








生物技术通报 ›› 2024, Vol. 40 ›› Issue (11): 285-295.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0286
吴永娜1( ), 滕文龙1, 张磊2, 王德富1, 牛颜冰1(
), 滕文龙1, 张磊2, 王德富1, 牛颜冰1( )
)
收稿日期:2024-03-22
出版日期:2024-11-26
发布日期:2024-12-19
通讯作者:
牛颜冰,女,博士,教授,研究方向:药用植物遗传学;E-mail: niuyanbingbest@163.com作者简介:吴永娜,女,博士,研究方向:药用植物生态学、分子生物学;E-mail: gpwzq0130@126.com
基金资助:
WU Yong-na1( ), TENG Wen-long1, ZHANG Lei2, WANG De-fu1, NIU Yan-bing1(
), TENG Wen-long1, ZHANG Lei2, WANG De-fu1, NIU Yan-bing1( )
)
Received:2024-03-22
Published:2024-11-26
Online:2024-12-19
摘要:
【目的】 研究连翘叶茶粗提物及其茶水对四氯化碳(CCl4)诱导大鼠肝硬化的影响,阐明不同剂量连翘叶茶粗提物及茶水对肠道菌群多样性的影响及其机理。【方法】 30只雄性SD大鼠按照平均体重相近的原则随机分为对照组、模型组、低、中、高剂量连翘叶茶灌胃组和茶水组,采用皮下注射CCl4+灌胃CCl4橄榄油混合液的方法构建肝硬化大鼠模型,建模成功后给予不同剂量连翘叶茶粗提物灌胃及茶水组干预10 d后,收取血清进行ELISA检测,肝脏组织通过HE染色和提取RNA进行RT-PCR检测,粪便样本进行16S rRNA测序。【结果】 连翘叶茶粗提物及茶水组均能显著改善大鼠肝硬化形态,尤其茶水组可明显逆转肝硬化状态,肝细胞脂肪变性、中央静脉周围炎性细胞浸润和汇管区纤维结缔组织增生较模型组均明显改善;连翘叶茶粗提物及茶水组可显著提高益生菌Rothia、Parabacteroides和Lactobacillus丰度,促进不饱和脂肪酸合成和酸呼吸通路的富集;连翘叶茶粗提物及茶水组可抑制血液中DAO、TLR-4、LPS内毒素因子的水平,促进抑炎因子1L-4及肠道屏障功能因子ZO-1表达,各组之间肠道微生物组成与DAO、LPS、1L-4和TLR4相关,其中与肠道损伤指标DAO相关性最大。【结论】 连翘叶茶粗提物及茶水组可能通过改善大鼠肝硬化形态、减弱炎症反应、改善肠道微生态功能失调及保护肠屏障功能抑制肝硬化发生。
吴永娜, 滕文龙, 张磊, 王德富, 牛颜冰. 连翘叶茶对大鼠肝硬化的影响及其机理研究[J]. 生物技术通报, 2024, 40(11): 285-295.
WU Yong-na, TENG Wen-long, ZHANG Lei, WANG De-fu, NIU Yan-bing. Effect of Forsythia suspensa Leaf Tea on with Cirrhosis and Its Mechanism in Rats[J]. Biotechnology Bulletin, 2024, 40(11): 285-295.

图1 肝硬化和正常大鼠肝脏Masson染色 A:对照组大鼠,肝小叶正常,中央静脉无炎症浸润,汇管区无结缔组织; B:模型组大鼠,纤维组织增生明显,假小叶形成
Fig. 1 Masson staining of liver between cirrhosis and normal rats A: The rats in the control group had normal liver lobules, no inflammatory infiltration of the central vein, and no connective tissue in the manifold area. B: Rats in the model group had obvious fibrous tissue hyperplasia and pseudolobule formation

图2 肝硬化大鼠肝脏形态特征 Control:对照组;Model:肝硬化模型组;Tea:连翘茶水组;Low、Medium和High:分别为连翘叶茶粗提物0.5、0.7和1 g/mL。下同
Fig. 2 Morphological characteristics of livers in cirrhotic rats Control: Control group. Model: Cirrhosis model group. Tea: Forsythia tea group. Low, Medium and High: 0.5, 0.7 and 1 g/mL crude extract of Forsythia leaf tea, respectively. The same below

图4 不同处理组间 β多样性特征 A:β多样性PLSDA模型聚类分析;B:维恩图特征分析
Fig. 4 β diversity characteristics among different treatments A: Cluster analysis of β diversity via PLSDA mode. B: Characteristic analysis via the Venn diagram

图5 不同处理组间TOP 10的肠道细菌门(A)和属(B)水平上的组成及差异
Fig. 5 Composition and difference of TOP 10 gut bacteria among different treatments at phylum(A)and genus(B)level

图9 不同处理组间差异功能通路分析 A:不饱和脂肪酸代谢通路;B:阿拉伯糖转运结合蛋白通路;C:延胡索酸呼吸通路;D:β-木糖苷水解酶GH43;不同字母代表P<0.05水平上差异显著,下同
Fig. 9 Analysis of differential functional pathways in different treatments A: Metabolic pathways of unsaturated fatty acids. B: Pathways of arabinosaccharide transport system substrate-binding protein. C: Respiratory pathway of fumaric acid. D: β-xylosidase GH43. Different letters indicate significant differences at the P<0.05 level. The same below

图10 不同处理组间血清中炎症因子的分析 1L-4:白介素4;DAO:二胺氧化酶;LPS:脂多糖;TLR4:Toll样受体(TLR)家族成员
Fig. 10 Analysis of inflammatory factor in serum in different treatments 1L-4: Interleukin 4. DAO: Diamine oxidase. LPS: Lipopolysaccharide. TLR4: A member of the toll-like receptor(TLR)family
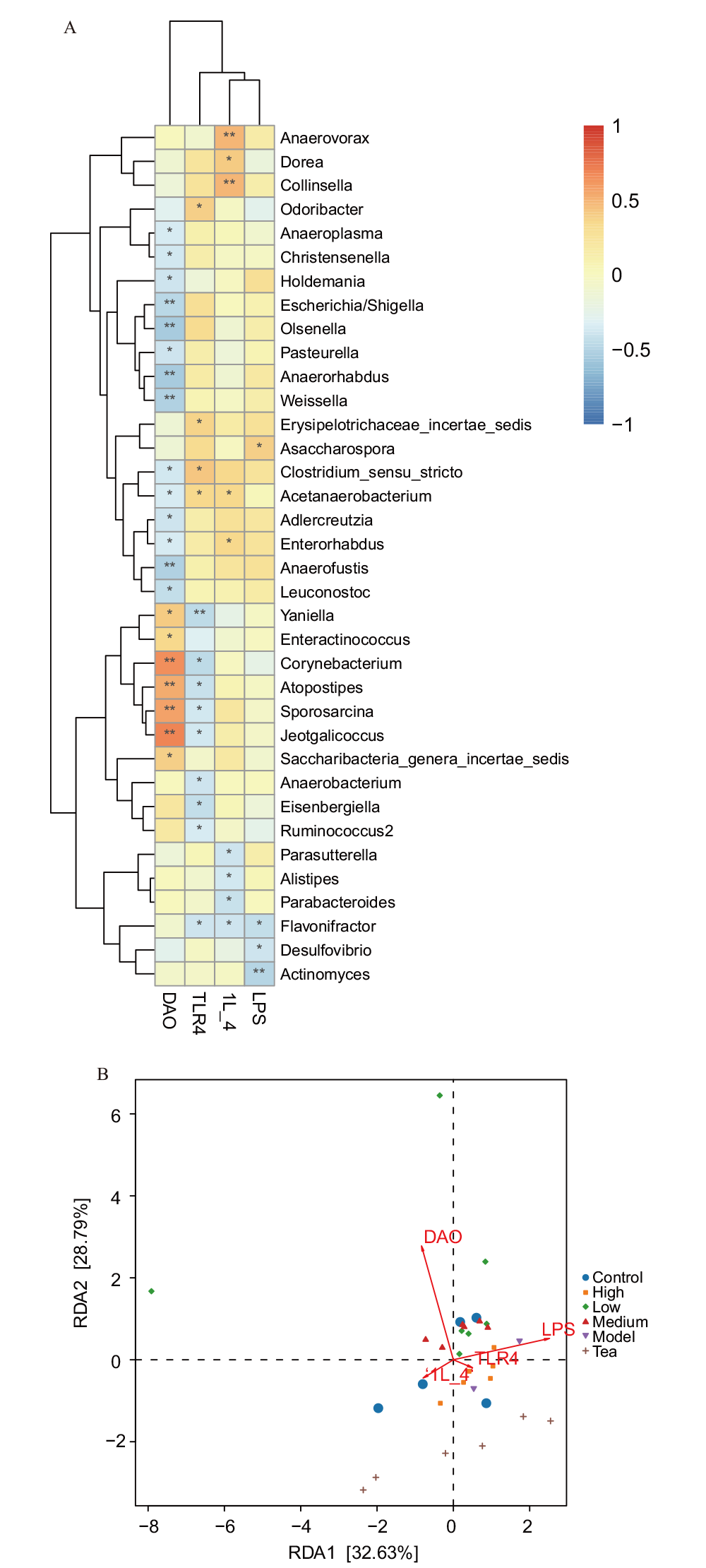
图12 不同处理组间肠道微生物与临床因子相关性分析 A:差异菌群与差异炎症因子斯皮尔曼相关性分析;B:差异菌群与差异炎症因子CCA分析
Fig. 12 Correlation analysis between intestinal microbes and clinical factors among different treatments A: Spearman correlation analysis between differential microbiota and differential inflammatory factors. B: CCA analysis of differential microbiota and differential inflammatory cytokines
| [1] | Blumberg R, Powrie F. Microbiota, disease, and back to health: a metastable journey[J]. Sci Transl Med, 2012, 4(137): 137rv7. |
| [2] |
Smith MI, Yatsunenko T, Manary MJ, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor[J]. Science, 2013, 339(6119): 548-554.
doi: 10.1126/science.1229000 pmid: 23363771 |
| [3] | Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism[J]. Nature, 2016, 535(7610): 56-64. |
| [4] | Węgielska I, Suliburska J. The role of intestinal microbiota in the pathogenesis of metabolic diseases[J]. Acta Sci Pol Technol Aliment, 2016, 15(2): 201-211. |
| [5] | Tilg H. A gut feeling about thrombosis[J]. N Engl J Med, 2016, 374(25): 2494-2496. |
| [6] | 郭慧玲, 邵玉宇, 孟和毕力格, 等. 肠道菌群与疾病关系的研究进展[J]. 微生物学通报, 2015, 42(2): 400-410. |
| Guo HL, Shao YY, Menghe B, et al. Research on the relation between gastrointestinal microbiota and disease[J]. Microbiol China, 2015, 42(2): 400-410. | |
| [7] | Claesson MJ, Cusack S, O'Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly[J]. Proc Natl Acad Sci USA, 2011, 108(Suppl 1): 4586-4591. |
| [8] | Usami M, Miyoshi M, Yamashita H. Gut microbiota and host metabolism in liver cirrhosis[J]. World J Gastroenterol, 2015, 21(41): 11597-11608. |
| [9] | Scharlau D, Borowicki A, Habermann N, et al. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre[J]. Mutat Res, 2009, 682(1): 39-53. |
| [10] | Wu YN, Zhang L, Chen T, et al. Granulocyte-macrophage colony-stimulating factor protects mice against hepatocellular carcinoma by ameliorating intestinal dysbiosis and attenuating inflammation[J]. World J Gastroenterol, 2020, 26(36): 5420-5436. |
| [11] | Zhang L, Wu YN, Chen T, et al. Relationship between intestinal microbial dysbiosis and primary liver cancer[J]. Hepatobiliary Pancreat Dis Int, 2019, 18(2): 149-157. |
| [12] | Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65(2): 87-108. |
| [13] | Tao XM, Wang N, Qin WX. Gut microbiota and hepatocellular carcinoma[J]. Gastrointest Tumors, 2015, 2(1): 33-40. |
| [14] |
Lange CM, Bojunga J, Hofmann WP, et al. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function[J]. Hepatology, 2009, 50(6): 2001-2006.
doi: 10.1002/hep.23346 pmid: 19937695 |
| [15] | 冯硕. 中药治疗慢性乙肝病毒携带者、肝纤维化和肝硬化的系统评价及结论偏倚方法学研究[D]. 北京: 北京中医药大学, 2017. |
| Feng S. A systematic review of traditional Chinese medicine in the treatment of chronic hepatitis B virus carriers, liver fibrosis and liver cirrhosis, and a methodological study of bias in conclusions[D]. Beijing: Beijing University of Chinese Medicine, 2017. | |
| [16] |
梁文欧, 赵力超, 方祥, 等. 大豆异黄酮与肠道微生物相互作用研究进展[J]. 食品科学, 2019, 40(9): 283-289.
doi: 10.7506/spkx1002-6630-20180119-261 |
| Liang WO, Zhao LC, Fang X, et al. Progress in the research of the interactions of soy isoflavones with gut microbiota[J]. Food Sci, 2019, 40(9): 283-289. | |
| [17] |
Li DT, Feng Y, Tian ML, et al. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation[J]. Microbiome, 2021, 9(1): 83.
doi: 10.1186/s40168-021-01028-7 pmid: 33820558 |
| [18] | 曲欢欢, 翟西峰, 李白雪, 等. 连翘不同部位中连翘酯苷和连翘苷的含量分析[J]. 药物分析杂志, 2008, 28(3): 382-385. |
| Qu HH, Zhai XF, Li BX, et al. Study of the content of forsythiaside and forsythin from different parts of Forsythia suspensa(Thunb.) Vahl[J]. Chin J Pharm Anal, 2008, 28(3): 382-385. | |
| [19] | 张炜, 张汉明, 郭美丽, 等. 连翘的药理学研究[J]. 中国现代应用药学, 2000, 17(1): 7-10. |
| Zhang W, Zhang HM, Guo ML, et al. Pharmacological studies of forsythia[J]. Chin J Mod Appl Pharm, 2000, 17(1): 7-10. | |
| [20] |
滕文龙, 吴永娜, 王德富, 等. 连翘叶茶对肝癌细胞增殖和迁移功能的影响及其作用机制[J]. 生物技术通报, 2024, 40(4): 287-296.
doi: 10.13560/j.cnki.biotech.bull.1985.2023-0935 |
| Teng WL, Wu YN, Wang DF, et al. Effect of Forsythia suspensa leaves tea on HCC proliferation and migration function and its mechanism of action[J]. Biotechnol Bull, 2024, 40(4): 287-296. | |
| [21] | 白美美. 连翘叶茶保肝作用研究[D]. 太原: 山西大学, 2018. |
| Bai MM. Study on hepatoprotective effect of Forsythia suspensa leaves tea[D]. Taiyuan: Shanxi University, 2018. | |
| [22] | 袁盛榕, 库宝善. 药理学实习教程[M]. 2版. 北京: 世界图书出版公司, 1994: 12-13. |
| Yuan SR, Ku BS. Pharmacology Internship Tutorial[M]. 2nd ed. Beijing: World Publishing Corportion, 1994: 12-13. | |
| [23] | Lin ZS, Wu JM, Wang JP, et al. Dietary Lactobacillus reuteri prevent from inflammation mediated apoptosis of liver via improving intestinal microbiota and bile acid metabolism[J]. Food Chem, 2023, 404(Pt B): 134643. |
| [24] | Zhao CJ, Bao LJ, Qiu M, et al. Commensal cow Roseburia reduces gut-dysbiosis-induced mastitis through inhibiting bacterial translocation by producing butyrate in mice[J]. Cell Rep, 2022, 41(8): 111681. |
| [25] | Sang JN, Zhuang DH, Zhang T, et al. Convergent and divergent age patterning of gut microbiota diversity in humans and nonhuman primates[J]. mSystems, 2022, 7(4): e0151221. |
| [26] | Dai SP, Wang ZL, Yang Y, et al. PM2.5 induced weight loss of mice through altering the intestinal microenvironment: mucus barrier, gut microbiota, and metabolic profiling[J]. J Hazard Mater, 2022, 431: 128653. |
| [27] | Teng SS, Zhang YF, Jin XH, et al. Structure and hepatoprotective activity of Usp10/NF-κB/Nrf2 pathway-related Morchella esculenta polysaccharide[J]. Carbohydr Polym, 2023, 303: 120453. |
| [28] |
Spinelli JB, Rosen PC, Sprenger HG, et al. Fumarate is a terminal electron acceptor in the mammalian electron transport chain[J]. Science, 2021, 374(6572): 1227-1237.
doi: 10.1126/science.abi7495 pmid: 34855504 |
| [29] | Zecchini V, Paupe V, Herranz-Montoya I, et al. Fumarate induces vesicular release of mtDNA to drive innate immunity[J]. Nature, 2023, 615(7952): 499-506. |
| [30] |
Schubert K, Olde Damink SWM, von Bergen M, et al. Interactions between bile salts, gut microbiota, and hepatic innate immunity[J]. Immunol Rev, 2017, 279(1): 23-35.
doi: 10.1111/imr.12579 pmid: 28856736 |
| [31] |
Guo Z, Zhang JC, Wang ZL, et al. Intestinal microbiota distinguish gout patients from healthy humans[J]. Sci Rep, 2016, 6: 20602.
doi: 10.1038/srep20602 pmid: 26852926 |
| [32] | Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria TH17 cell differentiation[J]. Nature, 2008, 455(7214): 808-812. |
| [33] |
Abu-Shanab A, Quigley EMM. The role of the gut microbiota in nonalcoholic fatty liver disease[J]. Nat Rev Gastroenterol Hepatol, 2010, 7(12): 691-701.
doi: 10.1038/nrgastro.2010.172 pmid: 21045794 |
| [34] |
Ye JZ, Lv LX, Wu WR, et al. Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels[J]. Front Microbiol, 2018, 9: 1967.
doi: 10.3389/fmicb.2018.01967 pmid: 30186272 |
| [35] | Sun SS, Wang K, Ma K, et al. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota[J]. Chin J Nat Med, 2019, 17(1): 3-14. |
| [36] |
Gadde U, Oh ST, Lee YS, et al. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens[J]. Probiotics Antimicrob Proteins, 2017, 9(4): 397-405.
doi: 10.1007/s12602-017-9275-9 pmid: 28421423 |
| [37] |
Mridha AR, Wree A, Robertson AAB, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice[J]. J Hepatol, 2017, 66(5): 1037-1046.
doi: S0168-8278(17)30056-9 pmid: 28167322 |
| [38] | 姜冰. 肝硬化患者DAO、内毒素、D-乳酸变化及在肠黏膜屏障功能评估中的作用[J]. 中国实用医药, 2017, 12(33): 33-34. |
| Jiang B. Changes in DAO, endotoxin, D-lactate and intestinal mucosa in patients with cirrhosis role in the assessment of barrier function[J]. China Pract Med, 2017, 12(33): 33-34. | |
| [39] | 王海娟, 戴雨珂, 潘渠. 魏斯氏菌的研究现状[J]. 成都医学院学报, 2014, 9(6): 747-750. |
| Wang HJ, Dai YK, Pan Q. Current status of Weissella research[J]. J Chengdu Med Coll, 2014, 9(6): 747-750. | |
| [40] | 克里斯·韦伯. 长寿密码: 来自科学前沿的健康长寿秘诀[M]. 王钊, 范丽, 译. 北京: 科学出版社, 2019. |
| Verburgh K. The longevity code: secrets to living well for longer from the front lines of science[M]. Wang Z, Fan L, translated. Beijing: Science Press, 2019. |
| [1] | 刘璐, 朱哲远, 李颖曦, 王颉, 彭迪. 微生物除草剂的研究进展[J]. 生物技术通报, 2024, 40(9): 161-171. |
| [2] | 滕文龙, 吴永娜, 王德富, 牛颜冰. 连翘叶茶对肝癌细胞增殖和迁移功能的影响及其作用机制[J]. 生物技术通报, 2024, 40(4): 287-296. |
| [3] | 赵睿萌, 王梦雨, 吕国英, 宋婷婷, 张作法. 药用真菌桑黄中多酚类成分药用机理研究进展[J]. 生物技术通报, 2024, 40(11): 3-13. |
| [4] | 王雨晴, 马子奇, 侯嘉欣, 宗钰琪, 郝晗睿, 刘国元, 魏辉, 连博琳, 陈艳红, 张健. 盐胁迫下植物根系分泌物的成分分析与生态功能研究进展[J]. 生物技术通报, 2024, 40(1): 12-23. |
| [5] | 李玉岭, 毛欣, 张元帅, 董元夫, 刘翠兰, 段春华, 毛秀红. 辐射诱变技术在木本植物育种中的应用及展望[J]. 生物技术通报, 2023, 39(6): 12-30. |
| [6] | 陈泉冰, 曹伟洁, 李春, 吕波. GH79家族糖苷水解酶分子进化关系和蛋白结构研究[J]. 生物技术通报, 2023, 39(1): 104-114. |
| [7] | 颜珲璘, 芦光新, 邓晔, 顾松松, 颜程良, 马坤, 赵阳安, 张海娟, 王英成, 周学丽, 窦声云. 高寒地区根瘤菌拌种对禾/豆混播土壤微生物群落的影响[J]. 生物技术通报, 2022, 38(10): 204-215. |
| [8] | 辛亚芬, 陈晨, 曾泰儒, 杜昭昌, 倪浩然, 钟怡豪, 谭小平, 闫艳红. 青贮添加剂对微生物多样性影响的研究进展[J]. 生物技术通报, 2021, 37(9): 24-30. |
| [9] | 王婷, 杨阳, 李金萍, 杜坤. 转基因作物对土壤微生物群落影响的研究进展[J]. 生物技术通报, 2021, 37(9): 255-265. |
| [10] | 张颖超, 尹守亮, 王一炜, 王学凯, 杨富裕. 木本饲料青贮研究进展[J]. 生物技术通报, 2021, 37(9): 48-57. |
| [11] | 姜富贵, 成海建, 魏晨, 张召坤, 苏文政, 时光, 宋恩亮. 糖蜜添加量对杂交构树青贮发酵品质和微生物多样性的影响[J]. 生物技术通报, 2021, 37(9): 68-76. |
| [12] | 张秀民, 马绍英, 杨洁, 包金玉, 张晓玲, 田鹏, 路亚琦, 李胜. 以次生代谢物产量为目标的西兰花毛状根培养技术体系优化[J]. 生物技术通报, 2021, 37(8): 75-84. |
| [13] | 张洁, 夏明聪, 朱文倩, 梁娟, 孙润红, 徐文, 武超, 杨丽荣. 蔬菜根结线虫生防芽胞杆菌的筛选及作用机理研究[J]. 生物技术通报, 2021, 37(7): 175-182. |
| [14] | 陈明雨, 倪烜, 司友斌, 孙凯. 固定化真菌漆酶在环境有机污染修复中的应用研究进展[J]. 生物技术通报, 2021, 37(6): 244-258. |
| [15] | 龚晓惠, 杨敏, 李舒婷, 林晟豪, 许文涛. 银纳米簇抗菌机理、活性及其应用的研究进展[J]. 生物技术通报, 2021, 37(5): 212-220. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||