳浩峰, 辛佳瑜, 刘宁, 曹志艳. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135. |
| [5] | 陈楠楠, 王春来, 蒋振忠, 焦鹏, 关淑艳, 马义勇. 玉米ZmDHN15基因在烟草中的遗传转化及抗冷性分析[J]. 生物技术通报, 2023, 39(4): 259-267. |
| [6] | 赵昕, 杜玉瑶, 殷子扬, 毛淑红. 胆固醇7α-羟化酶在毕赤酵母中的异源表达[J]. 生物技术通报, 2023, 39(10): 304-310. |
| [7] | 牛馨, 张莹, 王茂军, 刘文龙, 路福平, 李玉. 解淀粉芽胞杆菌不同整合位点对外源碱性蛋白酶表达的影响[J]. 生物技术通报, 2022, 38(4): 253-260. |
| [8] | 王玥, 高庆华, 董聪, 罗同阳, 王庆庆. 密码子优化的吡喃糖氧化酶基因在毕赤酵母中的表达[J]. 生物技术通报, 2022, 38(4): 269-277. |
| [9] | 王博雅, 姜勇, 黄艳, 曹颖, 胡尚连. 慈竹纤维素合酶BeCesA4的克隆及功能分析[J]. 生物技术通报, 2022, 38(11): 185-193. |
| [10] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [11] | 张彤彤, 郑登俞, 吴忠义, 张中保, 于荣. 玉米NF-Y转录因子基因ZmNF-YB13响应干旱和盐胁迫的功能分析[J]. 生物技术通报, 2022, 38(10): 115-123. |
| [12] | 杨威, 伍茜, 程建国, 罗燕, 王印, 杨泽晓, 姚学萍. 林麝干扰素α基因克隆、表达及转录调控分析[J]. 生物技术通报, 2022, 38(1): 194-204. |
| [13] | 杨悦, 陶妍, 谢晶, 钱韻芳. 基于重组毕赤酵母的草鱼C型溶菌酶生物合成及其抑菌活性[J]. 生物技术通报, 2021, 37(12): 169-179. |
| [14] | 刘珊, 叶伟, 朱牧孜, 李赛妮, 邓张双, 章卫民. 一种新型酰基转移酶GPAT的克隆、表达与酶学性质研究[J]. 生物技术通报, 2021, 37(11): 257-266. |
| [15] | 赵海燕, 宋晨斌, 刘正亚, 马兴荣, 尚会会, 李安华, 关现军, 王建设. 来源于Laceyella sp.的α-淀粉酶基因克隆、重组表达及酶学性质研究[J]. 生物技术通报, 2020, 36(8): 23-33. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||