








生物技术通报 ›› 2024, Vol. 40 ›› Issue (10): 296-304.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0541
收稿日期:2024-06-07
出版日期:2024-10-26
发布日期:2024-11-20
通讯作者:
高伟霞,女,博士,副教授,研究方向 :微生物 ;E-mail: gaoweixia@tust.edu.cn作者简介:赵锐,男,研究方向:微生物与生化药学;E-mail: zr1375649659@163.com
基金资助:
ZHAO Rui( ), DI Jing-yi, ZHANG Guang-tong, LIU Hao, GAO Wei-xia(
), DI Jing-yi, ZHANG Guang-tong, LIU Hao, GAO Wei-xia( )
)
Received:2024-06-07
Published:2024-10-26
Online:2024-11-20
摘要:
【目的】构建兽疫链球菌内源性表达元件文库,并探究其在提高透明质酸产量中的应用。【方法】通过对兽疫链球菌发酵指数期和平台期进行转录组测序分析,初步筛选32种高、中、低3种强度的启动子与RBS组合(表达元件PR);进一步利用绿色荧光蛋白基因转录水平及荧光强度来验证PR元件的强度;最后,利用筛选到的较强元件PR31过表达透明质酸合成关键基因hasA, hasB, hasC, hasD, hasE,并采用2 L发酵罐评价强表达元件在提高透明质酸产量的效果。【结果】上述基因的相对转录水平分别提高至原来的8.17、7.32、3.72、39.48、9倍。其中,过表达hasA及hasD后,透明质酸产量相对于野生型分别提高了43%和31%,达到5.654 g/L和5.283 g/L。【结论】成功构建了兽疫链球菌内源表达元件文库,可用于透明质酸合成途径强化及竞争途径弱化等代谢工程改造。
赵锐, 狄靖宜, 张广通, 刘浩, 高伟霞. 基于转录组学挖掘兽疫链球菌内源表达元件及高产透明质酸应用[J]. 生物技术通报, 2024, 40(10): 296-304.
ZHAO Rui, DI Jing-yi, ZHANG Guang-tong, LIU Hao, GAO Wei-xia. Screening Endogenous Expression Elements in Streptococcus zooepidemicus via Transcriptomics Analysis and Applications for High Yield of Hyaluronic Acid[J]. Biotechnology Bulletin, 2024, 40(10): 296-304.
| 表达元件Expression element | 表达元件控制的基因功能Gene functions controlled by expression elements | FPKM | 大小Size/bp |
|---|---|---|---|
| PR0 | cp25(常见的用于兽疫链球菌的异源表达元件)[ | - | 59 |
| PR1 | 3-phosphoshikimate 1-carboxyvinyltransferase | 65.45 | 130 |
| PR2 | Conserved hypothetical protein | 65.68 | 423 |
| PR3 | tRNA-specific 2-thiouridylase MnmA | 65.97 | 283 |
| PR4 | Maltodextrin transport system permease protein MalF | 89.14 | 299 |
| PR5 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 97.38 | 149 |
| PR6 | Sugar uptake protein | 105.48 | 500 |
| PR7 | 16S rRNA m(2)G 1207 methyltransferase | 117.68 | 233 |
| PR8 | Glutamate dehydrogenase | 137.6 | 500 |
| PR9 | UDP-N-acetylmuramoyl-L-alanyl-D-glutamate synthetase | 430.41 | 152 |
| PR10 | Putative cell-cycle regulation histidine triad protein HIT | 436.44 | 472 |
| PR11 | L-lactate dehydrogenase | 561.89 | 191 |
| PR12 | ABC transporter ATP-binding protein | 644.25 | 120 |
| PR13 | S-adenosylmethionine:tRNA ribosyltransferase-isomerase | 708.09 | 268 |
| PR14 | Methylated-DNA--protein-cysteine methyltransferase | 709.89 | 492 |
| PR15 | 30S ribosomal protein S4 | 855.87 | 500 |
| PR16 | 50S ribosomal protein L28 | 1029.4 | 500 |
| PR17 | Cell division protein | 1152.84 | 211 |
| PR18 | 50S ribosomal protein L32 | 1734.26 | 269 |
| PR19 | NADH oxidase | 1980.71 | 249 |
| PR20 | 30S ribosomal protein S13 | 2550.44 | 159 |
| PR21 | Fructose-bisphosphate aldolase | 4387.94 | 200 |
| PR22 | 6-phosphofructokinase | 4644.17 | 79 |
| PR23 | phosphoglycerate kinase | 4912.63 | 200 |
| PR24 | C3-bisphosphoglycerate-dependent phosphoglycerate mutase GpmA | 4918.12 | 122 |
| PR25 | UDP-glucose 6-dehydrogenase HasB | 6662.7 | 253 |
| PR26 | Hyaluronan synthase HasA | 7891.36 | 396 |
| PR27 | Glyceraldehyde-3-phosphate dehydrogenase | 9036.98 | 112 |
| PR28 | Cold shock protein | 10105.49 | 206 |
| PR29 | Conserved hypothetical protein | 11533.49 | 500 |
| PR30 | Hypothetical protein | 12563.03 | 340 |
| PR31 | Szp protein | 13899.81 | 384 |
| PR32 | Phosphopyruvate hydratase | 20029.66 | 213 |
表1 兽疫链球菌表达元件信息
Table 1 Information about the expression elements in S. zooepidemicus
| 表达元件Expression element | 表达元件控制的基因功能Gene functions controlled by expression elements | FPKM | 大小Size/bp |
|---|---|---|---|
| PR0 | cp25(常见的用于兽疫链球菌的异源表达元件)[ | - | 59 |
| PR1 | 3-phosphoshikimate 1-carboxyvinyltransferase | 65.45 | 130 |
| PR2 | Conserved hypothetical protein | 65.68 | 423 |
| PR3 | tRNA-specific 2-thiouridylase MnmA | 65.97 | 283 |
| PR4 | Maltodextrin transport system permease protein MalF | 89.14 | 299 |
| PR5 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 97.38 | 149 |
| PR6 | Sugar uptake protein | 105.48 | 500 |
| PR7 | 16S rRNA m(2)G 1207 methyltransferase | 117.68 | 233 |
| PR8 | Glutamate dehydrogenase | 137.6 | 500 |
| PR9 | UDP-N-acetylmuramoyl-L-alanyl-D-glutamate synthetase | 430.41 | 152 |
| PR10 | Putative cell-cycle regulation histidine triad protein HIT | 436.44 | 472 |
| PR11 | L-lactate dehydrogenase | 561.89 | 191 |
| PR12 | ABC transporter ATP-binding protein | 644.25 | 120 |
| PR13 | S-adenosylmethionine:tRNA ribosyltransferase-isomerase | 708.09 | 268 |
| PR14 | Methylated-DNA--protein-cysteine methyltransferase | 709.89 | 492 |
| PR15 | 30S ribosomal protein S4 | 855.87 | 500 |
| PR16 | 50S ribosomal protein L28 | 1029.4 | 500 |
| PR17 | Cell division protein | 1152.84 | 211 |
| PR18 | 50S ribosomal protein L32 | 1734.26 | 269 |
| PR19 | NADH oxidase | 1980.71 | 249 |
| PR20 | 30S ribosomal protein S13 | 2550.44 | 159 |
| PR21 | Fructose-bisphosphate aldolase | 4387.94 | 200 |
| PR22 | 6-phosphofructokinase | 4644.17 | 79 |
| PR23 | phosphoglycerate kinase | 4912.63 | 200 |
| PR24 | C3-bisphosphoglycerate-dependent phosphoglycerate mutase GpmA | 4918.12 | 122 |
| PR25 | UDP-glucose 6-dehydrogenase HasB | 6662.7 | 253 |
| PR26 | Hyaluronan synthase HasA | 7891.36 | 396 |
| PR27 | Glyceraldehyde-3-phosphate dehydrogenase | 9036.98 | 112 |
| PR28 | Cold shock protein | 10105.49 | 206 |
| PR29 | Conserved hypothetical protein | 11533.49 | 500 |
| PR30 | Hypothetical protein | 12563.03 | 340 |
| PR31 | Szp protein | 13899.81 | 384 |
| PR32 | Phosphopyruvate hydratase | 20029.66 | 213 |
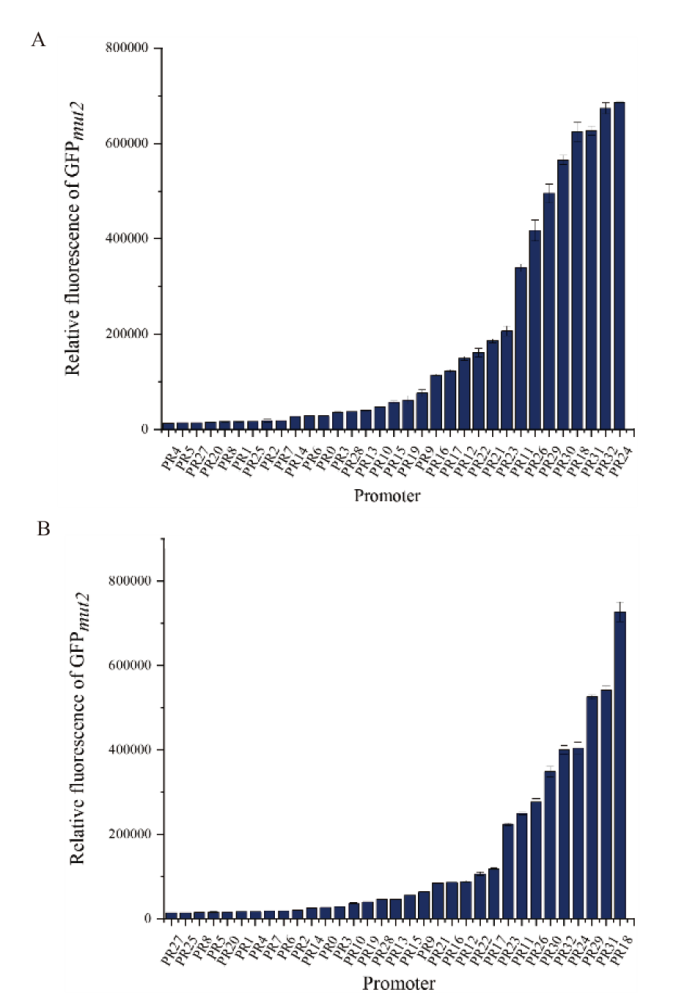
图2 不同启动子表达GFP的相对荧光强度 THY培养基(A)及FSB培养基(B)中培养8 h后GFP的相对荧光强度(荧光值/OD600)
Fig. 2 Relative fluorescence intensity of GFP expressed by different promoter Relative fluorescence intensity of GFP(fluorescence intensity/OD600)cultured in THY medium(A)or FSB medium(B)for 8 h

图3 gfp基因在不同培养基不同发酵时间下的相对转录水平 A,B:分别表示不同表达元件控制下gfp在THY培养基中培养4 h和8 h的相对转录水平;C,D:分别表示不同表达元件控制下gfp在FSB培养基中培养4 h和8 h的相对转录水平
Fig. 3 Relative transcriptions of gfp gene in different medium and fermentation time A, B: The relative transcriptions of gfp controlled by different expression elements cultured in THY medium for 4 h and 8 h, respectively. C, D: The relative transcriptions of gfp controlled by different expression elements cultured in FSB medium for 4 h and 8 h, respectively
| 培养基 Medium | 组成型强表达元件 Constitutive strongly expressive elements | 指数期强表达元件 Strongly expressed elements in exponential phase | 稳定期强表达元件 Strongly expressed elements in stable phase |
|---|---|---|---|
| THY | PR15、PR17、PR26、PR30、PR32 | PR8、PR11、PR16、PR18、PR19、PR21、PR24、PR29 | PR12、PR31 |
| FSB | PR8、PR16、PR24、PR26、PR28、PR30、PR31 | PR5、PR13 | PR6、PR12、PR21、PR22、PR23、PR32 |
表2 各类强表达元件
Table 2 Various strong expression elements
| 培养基 Medium | 组成型强表达元件 Constitutive strongly expressive elements | 指数期强表达元件 Strongly expressed elements in exponential phase | 稳定期强表达元件 Strongly expressed elements in stable phase |
|---|---|---|---|
| THY | PR15、PR17、PR26、PR30、PR32 | PR8、PR11、PR16、PR18、PR19、PR21、PR24、PR29 | PR12、PR31 |
| FSB | PR8、PR16、PR24、PR26、PR28、PR30、PR31 | PR5、PR13 | PR6、PR12、PR21、PR22、PR23、PR32 |
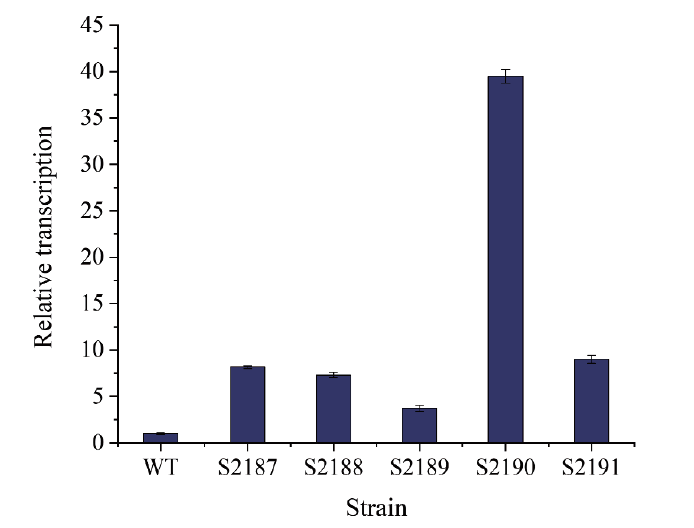
图4 PR31控制下hasA, hasB, hasC, hasD, hasE在FSB培养基中培养8 h相对于野生型的相对转录水平
Fig. 4 Relative transcriptions of hasA, hasB, hasC, hasD, and hasE controlled by PR31 cultured in FSB medium for 8 h, compared to the wild-type
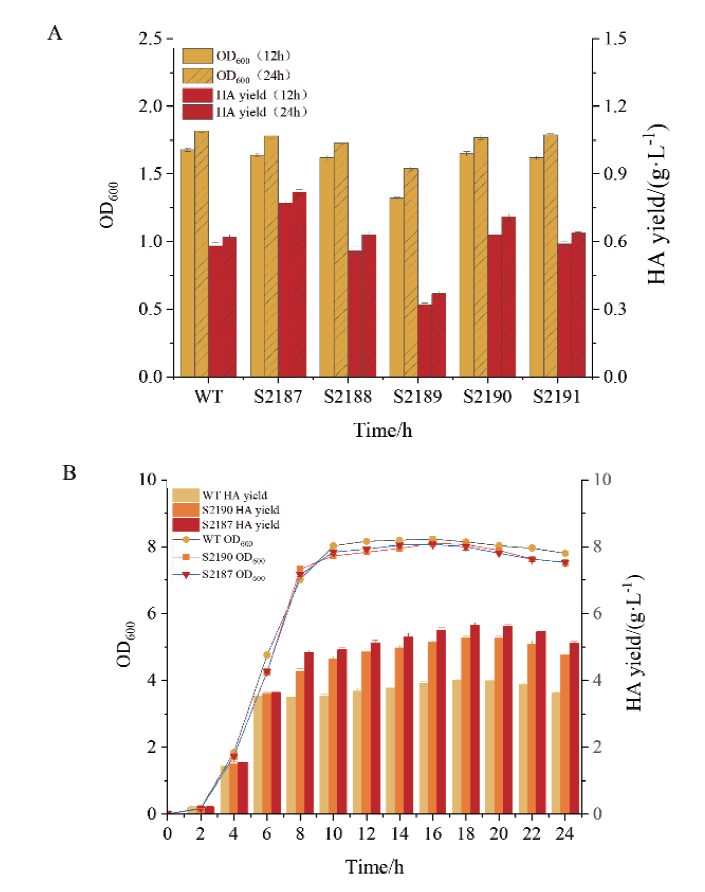
图5 菌株生长情况及HA产量 A:S2187(hasA), S2188(hasB), S2189(hasC), S2190(hasD), S2191(hasE)过表达菌株在摇瓶发酵12 h、24 h时的菌株生长情况以及HA产量;B:S2187(hasA),S2190(hasD)在2 L发酵罐中的生长曲线与HA产量
Fig. 5 Growth and HA yield of strains A: Growth and HA yield of hasA, hasB, hasC, hasD, and hasE overexpressing strains shaken at 12 h and 24 h. B: Growth curve and HA yield of S2187 (hasA) and S2190 (hasD) overexpressing strains cultured in 2 L fermenter
| [1] |
Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system[J]. Eur J Cell Biol, 2006, 85(8): 699-715.
doi: 10.1016/j.ejcb.2006.05.009 pmid: 16822580 |
| [2] | Graça MFP, Miguel SP, Cabral CSD, et al. Hyaluronic acid—based wound dressings: a review[J]. Carbohydr Polym, 2020, 241: 116364. |
| [3] | Diane R, Leonardo MS, Bruno F, et al. Hyaluronic acid-from production to application: a review[J]. Biointerface Res Appl Chem, 2022, 13(3): 211-233. |
| [4] | Zheng XL, Wang BT, Tang X, et al. Absorption, metabolism, and functions of hyaluronic acid and its therapeutic prospects in combination with microorganisms: a review[J]. Carbohydr Polym, 2023, 299: 120153. |
| [5] | Johnson ME, Murphy PJ, Boulton M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye[J]. Graefes Arch Clin Exp Ophthalmol, 2006, 244(1): 109-112. |
| [6] | Armstrong DC, Johns MR. Culture conditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepidemicus[J]. Appl Environ Microbiol, 1997, 63(7): 2759-2764. |
| [7] |
Pummill PE, DeAngelis PL. Alteration of polysaccharide size distribution of a vertebrate hyaluronan synthase by mutation[J]. J Biol Chem, 2003, 278(22): 19808-19814.
doi: 10.1074/jbc.M301097200 pmid: 12654925 |
| [8] |
Keasling JD. Manufacturing molecules through metabolic engineering[J]. Science, 2010, 330(6009): 1355-1358.
doi: 10.1126/science.1193990 pmid: 21127247 |
| [9] | Manfrão-Netto JHC, Queiroz EB, de Oliveira Junqueira AC, et al. Genetic strategies for improving hyaluronic acid production in recombinant bacterial culture[J]. J Appl Microbiol, 2022, 132(2): 822-840. |
| [10] |
Liu L, Liu YF, Li JH, et al. Microbial production of hyaluronic acid: current state, challenges, and perspectives[J]. Microb Cell Fact, 2011, 10: 99.
doi: 10.1186/1475-2859-10-99 pmid: 22088095 |
| [11] |
Afrasiabi S, Zanjani FSA, Ahmadian G, et al. The effect of manipulating glucuronic acid biosynthetic pathway in Bacillus subtilis strain on hyaluronic acid production[J]. AMB Express, 2023, 13(1): 63.
doi: 10.1186/s13568-023-01567-2 pmid: 37354246 |
| [12] | Woo JE, Seong HJ, Lee SY, et al. Metabolic engineering of Escher-ichia coli for the production of hyaluronic acid from glucose and galactose[J]. Front Bioeng Biotechnol, 2019, 7: 351. |
| [13] | Gomes AMV, Netto JHCM, Carvalho LS, et al. Heterologous hyaluronic acid production in Kluyveromyces lactis[J]. Microorganisms, 2019, 7(9): 294. |
| [14] | Chien LJ, Lee CK. Hyaluronic acid production by recombinant Lactococcus lactis[J]. Appl Microbiol Biotechnol, 2007, 77(2): 339-346. |
| [15] | Wang Y, Hu LT, Huang H, et al. Eliminating the capsule-like layer to promote glucose uptake for hyaluronan production by engineered Corynebacterium glutamicum[J]. Nat Commun, 2020, 11(1): 3120. |
| [16] |
Chong BF, Blank LM, McLaughlin R, et al. Microbial hyaluronic acid production[J]. Appl Microbiol Biotechnol, 2005, 66(4): 341-351.
doi: 10.1007/s00253-004-1774-4 pmid: 15599518 |
| [17] |
Hynes WL, Walton SL. Hyaluronidases of Gram-positive bacteria[J]. FEMS Microbiol Lett, 2000, 183(2): 201-207.
pmid: 10675584 |
| [18] | Mohan N, Pavan SS, Achar A, et al. Calorespirometric investigation of Streptococcus zooepidemicus metabolism: Thermodynamics of anabolic payload contribution by growth and hyaluronic acid synthesis[J]. Biochem Eng J, 2019, 152: 107367. |
| [19] | Beres SB, Sesso R, Pinto SWL, et al. Genome sequence of a Lancefield Group C Streptococcus zooepidemicus strain causing epidemic nephritis: new information about an old disease[J]. PLoS One, 2008, 3(8): e3026. |
| [20] | Cimini D, Iacono ID, Carlino E, et al. Engineering S. equi subsp. zooepidemicus towards concurrent production of hyaluronic acid and chondroitin biopolymers of biomedical interest[J]. AMB Express, 2017, 7(1): 61. |
| [21] | Chen WY, Marcellin E, Hung J, et al. Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus[J]. J Biol Chem, 2009, 284(27): 18007-18014. |
| [22] | Chen WY, Marcellin E, Steen JA, et al. The role of hyaluronic acid precursor concentrations in molecular weight control in Streptococcus zooepidemicus[J]. Mol Biotechnol, 2014, 56(2): 147-156. |
| [23] | Sun XY, Yang DD, Wang YY, et al. Development of a markerless gene deletion system for Streptococcus zooepidemicus: functional characterization of hyaluronan synthase gene[J]. Appl Microbiol Biotechnol, 2013, 97(19): 8629-8636. |
| [24] | Sun XQ, Wang Z, Bi YL, et al. Genetic and functional characterization of the hyaluronate lyase HylB and the beta-N-acetylglucosaminidase HylZ in Streptococcus zooepidemicus[J]. Curr Microbiol, 2015, 70(1): 35-42. |
| [25] | Gao WX, Xie YY, Zuo M, et al. Improved genetic transformation by disarmament of type II Restriction-Modification system in Streptococcus zooepidemicus[J]. 3 Biotech, 2022, 12(9): 192. |
| [26] | Sullivan MJ, Ulett GC. Stable expression of modified green fluorescent protein in group B streptococci to enable visualization in experimental systems[J]. Appl Environ Microbiol, 2018, 84(18): e01262-18. |
| [27] | Amado IR, Vázquez JA, Pastrana L, et al. Microbial production of hyaluronic acid from agro-industrial by-products: molasses and corn steep liquor[J]. Biochem Eng J, 2017, 117: 181-187. |
| [28] | de Macedo AC, Santana MHA. Hyaluronic acid depolymerization by ascorbate-redox effects on solid state cultivation of Streptococcus zooepidemicus in cashew apple fruit bagasse[J]. World J Microbiol Biotechnol, 2012, 28(5): 2213-2219. |
| [29] | Pires AMB, Macedo AC, Eguchi SY, et al. Microbial production of hyaluronic acid from agricultural resource derivatives[J]. Bioresour Technol, 2010, 101(16): 6506-6509. |
| [30] | Shah MV, Badle SS, Ramachandran KB. Hyaluronic acid production and molecular weight improvement by redirection of carbon flux towards its biosynthesis pathway[J]. Biochem Eng J, 2013, 80: 53-60. |
| [31] | Zhou SH, Ding RP, Chen J, et al. Obtaining a panel of cascade promoter-5'-UTR complexes in Escherichia coli[J]. ACS Synth Biol, 2017, 6(6): 1065-1075. |
| [32] | Song YF, Nikoloff JM, Fu G, et al. Promoter screening from Bacillus subtilis in various conditions hunting for synthetic biology and industrial applications[J]. PLoS One, 2016, 11(7): e0158447. |
| [33] | 刘秀霞, 赵子豪, 孙杨, 等. 谷氨酸棒杆菌内源表达元件的筛选[J]. 微生物学通报, 2016, 43(8): 1671-1678. |
| Liu XX, Zhao ZH, Sun Y, et al. Selection of endogenous expression elements from Corynebactrium glutamicum[J]. Microbiol China, 2016, 43(8): 1671-1678. | |
| [34] | 郑小娥, 王震, 刘浩. 兽疫链球菌 ATCC39920可控诱导表达系统的构建及其应用[J]. 生物技术通报, 2016, 32(12): 130-136. |
| Zheng XE, Wang Z, Liu H. Construction of controlled inducible expression system in Streptococcus zooepidemicus ATCC39920 and its application[J]. Biotechnol Bull, 2016, 32(12): 130-136. | |
| [35] | Chahuki FF, Aminzadeh S, Jafarian V, et al. Hyaluronic acid production enhancement via genetically modification and culture medium optimization in Lactobacillus acidophilus[J]. Int J Biol Macromol, 2019, 121: 870-881. |
| [36] | Jin P, Kang Z, Yuan PH, et al. Production of specific-molecular-weight hyaluronan by metabolically engineered Bacillus subtilis 168[J]. Metab Eng, 2016, 35: 21-30. |
| [1] | 岳丽昕, 王清华, 刘泽洲, 孔素萍, 高莉敏. 基于转录组和WGCNA筛选大葱雄性不育相关基因[J]. 生物技术通报, 2024, 40(9): 212-224. |
| [2] | 毋舒宁, 苏永平, 李冬雪, 柏映国, 刘波, 张志伟. 一种谷氨酸棒杆菌4-异丙基苯甲酸诱导型启动子的设计与应用[J]. 生物技术通报, 2024, 40(7): 108-116. |
| [3] | 高萌萌, 赵天宇, 焦馨悦, 林春晶, 关哲允, 丁孝羊, 孙妍妍, 张春宝. 大豆细胞质雄性不育系及其恢复系的比较转录组分析[J]. 生物技术通报, 2024, 40(7): 137-149. |
| [4] | 白志元, 徐菲, 杨午, 王明贵, 杨玉花, 张海平, 张瑞军. 大豆细胞质雄性不育弱恢复型杂种F1育性转变的转录组分析[J]. 生物技术通报, 2024, 40(6): 134-142. |
| [5] | 李梦然, 叶伟, 李赛妮, 张维阳, 李建军, 章卫民. Lithocarols类化合物生物合成基因litI的表达及其启动子功能分析[J]. 生物技术通报, 2024, 40(6): 310-318. |
| [6] | 秦健, 李振月, 何浪, 李俊玲, 张昊, 杜荣. 肌源性细胞分化的单细胞转录谱变化及细胞间通讯分析[J]. 生物技术通报, 2024, 40(6): 330-342. |
| [7] | 王周, 余杰, 王金华, 王永泽, 赵筱. 厌氧表达乳酸脱氢酶以提高大肠杆菌产D-乳酸光学纯度[J]. 生物技术通报, 2024, 40(5): 290-299. |
| [8] | 张清兰, 张亚冉, 鞠志花, 王秀革, 肖遥, 王金鹏, 魏晓超, 高亚平, 白福恒, 王洪程. 牛TARDBP基因核心启动子鉴定与转录调控分析[J]. 生物技术通报, 2024, 40(4): 306-318. |
| [9] | 杨淇, 魏子迪, 宋娟, 童堃, 杨柳, 王佳涵, 刘海燕, 栾维江, 马轩. 水稻组蛋白H1三突变体的创建和转录组学分析[J]. 生物技术通报, 2024, 40(4): 85-96. |
| [10] | 张洁萍, 关跃峰. 基于启动子编辑的作物育种[J]. 生物技术通报, 2024, 40(10): 53-61. |
| [11] | 朱毅, 柳唐镜, 宫国义, 张洁, 王晋芳, 张海英. 西瓜ClPP2C3克隆及表达分析[J]. 生物技术通报, 2024, 40(1): 243-249. |
| [12] | 刘玉玲, 王梦瑶, 孙琦, 马利花, 朱新霞. 启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响[J]. 生物技术通报, 2023, 39(9): 168-175. |
| [13] | 赵金玲, 安磊, 任晓亮. 单细胞转录组测序技术及其在秀丽隐杆线虫中的应用[J]. 生物技术通报, 2023, 39(6): 158-170. |
| [14] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [15] | 杨岚, 张晨曦, 樊学伟, 王阳光, 王春秀, 李文婷. 鸡 BMP15 基因克隆、表达模式及启动子活性分析[J]. 生物技术通报, 2023, 39(4): 304-312. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||