








生物技术通报 ›› 2023, Vol. 39 ›› Issue (2): 193-202.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0506
撒世娟1,2( ), 伍涵宇1,2, 温媛1,2, 陈雪娜1,2, 郑蕊1,2, 姚新灵1,2(
), 伍涵宇1,2, 温媛1,2, 陈雪娜1,2, 郑蕊1,2, 姚新灵1,2( )
)
收稿日期:2022-04-24
出版日期:2023-02-26
发布日期:2023-03-07
作者简介:撒世娟,女,硕士研究生,研究方向:植物分子遗传学;E-mail: 基金资助:
SA Shi-juan1,2( ), WU Han-yu1,2, WEN Yuan1,2, CHEN Xue-na1,2, ZHENG Rui1,2, YAO Xin-ling1,2(
), WU Han-yu1,2, WEN Yuan1,2, CHEN Xue-na1,2, ZHENG Rui1,2, YAO Xin-ling1,2( )
)
Received:2022-04-24
Published:2023-02-26
Online:2023-03-07
摘要:
CO2通过气孔进入叶绿体,叶绿体如何在分子水平响应气孔密度变化的研究鲜有报道。本研究通过调控本氏烟草NtEPF 2(Nicotiana benthamiana EPIDERMAL PATTERNING FACTOR 2)体内表达,分别得到气孔密度增加和降低的本氏烟草转化株系,用叶绿素积累、光合参数和iTRAQ(isobaric tags for relative and absolute quantification)蛋白质表达谱,表征气孔关闭和不同气孔密度开张株系,揭示叶绿体组成动态对气孔密度变化的响应。结果显示,气孔密度增加株系形成PSI-PSII-LHCII超复合体和加速电子传递的分化表达蛋白质(differential expression proteins, DEPs)显著上调积累,PSI光保护与光损伤修复DEPs则显著下调,叶绿素积累和净光合速率均值分别高于对照43%和67%。气孔密度降低株系叶片发育初期,由于高水平的光合磷酸化ATP合成代谢,叶绿素积累和光合参数显著高于对照,但随叶龄增加,叶绿素积累显著降低。结果表明,气孔密度增加,加速了PSII-PSI复合体形成和其电子传递,降低了光损伤修复水平,保持了不同叶龄叶绿素高水平积累;气孔密度降低,ATP合成和碳固定失衡,随叶龄增加,叶绿素积累模式发生了倒置。研究结果有助于更深入理解气孔的工作原理,拓宽通过调控气孔增加碳固定的视野。
撒世娟, 伍涵宇, 温媛, 陈雪娜, 郑蕊, 姚新灵. 叶绿体特异蛋白质表达谱对本氏烟不同气孔密度的响应[J]. 生物技术通报, 2023, 39(2): 193-202.
SA Shi-juan, WU Han-yu, WEN Yuan, CHEN Xue-na, ZHENG Rui, YAO Xin-ling. Responses of Choloroplast Specific Protein Profile to Different Stomatal Densities in Nicotiana benthamiana[J]. Biotechnology Bulletin, 2023, 39(2): 193-202.
| 名称Name | 序列Sequence(5'-3') | |
|---|---|---|
| EP | EPF | TCCCGCCTTCAGTTTAGC |
| EPR | CCCTTACGTCAGTGGAGATATC | |
| EP+ | EP+F | TCTAGCCAAAGCCTACGTCCAT |
| EP+R | AGGGAAACAAGGTCCACAAGCA | |
| EP- | EP-F | AGCCAAAGCCTACGTCCATAT |
| EP-R | TTGGATACATCTCCATTCCTAACA | |
表1 实验所用引物及序列
Table 1 Primers and sequences used in the study
| 名称Name | 序列Sequence(5'-3') | |
|---|---|---|
| EP | EPF | TCCCGCCTTCAGTTTAGC |
| EPR | CCCTTACGTCAGTGGAGATATC | |
| EP+ | EP+F | TCTAGCCAAAGCCTACGTCCAT |
| EP+R | AGGGAAACAAGGTCCACAAGCA | |
| EP- | EP-F | AGCCAAAGCCTACGTCCATAT |
| EP-R | TTGGATACATCTCCATTCCTAACA | |
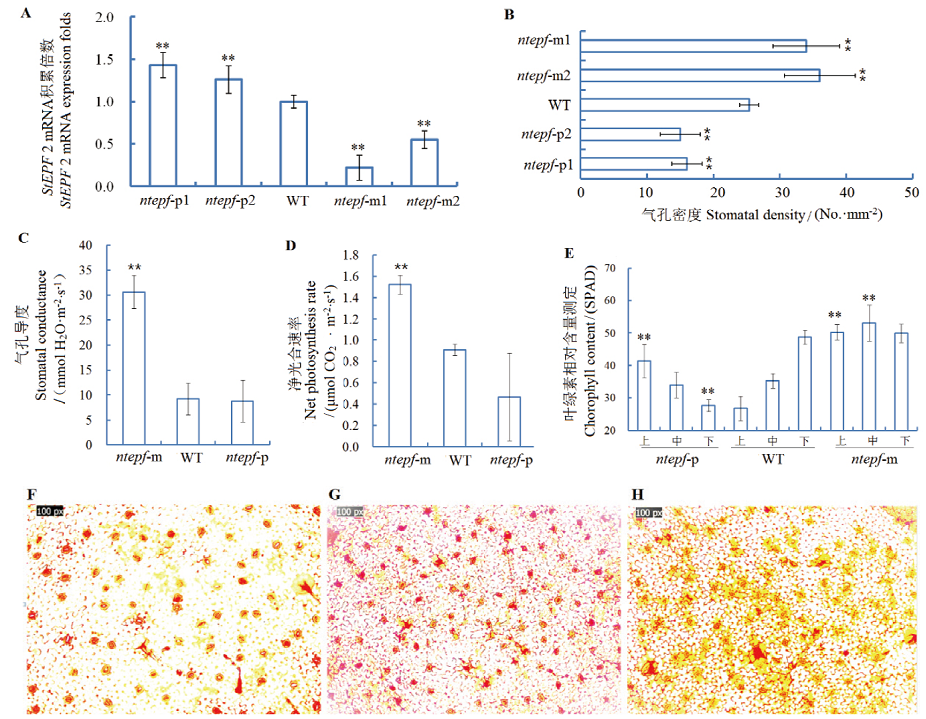
图1 株系 ntepf-m和ntepf-p气孔密度、叶绿素积累、光合测定和气孔观察结果 A:StEPF 2 mRNA积累相对定量;B:叶片气孔密度测定;C:气孔导度测定;D:净光合速率测定;E:叶绿素含量测定;F:StEPF 2过量表达株系气孔观察;G:对照株系气孔观察;H:StEPF 2表达抑制株系气孔观察。统计分析数据来自3次重复测定,** 代表P<0.05的差异显著水平
Fig. 1 Assay on stomatal density, chlorophyll accumulation and photosynthesis as well as stomata observation for ntepf-m and ntepf-p lines A: Relative accumulation of StEPF 2 mRNA. B: Assay on leaf stomatal density. C: Assay on stomatal conductance. D: Assay on net photosynthesis rate. E: Assay on chorophyll content. F: Stomatal observation for StEPF 2 overexpression lines. G: Stomatal observation for control lines. H: Stomatal observation for StEPF 2 inhibition expression lines. Data in statistics analysis from 3 repetitions, ** for P<0.05 difference significant level
| 生物过程 Biological process | 分子功能 Molecular function | 细胞组分 Cellular components | |
|---|---|---|---|
| ntepf-m | 120 | 69 | 72 |
| ntepf-p | 123 | 55 | 98 |
表2 与拟南芥同源物相似性大于60% DEPs的GO富集结果
Table 2 GO enrichment with DEPs sharing >60% similarity with orthologs in Arabidopsis
| 生物过程 Biological process | 分子功能 Molecular function | 细胞组分 Cellular components | |
|---|---|---|---|
| ntepf-m | 120 | 69 | 72 |
| ntepf-p | 123 | 55 | 98 |
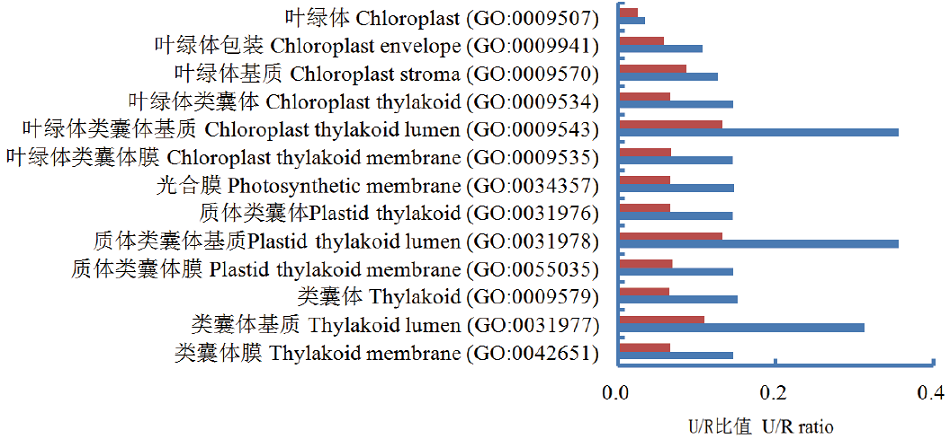
图2 气孔密度上、下调叶片DEPs GO富集中共同出现的叶绿体GO IDs
Fig. 2 Chloroplast common GO IDs in GO enrichment of DEPs identified from the leaves of up- or down-stomatal density
| GO ID | U/R比值U/R ratio |
|---|---|
| 光系统II反应中心Photosystem II reaction center(GO:0009539) | 0.30 |
| 光系统II放氧复合物Photosystem II oxygen evolving complex(GO:0009654) | 0.33 |
| 光系统II Photosystem II(GO:0009523) | 0.27 |
| 光系统I反应中心Photosystem I reaction center(GO:0009538) | 0.36 |
| 光系统I Photosystem I(GO:0009522) | 0.36 |
| 光系统Photosystem(GO:0009521) | 0.31 |
| 细胞色素b6f复合物Cytochrome b6f complex(GO:0009512) | 0.33 |
| 叶绿体Chromoplast(GO:0009509) | 0.67 |
| 叶绿体类囊体膜蛋白复合物Chloroplast thylakoid membrane protein complex(GO:0098807) | 0.12 |
| 叶绿体基质类囊体 Chloroplast stromal thylakoid(GO:0009533) | 0.30 |
表3 气孔密度增加特异DEPs GO富集
Table 3 GO enrichment with stomatal density rising specific DEPs
| GO ID | U/R比值U/R ratio |
|---|---|
| 光系统II反应中心Photosystem II reaction center(GO:0009539) | 0.30 |
| 光系统II放氧复合物Photosystem II oxygen evolving complex(GO:0009654) | 0.33 |
| 光系统II Photosystem II(GO:0009523) | 0.27 |
| 光系统I反应中心Photosystem I reaction center(GO:0009538) | 0.36 |
| 光系统I Photosystem I(GO:0009522) | 0.36 |
| 光系统Photosystem(GO:0009521) | 0.31 |
| 细胞色素b6f复合物Cytochrome b6f complex(GO:0009512) | 0.33 |
| 叶绿体Chromoplast(GO:0009509) | 0.67 |
| 叶绿体类囊体膜蛋白复合物Chloroplast thylakoid membrane protein complex(GO:0098807) | 0.12 |
| 叶绿体基质类囊体 Chloroplast stromal thylakoid(GO:0009533) | 0.30 |
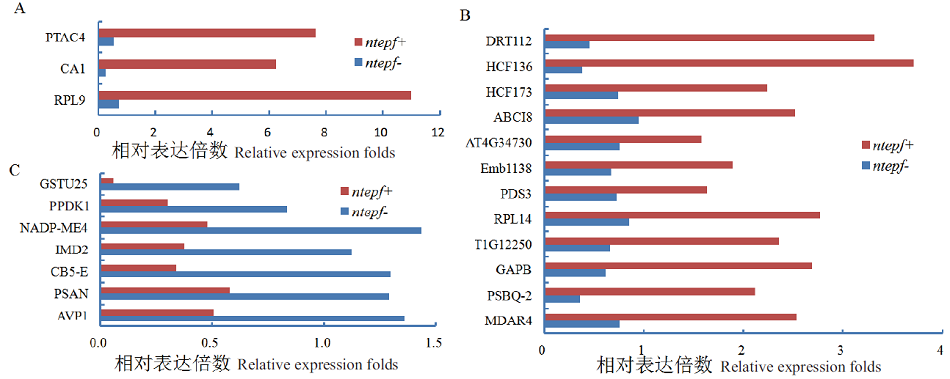
图3 气孔密度上、下调叶片共表达DEPs积累变化 A:随气孔密度增加表达量降低的3个DEPs;B:随气孔密度增加表达量降低的12个DEPs;C:表达量随气孔密度增加而升高的7个DEPs
Fig. 3 Accumulation variation of co-expreesion DEPs resulting from the leaves of up- or down-stomatal density A: Expression-lowing 3 DEPs along with rising stomatal density. B: Expression-lowing 12 DEPs along with rising stomatal density. C: Expression-rising 7 DEPs along with increased stomatal density

图4 气孔密度增加特异响应DEPs积累变化 A:高气孔密度下特异上调表达PSII-PSI复合体DEPs;B:高气孔密度下特异下调表达的电子传递DEPs;C:高气孔密度下特异上调表达的PSII组分DEPs
Fig. 4 Accumulation variation of specific DEPs in response to rising stomatal density A: PSII-PSI complex DEP accumulations up-regulated by high stomatal density. B: Electron transfer DEP accumulations down-regulated by high stomatal density. C: PSII component DEP accumulations down-regulated by high stomatal density
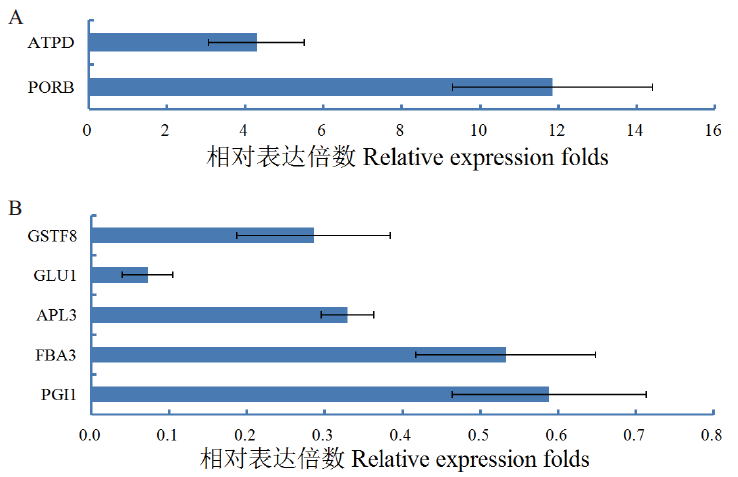
图5 气孔密度降低特异响应DEPs积累变化 A:低气孔密度下特异上调表达DEPs;B:低气孔密度下特异下调表达DEPs
Fig. 5 Accumulation variation of specific DEPs in responses to lowering stomatal density A: DEP accumulations up-regulated by low stomatal density. B: DEP accumulations down-regulated by low stomatal density
| [1] |
Engineer CB, Ghassemian M, Anderson JC, et al. Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development[J]. Nature, 2014, 513(7517): 246-250.
doi: 10.1038/nature13452 URL |
| [2] |
Gao J, Han X, Seneweera S, et al. Leaf photosynthesis and yield components of mung bean under fully open-air elevated[CO2][J]. J Integr Agric, 2015, 14(5): 977-983.
doi: 10.1016/S2095-3119(14)60941-2 URL |
| [3] |
Zhang JB, De-Oliveira-Ceciliato P, Takahashi Y, et al. Insights into the molecular mechanisms of CO2 mediated regulation of stomatal movements[J]. Curr Biol, 2018, 28(23): R1356-R1363.
doi: 10.1016/j.cub.2018.10.015 URL |
| [4] |
Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising[CO2]: mechanisms and environmental interactions[J]. Plant Cell Environ, 2007, 30(3): 258-270.
doi: 10.1111/j.1365-3040.2007.01641.x URL |
| [5] |
Jumrani K, Bhatia VS, Pandey GP. Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean[J]. Photosynth Res, 2017, 131(3): 333-350.
doi: 10.1007/s11120-016-0326-y URL |
| [6] |
Azoulay-Shemer T, Palomares A, Bagheri A, et al. Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2 and ABA-induced stomatal closing[J]. Plant J, 2015, 83(4): 567-581.
doi: 10.1111/tpj.12916 URL |
| [7] |
Hippler M, Nelson N. The plasticity of photosystem I[J]. Plant Cell Physiol, 2021, 62(7): 1073-1081.
doi: 10.1093/pcp/pcab046 URL |
| [8] |
Pietrzykowska M, Suorsa M, Semchonok DA, et al. The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis[J]. Plant Cell, 2014, 26(9): 3646-3660.
doi: 10.1105/tpc.114.127373 URL |
| [9] |
Brestic M, Zivcak M, Kunderlikova K, et al. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines[J]. Photosynth Res, 2015, 125(1/2): 151-166.
doi: 10.1007/s11120-015-0093-1 URL |
| [10] |
Yokono M, Takabayashi A, Kishimoto J, et al. The PSI-PSII megacomplex in green plants[J]. Plant Cell Physiol. 2019, 60(5):1098-1108.
doi: 10.1093/pcp/pcz026 pmid: 30753722 |
| [11] | Bečková M, Sobotka R, Komenda J. Photosystem II antenna modules CP43 and CP47 do not form a stable ‘no reaction centre complex’ in the cyanobacterium Synechocystis sp. PCC 6803[J]. Photosynth Res, 2022: 2022 Jan 11. |
| [12] |
Chen H, Zhang DY, Guo JK, et al. A Psb27 homologue in Arabidopsis thaliana is required for efficient repair of photodamaged photosystem II[J]. Plant Mol Biol, 2006, 61(4/5): 567-575.
doi: 10.1007/s11103-006-0031-x URL |
| [13] |
Schult K, Meierhoff K, Paradies S, et al. The nuclear-encoded factor HCF173 is involved in the initiation of translation of the psbA mRNA in Arabidopsis thaliana[J]. Plant Cell, 2007, 19(4): 1329-1346.
doi: 10.1105/tpc.106.042895 URL |
| [14] |
Williams-Carrier R, Brewster C, Belcher SE, et al. The Arabidopsis pentatricopeptide repeat protein LPE1 and its maize ortholog are required for translation of the chloroplast psbJ RNA[J]. Plant J, 2019, 99(1): 56-66.
doi: 10.1111/tpj.14308 |
| [15] |
Swiatek M, Regel RE, Meurer J, et al. Effects of selective inactivation of individual genes for low-molecular-mass subunits on the assembly of photosystem II, as revealed by chloroplast transformation: the psbEFLJoperon in Nicotiana tabacum[J]. Mol Genet Genomics, 2003, 268(6): 699-710.
pmid: 12655396 |
| [16] |
Munekage Y, Takeda S, Endo T, et al. Cytochrome b(6)f mutation specifically affects thermal dissipation of absorbed light energy in Arabidopsis[J]. Plant J, 2001, 28(3): 351-359.
pmid: 11722777 |
| [17] |
Jahns P, Graf M, Munekage Y, et al. Single point mutation in the Rieske iron-sulfur subunit of cytochrome b6/f leads to an altered pH dependence of plastoquinol oxidation in Arabidopsis[J]. FEBS Lett, 2002, 519(1/2/3): 99-102.
doi: 10.1016/S0014-5793(02)02719-9 URL |
| [18] |
Maiwald D, Dietzmann A, Jahns P, et al. Knock-out of the genes coding for the Rieske protein and the ATP-synthase delta-subunit of Arabidopsis. Effects on photosynthesis, thylakoid protein composition, and nuclear chloroplast gene expression[J]. Plant Physiol, 2003, 133(1): 191-202.
doi: 10.1104/pp.103.024190 URL |
| [19] |
Zhang D, Li YH, Zhang XY, et al. The SWI2/SNF2 chromatin-remodeling ATPase BRAHMA regulates chlorophyll biosynthesis in Arabidopsis[J]. Mol Plant, 2017, 10(1): 155-167.
doi: S1674-2052(16)30272-6 pmid: 27865928 |
| [20] |
Bartley GE, Scolnik PA, Beyer P. Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and Zeta-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene[J]. Eur J Biochem, 1999, 259(1/2): 396-403.
doi: 10.1046/j.1432-1327.1999.00051.x URL |
| [21] |
Edelman M, Mattoo AK. D1-protein dynamics in photosystem II: the lingering enigma[J]. Photosynth Res, 2008, 98(1/2/3): 609-620.
doi: 10.1007/s11120-008-9342-x URL |
| [22] |
Yang HX, Liu J, Wen XG, et al. Molecular mechanism of photosystem I assembly in oxygenic organisms[J]. Biochim Biophys Acta, 2015, 1847(9): 838-848.
doi: 10.1016/j.bbabio.2014.12.011 pmid: 25582571 |
| [23] |
Lisenbee CS, Lingard MJ, Trelease RN. Arabidopsis peroxisomes possess functionally redundant membrane and matrix isoforms of monodehydroascorbate reductase[J]. Plant J, 2005, 43(6): 900-914.
pmid: 16146528 |
| [24] |
Eastmond PJ. MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis[J]. Plant Cell, 2007, 19(4): 1376-1387.
doi: 10.1105/tpc.106.043992 pmid: 17449810 |
| [25] |
Yu TS, Lue WL, Wang SM, et al. Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation[J]. Plant Physiol, 2000, 123(1): 319-326.
doi: 10.1104/pp.123.1.319 pmid: 10806248 |
| [26] |
Gardberg A, Abendroth J, Bhandari J, et al. Structure of fructose bisphosphate aldolase from Bartonella henselae bound to fructose 1, 6-bisphosphate[J]. Acta Crystallogr Sect F Struct Biol Cryst Commun, 2011, 67(Pt 9): 1051-1054.
doi: 10.1107/S174430911101894X pmid: 21904049 |
| [27] |
Ishizaki T, Ohsumi C, Totsuka K, et al. Analysis of glutamate homeostasis by overexpression of fd-GOGAT gene in Arabidopsis thaliana[J]. Amino Acids, 2010, 38(3): 943-950.
doi: 10.1007/s00726-009-0303-2 pmid: 19468822 |
| [28] |
Wagner U, Edwards R, Dixon DP, et al. Probing the diversity of the Arabidopsis glutathione S-transferase gene family[J]. Plant Mol Biol, 2002, 49(5): 515-532.
doi: 10.1023/A:1015557300450 URL |
| [29] |
Hara K, Yokoo T, Kajita R, et al. Epidermal cell density is autoregulated via a secretory peptide, epidermal patterning factor 2 in Arabidopsis leaves[J]. Plant Cell Physiol, 2009, 50(6): 1019-1031.
doi: 10.1093/pcp/pcp068 URL |
| [30] |
Ohki S, Takeuchi M, Mori M. The NMR structure of stomagen reveals the basis of stomatal density regulation by plant peptide hormones[J]. Nat Commun, 2011, 2: 512.
doi: 10.1038/ncomms1520 pmid: 22027592 |
| [31] |
Sugano SS, Shimada T, Imai Y, et al. Stomagen positively regulates stomatal density in Arabidopsis[J]. Nature, 2010, 463(7278): 241-244.
doi: 10.1038/nature08682 URL |
| [32] |
Wang YL, Xie T, Zhang CL, et al. Overexpression of the potato StEPF2 gene confers enhanced drought tolerance in Arabidop-sis[J]. Plant Biotechnol Rep, 2020, 14(4): 479-490.
doi: 10.1007/s11816-020-00627-4 URL |
| [33] |
Lima VF, Anjos LD, Medeiros DB, et al. The sucrose-to-malate ratio correlates with the faster CO2 and light stomatal responses of angiosperms compared to ferns[J]. New Phytol, 2019, 223(4): 1873-1887.
doi: 10.1111/nph.15927 URL |
| [34] |
Lim SL, Flütsch S, Liu JH, et al. Arabidopsis guard cell chloroplasts import cytosolic ATP for starch turnover and stomatal opening[J]. Nat Commun, 2022, 13(1): 652.
doi: 10.1038/s41467-022-28263-2 URL |
| [35] | 撒世娟, 殷倩, 伍涵宇, 等. 普通烟草体内过量表达St536基因对纤维素积累的影响[J]. 农业生物技术学报, 2021, 29(5): 915-923. |
| Sa SJ, Yin Q, Wu HY, et al. Effects of overexpression of St536 gene on cellulose accumulation in Nicotiana tabacum[J]. J Agric Biotechnol, 2021, 29(5): 915-923. | |
| [36] |
Xu YH, Liu R, Yan L, et al. Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis[J]. J Exp Bot, 2012, 63(3): 1095-1106.
doi: 10.1093/jxb/err315 pmid: 22143917 |
| [37] |
Tyutereva EV, Dmitrieva VA, Shavarda AL, et al. Stomata control is changed in a chlorophyll b-free barley mutant[J]. Funct Plant Biol, 2018, 45(4): 453-463.
doi: 10.1071/FP17056 pmid: 32290984 |
| [38] |
撒世娟, 伍涵宇, 张晓萍, 等. 叶绿素结合蛋白CP24介导光照响应基因StRSM 1调控叶绿素积累[J]. 生物技术通报, 2021, 37(1): 198-204.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0502 |
| Sa SJ, Wu HY, Zhang XP, et al. Light-responding gene StRSM 1 mediated by chlorophyll-binding protein CP24 regulates chlorophyll accumulation[J]. Biotechnol Bull, 2021, 37(1): 198-204. | |
| [39] |
Spanos C, Moore JB. Sample preparation approaches for iTRAQ labeling and quantitative proteomic analyses in systems biology[J]. Methods Mol Biol, 2016, 1394: 15-24.
doi: 10.1007/978-1-4939-3341-9_2 pmid: 26700038 |
| [40] |
Vaudel M, Burkhart JM, Zahedi RP, et al. iTRAQ data interpretation[J]. Methods Mol Biol, 2012, 893: 501-509.
doi: 10.1007/978-1-61779-885-6_30 pmid: 22665319 |
| [41] |
Engineer CB, Hashimoto-Sugimoto M, Negi J, et al. CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions[J]. Trends Plant Sci, 2016, 21(1): 16-30.
doi: S1360-1385(15)00229-0 pmid: 26482956 |
| [42] |
Azoulay-Shemer T, Hsu PK, Schroeder JI. Seeing is believing[J]. Nat Plants, 2017, 3(10): 765-766.
doi: 10.1038/s41477-017-0025-5 pmid: 28970562 |
| [1] | 尹明华, 余锾媛, 肖心怡, 王玉婷. 江西铅山红芽芋叶绿体基因组特征及系统发育分析[J]. 生物技术通报, 2023, 39(6): 233-247. |
| [2] | 郁慧丽, 李爱涛. 细胞色素P450酶在香精香料绿色生物合成中的应用[J]. 生物技术通报, 2023, 39(4): 24-37. |
| [3] | 王慕镪, 陈琦, 马薇, 李春秀, 欧阳鹏飞, 许建和. 机器学习方法在酶定向进化中的应用进展[J]. 生物技术通报, 2023, 39(4): 38-48. |
| [4] | 周恒, 谢彦杰. 植物氧化胁迫信号应答的研究进展[J]. 生物技术通报, 2023, 39(11): 36-43. |
| [5] | 罗皓天, 王龙, 王禹茜, 王月, 李佳祯, 杨梦珂, 张杰, 邓欣, 王红艳. 青狗尾草RNAi途径相关基因的全基因组鉴定和表达分析[J]. 生物技术通报, 2023, 39(1): 175-186. |
| [6] | 刘雄伟, 刘畅, 曾宪法, 杨小英, 俸婷婷, 赵杰宏, 周英. 朱砂根叶绿体全基因组解析及系统发育分析[J]. 生物技术通报, 2023, 39(1): 232-242. |
| [7] | 钱方, 高作敏, 胡利娟, 王洪程. 海甘蓝(Crambe abyssinica)叶绿体基因组特征及其系统发育研究[J]. 生物技术通报, 2022, 38(6): 174-186. |
| [8] | 赵明明, 唐殷, 郭磊周, 韩佳慧, 葛佳茗, 孟勇, 平淑珍, 周正富, 王劲. Lon1蛋白酶参与耐辐射异常球菌高温胁迫及细胞分裂的功能研究[J]. 生物技术通报, 2022, 38(5): 149-158. |
| [9] | 易芳, 来鹏程, 郑希鳌, 胡帅, 高燕丽. Kod DNA聚合酶的制备及纯化研究[J]. 生物技术通报, 2022, 38(5): 183-190. |
| [10] | 祖国蔷, 胡哲, 王琪, 李光哲, 郝林. Burkholderia sp. GD17对水稻幼苗镉耐受的调节[J]. 生物技术通报, 2022, 38(4): 153-162. |
| [11] | 李兵娟, 郑璐, 沈仁芳, 兰平. 拟南芥RPP1A参与幼苗生长的蛋白质组学分析[J]. 生物技术通报, 2022, 38(2): 10-20. |
| [12] | 贾海红, 李冰清. 超氧化物歧化酶翻译后修饰的研究进展[J]. 生物技术通报, 2022, 38(2): 237-244. |
| [13] | 马荣, 尚方正, 潘剑锋, 戎友俊, 王敏, 李金泉, 张燕军. 细胞内mRNA翻译影响因素及翻译组学的研究进展[J]. 生物技术通报, 2022, 38(12): 115-126. |
| [14] | 王智博, 王道平, 苗兰, 李瑛, 潘映红, 刘建勋. 血液样本蛋白质组分析方法的比较研究[J]. 生物技术通报, 2021, 37(8): 307-318. |
| [15] | 康凌云, 陈建胜, 甘瀚凌, 韩露露, 冯海霞, 刁其玉, 邢凯, 崔凯. 基于转录组学技术分析蛋白质限制与补偿对羔羊肝脏抗氧化性能的影响[J]. 生物技术通报, 2021, 37(6): 171-180. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||