








生物技术通报 ›› 2024, Vol. 40 ›› Issue (6): 281-289.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0121
王秋月1( ), 段鹏亮1, 李海笑2, 刘宁1(
), 段鹏亮1, 李海笑2, 刘宁1( ), 曹志艳1,2(
), 曹志艳1,2( ), 董金皋1,2
), 董金皋1,2
收稿日期:2024-01-31
出版日期:2024-06-26
发布日期:2024-05-14
通讯作者:
刘宁,女,博士,副教授,研究方向:植物保护;E-mail: lning121@126.com;作者简介:王秋月,女,硕士,研究方向:植物保护;E-mail: 937813463@qq.com
基金资助:
WANG Qiu-yue1( ), DUAN Peng-liang1, LI Hai-xiao2, LIU Ning1(
), DUAN Peng-liang1, LI Hai-xiao2, LIU Ning1( ), CAO Zhi-yan1,2(
), CAO Zhi-yan1,2( ), DONG Jin-gao1,2
), DONG Jin-gao1,2
Received:2024-01-31
Published:2024-06-26
Online:2024-05-14
摘要:
【目的】 筛选玉米大斑病菌(Setosphaeria turcica)转录因子的互作蛋白,解析黑色素调控转录因子StMR1调控玉米大斑病菌致病性的分子机制。为解析玉米大斑病菌侵染过程中转录因子的调控网络,阐明病菌的致病机理提供参考。【方法】 收集玉米大斑病菌菌丝和孢子不同萌发阶段作为试验材料,采用Gateway方法构建玉米大斑病菌cDNA文库,使用同源重组的方法构建转录因子StMR1的诱饵载体,采用酵母双杂交技术筛选其互作蛋白并进行一对一验证。【结果】 构建的玉米大斑病菌文库插入的平均片段长度大于1 000 bp,初级文库及次级文库的库容量为1.2×107和1.04×107 CFU,重组率为100%,可以用于酵母双杂交筛选。成功构建可以用于筛库的诱饵载体pGBKT7-StMR1,经初筛与复筛得到3个互作蛋白,一对一验证短链脱氢酶、糖基转移酶、富含亮氨酸重复序列蛋白均与转录因子StMR1存在互作。【结论】 成功构建了丰富度高且质量好的玉米大斑病菌cDNA文库并筛选到了与转录因子StMR1互作的蛋白。
王秋月, 段鹏亮, 李海笑, 刘宁, 曹志艳, 董金皋. 玉米大斑病菌cDNA文库的构建及转录因子StMR1互作蛋白的筛选[J]. 生物技术通报, 2024, 40(6): 281-289.
WANG Qiu-yue, DUAN Peng-liang, LI Hai-xiao, LIU Ning, CAO Zhi-yan, DONG Jin-gao. Construction of cDNA Library of Setosphaeria turcica and Screening of Transcription Factor StMR1 Interacting Proteins[J]. Biotechnology Bulletin, 2024, 40(6): 281-289.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| pGBKT7-StMR1-F | TGGCCATGGAGGCCGAATTCCCGGGATGGTTTTCTGCACATACTGC |
| pGBKT7-StMR1-R | GGGGTTATGCTAGTTATGCGGCCGCTTAGCCGCGCGCAAGCTTTT |
| pGADT7-135640--F | CGACGTACCAGATTACGCTCATATGATGGCAGAGAAGAAGCAGCGC |
| pGADT7-135640-R | TGCAGCTCGAGCTCGATGGATCCTTATTGCTTCAGCGCGAGCA |
| pGADT7-169112-F | CGACGTACCAGATTACGCTCATATGATGCACCTCAACGACTTCCC |
| pGADT7-169112-R | TGCAGCTCGAGCTCGATGGATCCTCACGTCTGGATGGAATGGA |
| pGADT7-165582-F | TGGCCATGGAGGCCAGTGAATTCATGACATCACGACTCATCACC |
| pGADT7-165582-R | CTGCAGCTCGAGCTCGATGGATCCTTACCACTTCTCAACCG |
| M 13-F | GTAAAACGACGGCCAG |
| M 13-R | CAGGAAACAGCTATGAC |
| T7 | TAATACGACTCACTATAGGGC |
| 3-AD | AGATGGTGCACGATGCACAG |
表1 试验所用引物
Table 1 Primers used in the experiment
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| pGBKT7-StMR1-F | TGGCCATGGAGGCCGAATTCCCGGGATGGTTTTCTGCACATACTGC |
| pGBKT7-StMR1-R | GGGGTTATGCTAGTTATGCGGCCGCTTAGCCGCGCGCAAGCTTTT |
| pGADT7-135640--F | CGACGTACCAGATTACGCTCATATGATGGCAGAGAAGAAGCAGCGC |
| pGADT7-135640-R | TGCAGCTCGAGCTCGATGGATCCTTATTGCTTCAGCGCGAGCA |
| pGADT7-169112-F | CGACGTACCAGATTACGCTCATATGATGCACCTCAACGACTTCCC |
| pGADT7-169112-R | TGCAGCTCGAGCTCGATGGATCCTCACGTCTGGATGGAATGGA |
| pGADT7-165582-F | TGGCCATGGAGGCCAGTGAATTCATGACATCACGACTCATCACC |
| pGADT7-165582-R | CTGCAGCTCGAGCTCGATGGATCCTTACCACTTCTCAACCG |
| M 13-F | GTAAAACGACGGCCAG |
| M 13-R | CAGGAAACAGCTATGAC |
| T7 | TAATACGACTCACTATAGGGC |
| 3-AD | AGATGGTGCACGATGCACAG |

图1 文库材料选取 A-E:培养4、6、8、10和15 d;F:分生孢子;G:芽管;H:附着胞;I:侵入钉
Fig. 1 Library material selection A-E: Culture for 4, 6, 8, 10 and 15 d. F: Conidia. G: Germ tube. H: Appressorium.I: Penetration peg

图2 总RNA提取(A)及mRNA(B)和双链cDNA(C)电泳
Fig. 2 Total RNA extraction(A)and electrophoresis results of mRNA(B)and ds cDNA(C) (A) M: DL 5000 marker, 1-9: RNA;(B-C) M: DL 2000 marker, 1: mRNA,2: ds cDNA

图3 文库库容量及插入片段重组率鉴定 A:初级文库容量鉴定;B:1-24初级文库插入片段检测;C:核体系次级文库容量鉴定;D:1-24核体系次级文库插入片段检测;M:DL 2000 marker
Fig. 3 Identification of library capacity and insert recombination rate A: Identification of primary library capacity. B: 1-24 detection of primary library insert. C: Identification of the secondary library capacity of the nuclear system. D: 1-24 detection of nuclear system secondary library insert. M: DL 2000 marker
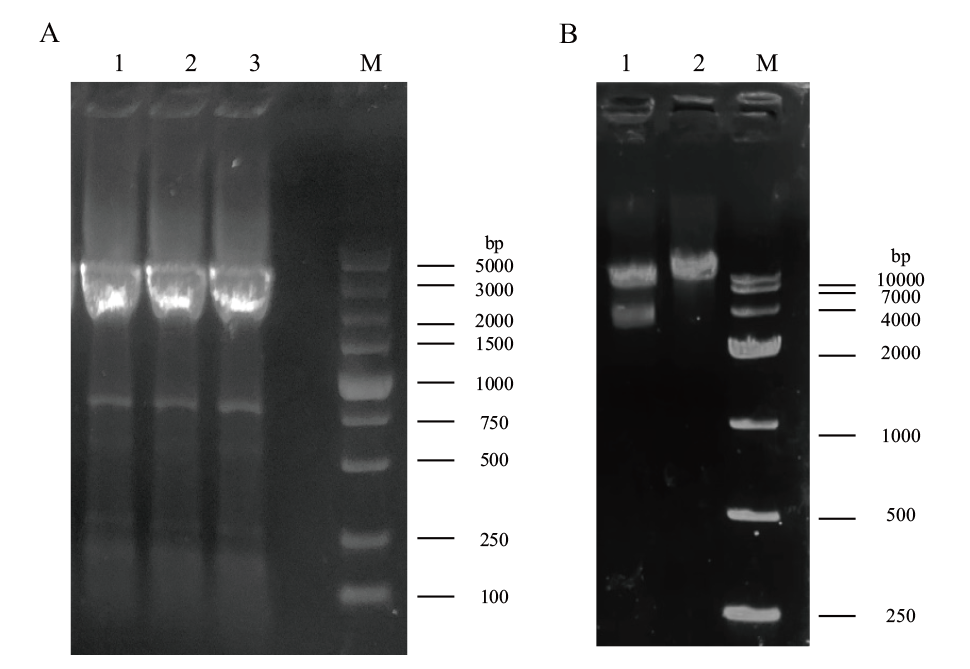
图4 诱饵载体pGBKT7-StMR1构建 A:目的片段扩增(1-3:目的片段;M:DL 5000 marker);B:诱饵载体双酶切验证(1:双酶切;2:单酶切;M:DL 10000 marker)
Fig. 4 Construction of bait vector pGBKT7-StMR1 A: Target fragment amplification(1-3: target fragment; M: DL 5000 marker). B: Verification of double digestion of bait vectors(1: digestion; 2: double single digestion; M: DL 10000 marker)
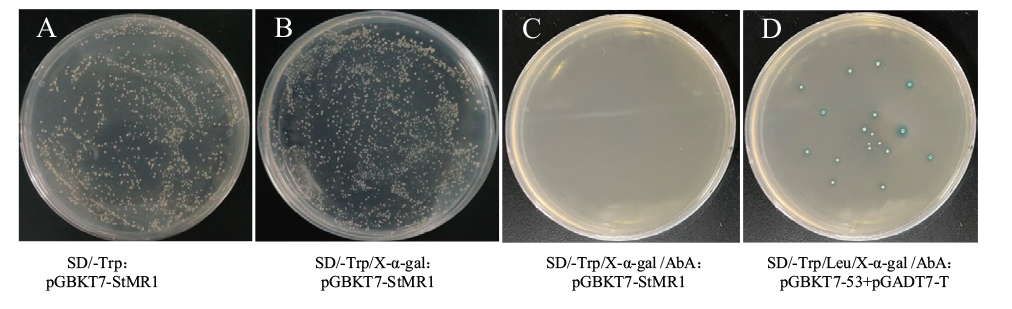
图5 诱饵载体pGBKT7-StMR1毒性及自激活活性检测
Fig. 5 Detection of toxicity and self-activating activity of bait vector pGBKT7-StMR1 A: SD/-Trp: pGBKT7-StMR1; B: SD/-Trp/X-α-gal: pGBKT7-StMR1; C: SD/-Trp/X-α-gal /AbA: pGBKT7-StMR1; D: SD/-Trp/Leu/X-α-gal/AbA: pGBKT7-53+pGADT7-T

图6 StMR1互作蛋白筛选 A:阳性克隆初筛;B:阳性克隆复筛;C:阳性克隆PCR验证
Fig. 6 StMR1 interacting protein screen A: Positive clone primary screening. B: Positive clone rescreen. C: PCR verification of positive cloning
| 基因号 Gene ID | 基因登录号 GenBank No. | 片段大小 Fragment size/bp | 编码蛋白 Encoded protein | 结构域 Pfam |
|---|---|---|---|---|
| SETTUDRAFT-165582 | XM_008032228.1 | 1 396 | 短链脱氢/还原酶 Short chain dehydrogenase/reductase | 短链脱氢酶|烯酰基-(酰基载体蛋白)还原酶|氪域 |
| SETTUDRAFT-135640 | XM_008027394.1 | 1 548 | 糖基转移酶 Glycosyltransferase | 酵母中ALG2是一种1,3-甘露糖基转移酶 |
| SETTUDRAFT-169112 | XM_008027772.1 | 1 920 | 富含亮氨酸重复序列蛋白 Leucine-rich repeat | LRR_AMN1;富含亮氨酸的重复结构 |
表2 StMR1候选蛋白
Table 2 StMR1 candidate protein
| 基因号 Gene ID | 基因登录号 GenBank No. | 片段大小 Fragment size/bp | 编码蛋白 Encoded protein | 结构域 Pfam |
|---|---|---|---|---|
| SETTUDRAFT-165582 | XM_008032228.1 | 1 396 | 短链脱氢/还原酶 Short chain dehydrogenase/reductase | 短链脱氢酶|烯酰基-(酰基载体蛋白)还原酶|氪域 |
| SETTUDRAFT-135640 | XM_008027394.1 | 1 548 | 糖基转移酶 Glycosyltransferase | 酵母中ALG2是一种1,3-甘露糖基转移酶 |
| SETTUDRAFT-169112 | XM_008027772.1 | 1 920 | 富含亮氨酸重复序列蛋白 Leucine-rich repeat | LRR_AMN1;富含亮氨酸的重复结构 |
| [1] | Mideros SX, Chung CL, Wiesner-Hanks T, et al. Determinants of virulence and in vitro development colocalize on a genetic map of Setosphaeria turcica[J]. Phytopathology, 2018, 108(2): 254-263. |
| [2] | Perkins JM, Pedersen WL. Disease development and yield losses associated with northern leaf blight on corn[J]. Plant Dis, 1987, 71(10): 940. |
| [3] | Dong JG, Fan YS, Gui XM, et al. Geographic distribution and genetic analysis of physiological races of Setosphaeria turcica in northern China[J]. Am J Agric Biol Sci, 2008, 3(1): 389-398. |
| [4] | 曹志艳, 贾慧, 朱显明, 等. DHN黑色素与玉米大斑病菌附着胞膨压形成的关系[J]. 中国农业科学, 2011, 44(5): 925-932. |
| Cao ZY, Jia H, Zhu XM, et al. Relationship between DHN melanin and formation of appressorium turgor pressure of Setosphaeria turcica[J]. Sci Agric Sin, 2011, 44(5): 925-932. | |
| [5] | Guo XY, Liu N, Liu BH, et al. Melanin, DNA replication, and autophagy affect appressorium development in Setosphaeria turcica by regulating glycerol accumulation and metabolism[J]. J integr Agric, 2022, 21(3): 762-773. |
| [6] | Zhang ZX, Jia H, Liu N, et al. The zinc finger protein StMR1 affects the pathoenicity and melanin synthesis of setosphaeria turcica and directly regulates the expression of DHN melanin synthesis pathway genes[J]. Mol Microbiol, 2022, 117(2): 261-273. |
| [7] | Sousa Melo B, Voltan AR, Arruda W, et al. Morphological and molecular aspects of sclerotial development in the phytopathogenic fungus Sclerotinia sclerotiorum[J]. Microbiol Res, 2019, 229: 126326. |
| [8] | Stappers MHT, Clark AE, Aimanianda V, et al. Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus[J]. Nature, 2018, 555(7696): 382-386. |
| [9] |
Tsuji G, Kenmochi Y, Takano Y, et al. Novel fungal transcriptional activators, Cmr1p of Colletotrichum lagenarium and pig1p of Magnaporthe grisea, contain Cys2His2 zinc finger and Zn(II)2Cys6 binuclear cluster DNA-binding motifs and regulate transcription of melanin biosynthesis genes in a developmentally specific manner[J]. Mol Microbiol, 2000, 38(5): 940-954.
pmid: 11123670 |
| [10] | 竺思仪. 稻瘟病菌黑色素合成和调控相关基因的功能研究[D]. 杭州: 浙江大学, 2021. |
| Zhu SY. The study on melanin synthesis and regulation related genes in Magnaporthe oryzae[D]. Hangzhou: Zhejiang University, 2021. | |
| [11] | Langfelder K, Jahn B, Gehringer H, et al. Identification of a polyketide synthase gene(pksP)of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence[J]. Med Microbiol Immunol, 1998, 187(2): 79-89. |
| [12] | Lin SY, Okuda S, Ikeda K, et al. LAC2 encoding a secreted laccase is involved in appressorial melanization and conidial pigmentation in Colletotrichum orbiculare[J]. Mol Plant Microbe Interact, 2012, 25(12): 1552-1561. |
| [13] |
Eliahu N, Igbaria A, Rose ms, et al. Melanin biosynthesis in the maize pathogen Cochliobolus heterostrophus depends on two mitogen-activated protein kinases, Chk1 and Mps1, and the transcription factor Cmr1[J]. Eukaryot Cell, 2007, 6(3): 421-429.
doi: 10.1128/EC.00264-06 pmid: 17237364 |
| [14] | Kihara J, Moriwaki A, Tanaka N, et al. Characterization of the BMR1 gene encoding a transcription factor for melanin biosynthesis genes in the phytopathogenic fungus Bipolaris oryzae[J]. FEMS Microbiol Lett, 2008, 281(2): 221-227. |
| [15] | Cho Y, Srivastava A, Ohm RA, et al. Transcription factor Amr1 induces melanin biosynthesis and suppresses virulence in Alternaria brassicicola[J]. PLoS Pathog, 2012, 8(10): e1002974. |
| [16] | Zhou YJ, Yang L, Wu MD, et al. A single-nucleotide deletion in the transcription factor gene bcsmr1 causes sclerotial-melanogenesis deficiency in Botrytis cinerea[J]. Front Microbiol, 2017, 8: 2492. |
| [17] | Wang YL, Hu XP, Fang YL, et al. Transcription factor VdCmr 1 is required for pigment production, protection from UV irradiation, and regulates expression of melanin biosynthetic genes in Verticillium dahliae[J]. Microbiology, 2018, 164(4): 685-696. |
| [18] | Carrillo AJ, Schacht P, Cabrera IE, et al. Functional profiling of transcription factor genes in Neurospora crassa[J]. G3, 2017, 7(9): 2945-2956. |
| [19] | 沈竹, 曹勤红. 酵母双杂交及其衍生技术应用研究进展[J]. 农业生物技术学报, 2022, 12: 2425-2433. |
| Shen Z, Cao QH. Research progress on application of yeast two hybrid system and y2h-derivated techniques[J]. J Agric Biotechnol, 2022, 12: 2425-2433. | |
| [20] |
Choi sG, Richardson A, Lambourne L, et al. Protein interactomics by two-hybrid methods[J]. Methods Mol Biol, 2018, 1794: 1-14.
doi: 10.1007/978-1-4939-7871-7_1 pmid: 29855947 |
| [21] | 韦吉, 栾宏瑛, 王荟洁, 等. 致病疫霉诱导的马铃薯酵母双杂交文库构建及无毒蛋白PiAVR3b寄主靶标筛选[J]. 植物保护, 2022, 4: 114-122, 130. |
| Wei J, Luan HY, Wang HJ, et al. Construction of yeast two-hybrid cDNA library induced by Phytophthorainfestans and screening host targets for avirulence protein PiAVR3b in potato[J]. Plant Prot, 2022, 4: 114-122, 130. | |
| [22] | 许艳, 李冉, 宋健, 等. 大丽轮枝菌及激素处理后棉花酵母双杂交文库的构建[J]. 植物保护, 2021, 47(2): 46-55. |
| Xu Y, Li R, Song J, et al. Construction of a sea-island cotton yeast two-hybrid library under the conditions of infection with Verticillium dahliae and treatment with hormones[J]. Plant Prot, 2021, 47(2): 46-55. | |
| [23] | Zhu JK. Salt and drought stress signal transduction in plants[J]. Annu Rev Plant Biol, 2002, 53: 247-273. |
| [24] | 李舒文, 董笛, 李殷睿智, 等. 蒺藜苜蓿酵母杂交cDNA文库的构建与分析[J]. 分子植物育种, 2021, 19(23): 7861-7866. |
| Li SW, Dong D, Li YRZ, et al. Construction and analysis of a yeast cDNA library from Medicago truncatula[J]. Mol Plant Breed, 2021, 19(23): 7861-7866. | |
| [25] |
Luo XM, Xie CJ, Dong JY, et al. Comparative transcriptome analysis reveals regulatory networks and key genes of microsclerotia formation in the cotton vascular wilt pathogen[J]. Fungal Genet Biol, 2019, 126: 25-36.
doi: S1087-1845(18)30217-2 pmid: 30710746 |
| [26] | Zhang L, Dong DH, Wang JF, et al. A zinc finger protein SlSZP1 protects SlSTOP1 from SlRAE1-mediated degradation to modulate aluminum resistance[J]. New Phytol, 2022, 236(1): 165-181. |
| [27] | Yu SH, Sun QG, Wu JX, et al. Genome-wide identificaiton and characterizaiton of short-chain dehydrogenase/reductase(SDR)gene family in Medicago truncatula[J]. Int J Mol Sci, 2021, 22(17): 9498. |
| [28] |
王帅乐, 肖珂雨, 王维东, 等. 苹果短链脱氢酶基因MdSDR响应腐烂病菌侵染的功能研究[J]. 核农学报, 2022, 36(11): 2158-2165.
doi: 10.11869/j.issn.100-8551.2022.11.2158 |
| Wang SL, Xiao KY, Wang WD, et al. Function exploration of apple short-chain dehydrogenases/reductase in response to valsa mali[J]. J Nucl Agric Sci, 2022, 36(11): 2158-2165. | |
| [29] | Huang LJ, Yang WH, Chen JL, et al. Molecular identification and functional characterization of an environmental stress responsive glutaredoxin gene ROXY1 in Quercus glauca[J]. Plant Physiol Biochem, 2024, 207: 108367. |
| [30] | Li J, Wen JQ, Lease KA, et al. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling[J]. Cell, 2002, 110(2): 213-222. |
| [31] | Gharabli H, Della Gala V, Welner DH. The function of UDP-glycosyltransferases in plants and their possible use in crop protection[J]. Biotechnol Adv, 2023, 67: 108182. |
| [32] |
Jo YK, Park NY, Park SJ, et al. O-GlcNAcylation of ATG4B positively regulates autophagy by increasing its hydroxylase activity[J]. Oncotarget, 2016, 7(35): 57186-57196.
doi: 10.18632/oncotarget.11083 pmid: 27527864 |
| [1] | 张迪, 鞠睿, 李丽梅, 王煜倩, 陈瑞, 王新一. 基于转录因子生物传感器在环境分析中的应用[J]. 生物技术通报, 2024, 40(6): 114-125. |
| [2] | 胡雅丹, 伍国强, 刘晨, 魏明. MYB转录因子在调控植物响应逆境胁迫中的作用[J]. 生物技术通报, 2024, 40(6): 5-22. |
| [3] | 王健, 杨莎, 孙庆文, 陈宏宇, 杨涛, 黄园. 金钗石斛bHLH转录因子家族全基因组鉴定及表达分析[J]. 生物技术通报, 2024, 40(6): 203-218. |
| [4] | 王迪, 张晓宇, 宋宇鑫, 郑东然, 田静, 李玉花, 王宇, 吴昊. 细胞全能性转录因子调控植物组培再生的分子机制研究进展[J]. 生物技术通报, 2024, 40(6): 23-33. |
| [5] | 李慧, 文钰芳, 王悦, 纪超, 石国优, 罗英, 周勇, 李志敏, 吴晓玉, 杨有新, 刘建萍. 盐胁迫下辣椒CaPIF4的表达特性与功能分析[J]. 生物技术通报, 2024, 40(4): 148-158. |
| [6] | 肖雅茹, 贾婷婷, 罗丹, 武喆, 李丽霞. 黄瓜CsERF025L转录因子的克隆及表达分析[J]. 生物技术通报, 2024, 40(4): 159-166. |
| [7] | 郭纯, 宋桂梅, 闫艳, 邸鹏, 王英平. 西洋参bZIP基因家族全基因组鉴定和表达分析[J]. 生物技术通报, 2024, 40(4): 167-178. |
| [8] | 谢倩, 江来, 贺进, 刘玲玲, 丁明月, 陈清西. 不同鲜食品质橄榄果实转录组测序及酚类代谢途径相关调控基因挖掘[J]. 生物技术通报, 2024, 40(3): 215-228. |
| [9] | 陈艳梅. 蛋白质翻译后修饰之间的互作关系及其协同调控机理[J]. 生物技术通报, 2024, 40(2): 1-8. |
| [10] | 杨艳, 胡洋, 刘霓如, 殷璐, 杨锐, 王鹏飞, 穆霄鹏, 张帅, 程春振, 张建成. ‘红满堂’苹果MbbZIP43基因的克隆与功能研究[J]. 生物技术通报, 2024, 40(2): 146-159. |
| [11] | 路喻丹, 刘晓驰, 冯新, 陈桂信, 陈义挺. 猕猴桃BBX基因家族成员鉴定与转录特征分析[J]. 生物技术通报, 2024, 40(2): 172-182. |
| [12] | 辛奇, 李压凡, 尹铮, 张晓丹, 陈霆, 刘晓华. 甘蔗CBL-CIPK基因家族的鉴定和表达分析[J]. 生物技术通报, 2024, 40(2): 197-211. |
| [13] | 任延靖, 张鲁刚, 赵孟良, 李江, 邵登魁. 白菜种子cDNA酵母文库的构建及BrTTG1互作蛋白的筛选及分析[J]. 生物技术通报, 2024, 40(2): 223-232. |
| [14] | 邹修为, 岳佳妮, 李志宇, 戴良英, 李魏. 水稻热激转录因子HsfA2b调控非生物胁迫抗性的功能分析[J]. 生物技术通报, 2024, 40(2): 90-98. |
| [15] | 周会汶, 吴兰花, 韩德鹏, 郑伟, 余跑兰, 吴杨, 肖小军. 甘蓝型油菜种子硫苷含量全基因组关联分析[J]. 生物技术通报, 2024, 40(1): 222-230. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||