








生物技术通报 ›› 2022, Vol. 38 ›› Issue (4): 278-287.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0860
收稿日期:2021-07-02
出版日期:2022-04-26
发布日期:2022-05-06
作者简介:何亚伦,男,硕士,研究方向:膳食功能因子与肠道微生态;E-mail: 674859113@qq.com
基金资助:
HE Ya-lun( ), ZENG Li-rong, LIU Xiong, ZHANG Ling, WANG Qiong
), ZENG Li-rong, LIU Xiong, ZHANG Ling, WANG Qiong
Received:2021-07-02
Published:2022-04-26
Online:2022-05-06
摘要:
以正常饮食小鼠和高脂饮食诱导肥胖模型小鼠为对象,研究高剂量单宁酸对小鼠肠道屏障及肠道菌群的影响。通过H&E染色、RT-qPCR、16S rRNA测序等方法进行检测分析。研究发现高剂量单宁酸(400 mg/kg BW)灌胃可使小鼠的体重和进食量降低,增加小鼠体内各肠段内容物的含量,其中结肠内容物的含量显著增加。并且高剂量单宁酸干预后可造成肠道功能损伤及肠道屏障破坏,如杯状细胞数量和隐窝长度减少,及肠道紧密连接蛋白(ZO-1,Occludin及Claudin)表达降低。此外,口服高剂量单宁酸会导致小鼠结肠肠道菌群的多样性发生变化,并增加了SCFA产生菌Alistipes,Ruminococcus和 Blautia以及肥胖负相关菌群Alistipes和Oscillibacter的含量。结果表明,高剂量单宁酸对肠黏膜屏障的破坏影响了小鼠对食物的消化吸收,这可能是高剂量单宁酸干预后小鼠体重快速下降的主要潜在原因。另一方面,高剂量单宁酸所造成的肠道菌群变化也会对小鼠的体重产生影响。
何亚伦, 曾丽荣, 刘雄, 张铃, 王琼. 高剂量单宁酸对小鼠肠道屏障和肠道菌群的影响[J]. 生物技术通报, 2022, 38(4): 278-287.
HE Ya-lun, ZENG Li-rong, LIU Xiong, ZHANG Ling, WANG Qiong. Effects of High-dose Tannic Acid on the Intestinal Barrier Function and Gut Microbiota in Mice[J]. Biotechnology Bulletin, 2022, 38(4): 278-287.
| Gene | Forward primer(5'-3') | Reverse primer(5'-3') |
|---|---|---|
| ZO 1 | GCTTTAGCGAACAGAAGGAGC | TTCATTTTTCCGAGACTTCACCA |
| Occludin | CACACTTGCTTGGGACAGAG | TAGCCATAGCCTCCATAGCC |
| GAPDH | TGGCCTTCCGTGTTCCTAC | GAGTTGCTGTTGAAGTCGCA |
表1 RT-qPCR引物序列
Table 1 Primers’ sequences for RT-qPCR
| Gene | Forward primer(5'-3') | Reverse primer(5'-3') |
|---|---|---|
| ZO 1 | GCTTTAGCGAACAGAAGGAGC | TTCATTTTTCCGAGACTTCACCA |
| Occludin | CACACTTGCTTGGGACAGAG | TAGCCATAGCCTCCATAGCC |
| GAPDH | TGGCCTTCCGTGTTCCTAC | GAGTTGCTGTTGAAGTCGCA |

图1 高剂量单宁酸对小鼠体重的影响 A:4组小鼠在实验期间的体重变化情况;B:实验结束后小鼠的体重增加量;C:实验期间各组小鼠的周均饮食摄入量;D:实验结束后各组小鼠的脂肪组织重量。误差线表示均值±标准误,*(0.01<P<0.05),**(0.001<P<0.01),***(P<0.001),下同
Fig. 1 Effects of high-dose tannic acid on the body weights of the mice A:Changes in the body weights of the four groups of mice during the experiment. B:Weight gains of mice after the end of the experiment. C:The weekly dietary intake of each group during the experiment. D:The weight of adipose tissue in each group after the end of the experiment. The error lines in the figure all are the mean ± standard error,and *(0.01<P<0.05),**(0.001<P<0.01),and ***(P<0.001),the same below
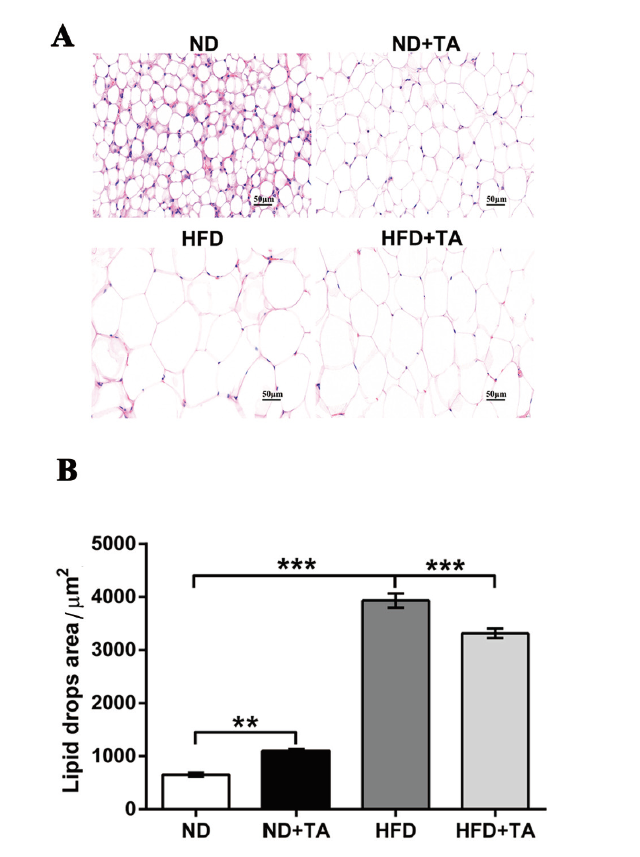
图2 高剂量单宁酸对小鼠体脂的影响 A:脂肪组织H&E染色切片;B:脂滴面积
Fig. 2 Effects of high-dose tannic acid on the body fats of the mice A:H&E staining sections of adipose tissue. B:Fat droplet area

图3 高剂量单宁酸对肠道结构的影响 A:小鼠肠道组织重量;B:小鼠肠道组织内容物重量;C:结肠组织H&E染色;D:杯状细胞数量;E:隐窝长度;F:组织损伤评分
Fig. 3 Effects of high-dose tannic acid on the intestinal structure A:Weights of intestinal tissues of the mice. B:Weights of intestinal tissue contents of the mice. C:H&E staining of colon tissue. D:Number of goblet cells. E:Crypt length. F:Tissue damage score
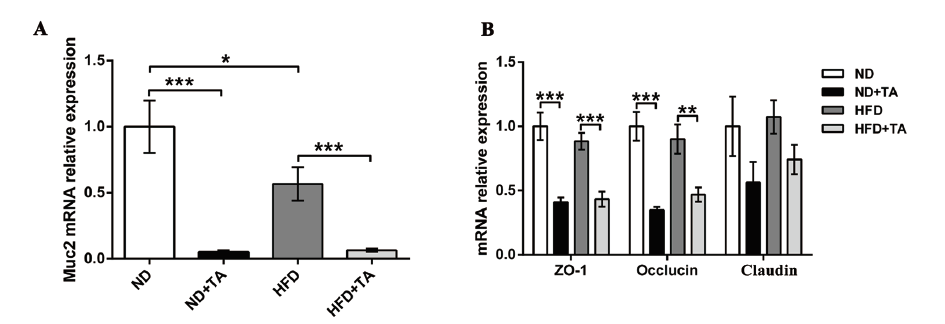
图4 高剂量单宁酸对肠道屏障的影响 A:肠道Muc2 mRNA含量的变化;B:肠道屏障功能相关基因mRNA含量的变化
Fig. 4 Effect of high dose tannic acid on the intestinal barrier A:Changes in intestinal Muc2 mRNA content. B:Changes in the mRNA levels of genes related to intestinal barrier function
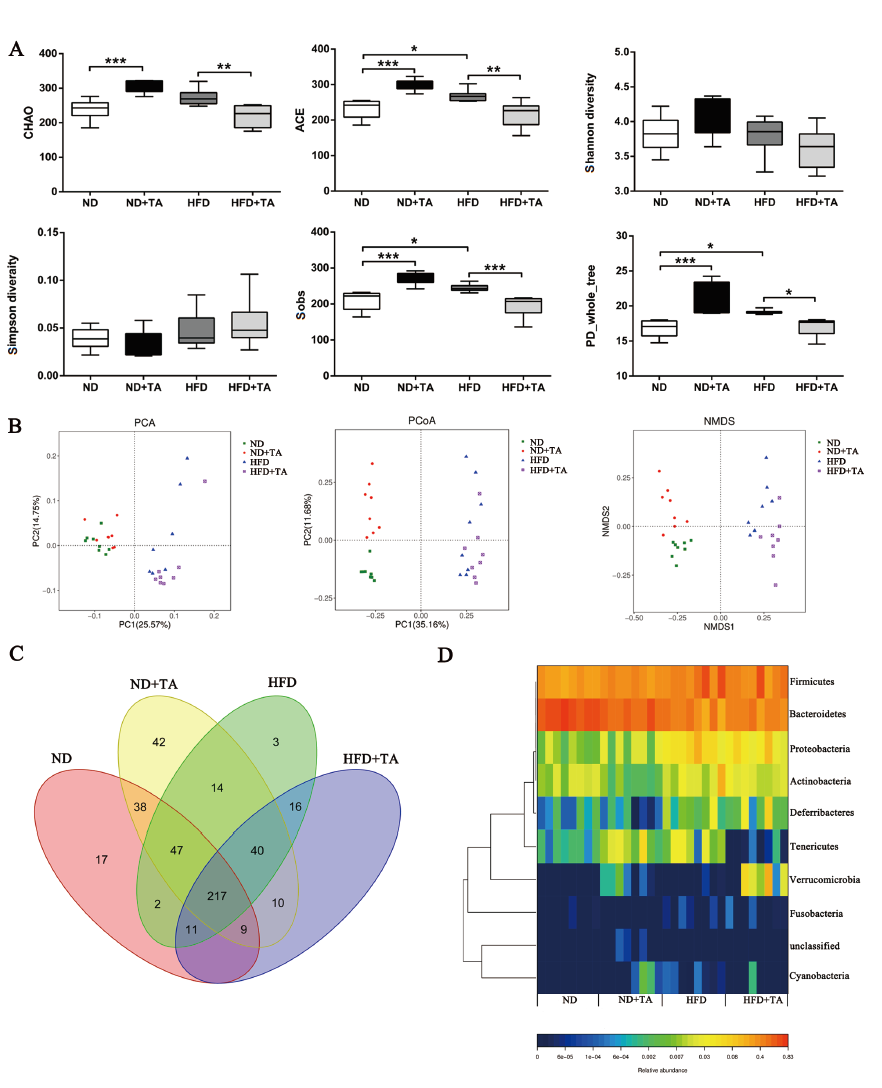
图5 高剂量单宁酸对肥胖小鼠肠道微生物多样性的影响 A:Alpha多样性指数分析;B:Beta多样性分析;C:Venn图;D:门水平热图
Fig. 5 Effects of high dose tannic acid on the intestinal microbial diversity of the obese mice A:Alpha diversity index analysis. B:Beta diversity analysis. C:Venn diagram. D:Heatmap at the phylum level

图6 在门水平上的物种组成成分及组间差异分析 A:门水平群落Bar图;B、C:组间差异分析-多组比较
Fig. 6 Analysis of species composition and inter-group differences at the phylum level A:Community bar plot at the phylum level. B and C:Intergroup difference analysis - multi-group comparison
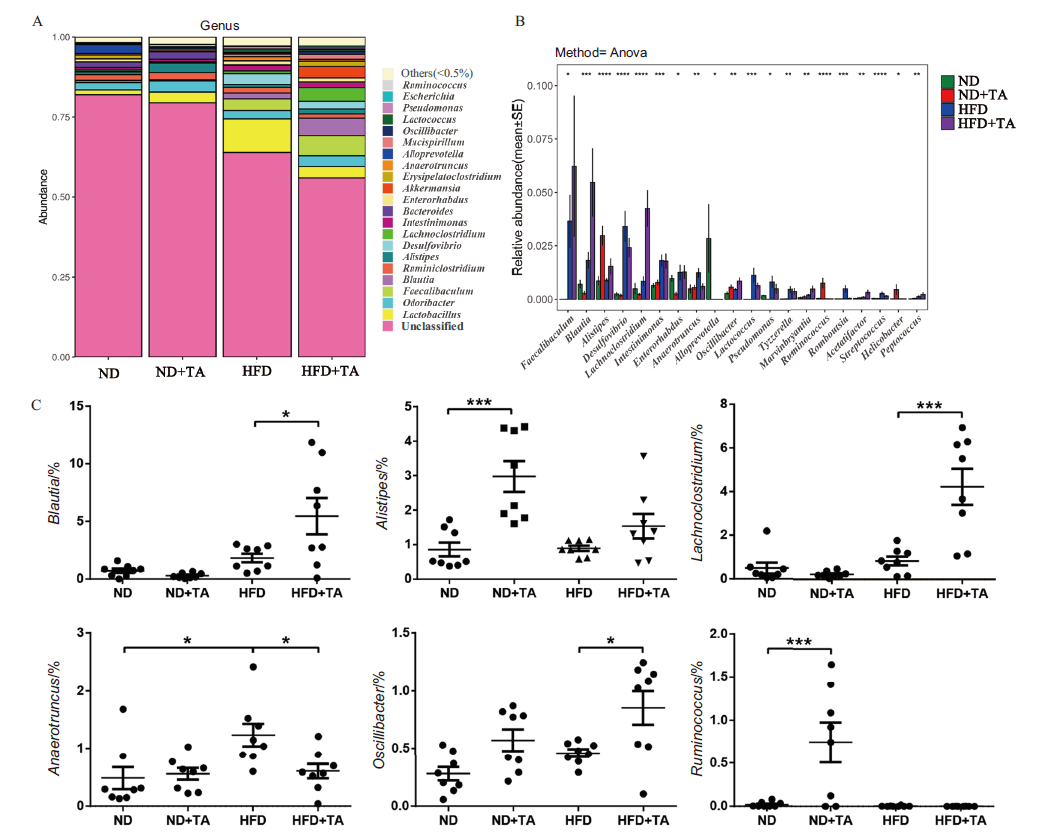
图7 在属水平上的物种组成成分及组间差异分析 A:属水平群落Bar图;B、C:组间差异分析-多组比较
Fig. 7 Analysis of species composition and inter-group differences at the genus level A:Community bar plot at the genus level. B and C:Intergroup difference analysis - multi-group comparison
| [1] |
Chung KT, Wong TY, Wei CI, et al. Tannins and human health:a review[J]. Crit Rev Food Sci Nutr, 1998, 38(6):421-464.
pmid: 9759559 |
| [2] |
Sanyal R, Darroudi F, et al. Inhibition of the genotoxic effects of heterocyclic amines in human derived hepatoma cells by dietary bioantimutagens[J]. Mutagenesis, 1997, 12(4):297-303.
doi: 10.1093/mutage/12.4.297 pmid: 9237777 |
| [3] |
Chen X, Beutler JA, McCloud TG, et al. Tannic acid is an inhibitor of CXCL12(SDF-1alpha)/CXCR4 with antiangiogenic activity[J]. Clin Cancer Res, 2003, 9(8):3115-3123.
pmid: 12912963 |
| [4] | Ngobili TA, Shah H, Park JP, et al. Remodeling of tannic acid crosslinked collagen type I induces apoptosis in ER+ breast cancer cells[J]. Anticancer Res, 2015, 35(3):1285-1290. |
| [5] |
Wang CC, Chen LG, Yang LL. Cuphiin D1, the macrocyclic hydrolyzable tannin induced apoptosis in HL-60 cell line[J]. Cancer Lett, 2000, 149(1/2):77-83.
doi: 10.1016/S0304-3835(99)00344-4 URL |
| [6] |
Yang LL, Lee CY, Yen KY. Induction of apoptosis by hydrolyzable tannins from Eugenia jambos L. on human leukemia cells[J]. Cancer Lett, 2000, 157(1):65-75.
pmid: 10893444 |
| [7] |
Uchiumi F, Sato T, Tanuma SI. Identification and characterization of a tannic acid-responsive negative regulatory element in the mouse mammary tumor virus promoter[J]. J Biol Chem, 1998, 273(20):12499-12508.
doi: 10.1074/jbc.273.20.12499 pmid: 9575208 |
| [8] |
Sun YY, Zhang TH, Wang BD, et al. Tannic acid, an inhibitor of poly(ADP-ribose)glycohydrolase, sensitizes ovarian carcinoma cells to cisplatin[J]. Anti Cancer Drugs, 2012, 23(9):979-990.
doi: 10.1097/CAD.0b013e328356359f URL |
| [9] |
Athar M, Khan WA, Mukhtar H. Effect of dietary tannic acid on epidermal, lung, and forestomach polycyclic aromatic hydrocarbon metabolism and tumorigenicity in Sencar mice[J]. Cancer Res, 1989, 49(21):5784-5788.
pmid: 2507136 |
| [10] |
Chen SC, Chung KT. Mutagenicity and antimutagenicity studies of tannic acid and its related compounds[J]. Food Chem Toxicol, 2000, 38(1):1-5.
pmid: 10685008 |
| [11] |
Ahmad ST, Sultana S. Tannic acid mitigates cisplatin-induced nephrotoxicity in mice[J]. Hum Exp Toxicol, 2012, 31(2):145-156.
doi: 10.1177/0960327111414282 pmid: 21724663 |
| [12] | Boyd EM, Bereczky K, Godi I. The acute toxicity of tannic acid administered intragastrically[J]. Can Med Assoc J, 1965, 92:1292-1297. |
| [13] |
Al-Mamary M, Molham AH, Abdulwali AA, et al. In vivo effects of dietary Sorghum tannins on rabbit digestive enzymes and mineral absorption[J]. Nutr Res, 2001, 21(10):1393-1401.
doi: 10.1016/S0271-5317(01)00334-7 URL |
| [14] |
Hervás G, Pérez V, et al. Intoxication of sheep with quebracho tannin extract[J]. J Comp Pathol, 2003, 129(1):44-54.
pmid: 12859907 |
| [15] |
Smith AH, Mackie RI. Effect of condensed tannins on bacterial diversity and metabolic activity in the rat gastrointestinal tract[J]. Appl Environ Microbiol, 2004, 70(2):1104-1115.
doi: 10.1128/AEM.70.2.1104-1115.2004 URL |
| [16] |
Larraín RE, Richards MP, Schaefer DM, et al. Growth performance and muscle oxidation in rats fed increasing amounts of high-tannin Sorghum[J]. J Anim Sci, 2007, 85(12):3276-3284.
pmid: 17709777 |
| [17] |
Molan AL, Waghorn GC, et al. The effect of condensed tannins from seven herbages on Trichostrongylus colubriformis larval migration in vitro[J]. Folia Parasitol, 2000, 47(1):39-44.
doi: 10.14411/fp.2000.007 URL |
| [18] |
Funatogawa K, Hayashi S, et al. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori[J]. Microbiol Immunol, 2004, 48(4):251-261.
pmid: 15107535 |
| [19] | 孙展英, 李建涛, 陈宝江. 单宁酸对仔猪生长性能、营养物质利用率及相关消化酶活性的影响[J]. 饲料研究, 2014(1):46-49. |
| Sun ZY, Li JT, Chen BJ. Effects of tannic acid on growth performance, nutrient utilization and related digestive enzyme activities of piglets[J]. Feed Res, 2014(1):46-49. | |
| [20] |
Schiavone A, Guo K, et al. Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks[J]. Poult Sci, 2008, 87(3):521-527.
doi: 10.3382/ps.2007-00113 URL |
| [21] | 刘起胜, 徐筱红, 刘怀, 等. 七味白术散对菌群失调腹泻小鼠肠绒毛和隐窝的影响[J]. 中国中医药现代远程教育, 2014, 12(23):154-155. |
| Liu QS, Xu XH, Liu H, et al. Effects on intestinal villi and crypts in flora diarrhea mice by qiweibaizhu powder[J]. Chin Med Mod Distance Educ China, 2014, 12(23):154-155. | |
| [22] | 黄永洁. 低聚木糖对断奶仔猪肠道pH和肠黏膜形态结构的影响[J]. 现代畜牧兽医, 2014(5):23-27. |
| Huang YJ. Effects of xylo-oligosaccharides on intestinal pH and intestinal mucosal morphological structure of weaned piglets[J]. Mod J Animal Husb Vet Med, 2014(5):23-27. | |
| [23] | 魏小兵, 张秀林, 等. 无抗发酵饲粮对猪小肠黏膜形态和杯状细胞的影响[J]. 动物营养学报, 2019, 31(4):1797-1805. |
| Wei XB, Zhang XL, et al. Effects of fermentation diet without antibiotic on intestinal mucosal morphology and goblet cells of piglets[J]. Chin J Animal Nutr, 2019, 31(4):1797-1805. | |
| [24] |
Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions[J]. PNAS, 2011, 108(Suppl 1):4659-4665.
doi: 10.1073/pnas.1006451107 URL |
| [25] |
Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population[J]. BMC Microbiol, 2017, 17(1):120.
doi: 10.1186/s12866-017-1027-1 URL |
| [26] | Liu X, Mao B, et al. Blautia-a new functional genus with potential probiotic properties?[J]. Gut Microbes, 2021, 13(1):1-21. |
| [27] | Benítez-Páez A, Gómez del Pugar EM, López-Almela I, et al. Depletion of Blautia species in the microbiota of obese children relates to intestinal inflammation and metabolic phenotype worsening[J]. mSystems, 2020, 5(2):e00857-19. |
| [28] |
Thingholm LB, Rühlemann MC, Koch M, et al. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition[J]. Cell Host Microbe, 2019, 26(2):252-264.
doi: S1931-3128(19)30348-8 pmid: 31399369 |
| [1] | 沙珊珊, 董世荣, 杨玉菊. 肠道菌群及代谢物调控宿主肠道免疫的研究进展[J]. 生物技术通报, 2023, 39(8): 126-136. |
| [2] | 熊淑琪. 胆汁酸生理功能及其与肠道微生物互作研究进展[J]. 生物技术通报, 2023, 39(4): 187-200. |
| [3] | 王松, 简晓平, 潘婉舒, 张永光, 王涛, 游玲. 玉米小曲酒糟发酵饲料对育肥猪肠道菌群的影响[J]. 生物技术通报, 2022, 38(9): 248-257. |
| [4] | 陈天赐, 武少兰, 杨国辉, 江丹霞, 江玉姬, 陈炳智. 无柄灵芝醇提物对小鼠睡眠及肠道菌群的影响[J]. 生物技术通报, 2022, 38(8): 225-232. |
| [5] | 钟明月, 刘春妍, 颜妍, 张晓慧, 原海升, 徐国全, 张和平, 王玉珍. 乳双歧杆菌V9对高脂饮食诱导的NAFLD大鼠的改善作用[J]. 生物技术通报, 2022, 38(3): 181-187. |
| [6] | 金秋霞, 王思宏, 金丽华. 果蝇肠道干细胞及肠道菌群的研究进展[J]. 生物技术通报, 2021, 37(4): 245-250. |
| [7] | 李海超, 谢飞, 张园琦, 关若冰. 不同抗、感水稻品种对褐飞虱肠道菌群的影响[J]. 生物技术通报, 2021, 37(3): 1-9. |
| [8] | 胡紫媛, 夏嫱. 昆虫肠道菌群组学研究及功能和应用进展[J]. 生物技术通报, 2021, 37(1): 102-112. |
| [9] | 杨立杰, 曾祥芳, 谯仕彦. 非淀粉多糖在猪肠道菌群调控中的研究进展[J]. 生物技术通报, 2020, 36(2): 9-16. |
| [10] | 吴芹, 许子洋, 刘丽萍, 张文英, 宋思远. 肠道菌群在应激诱发大鼠高血压中的作用[J]. 生物技术通报, 2020, 36(2): 83-90. |
| [11] | 陈玉青, 严新, 陈明军, 林娈, 杨成凤, 李秋哲, 刘斌, 赵超,. 淡黑巨藻醇提取物降血糖活性及其对小鼠肠道菌群的影响[J]. 生物技术通报, 2017, 33(12): 162-169. |
| [12] | 刘冬恋, 廖梦玲, 周欢. 基于高通量测序技术分析糖尿病与肠道菌群相关性究进展[J]. 生物技术通报, 2016, 32(9): 59-64. |
| [13] | 赵洁 ,马晨 ,席晓敏, 张和平. 实时荧光定量PCR技术在肠道微生物领域中的研究进展[J]. 生物技术通报, 2014, 0(12): 61-66. |
| [14] | 赵娜,刘社兰,路纪琪,何宏轩,赵宝华. 中国恒河猴肠道菌群特征揭示[J]. 生物技术通报, 2013, 0(7): 153-160. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||