








生物技术通报 ›› 2022, Vol. 38 ›› Issue (4): 269-277.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0148
收稿日期:2021-02-04
出版日期:2022-04-26
发布日期:2022-05-06
作者简介:王玥,女,硕士,助理研究员,研究方向:应用微生物学;E-mail: 597646928@qq.com;王玥同为本文通讯作者
基金资助:
WANG Yue( ), GAO Qing-hua, DONG Cong, LUO Tong-yang, WANG Qing-qing
), GAO Qing-hua, DONG Cong, LUO Tong-yang, WANG Qing-qing
Received:2021-02-04
Published:2022-04-26
Online:2022-05-06
摘要:
吡喃糖氧化酶(pyranose oxidase,PROD,简称P2O)在木质素降解、碳水化合物的生物转化合成中具有重要作用,还应用于生物燃料电池、生物传感器以及临床诊断分析中。本实验室进行了吡喃糖氧化酶在毕赤酵母中的高效表达及酶学性质研究,为以后吡喃糖氧化酶的工业化高效生产提供理论依据。利用生物技术方法,基于毕赤酵母密码子偏好性优化吡喃糖氧化酶基因密码子。通过基因外源表达技术构建吡喃糖氧化酶重组毕赤酵母GS115菌株以实现吡喃糖氧化酶的高效表达,并对重组吡喃糖氧化酶的酶学性质进行研究。优化高产重组菌株的发酵条件,在10 L发酵罐中进行扩大培养。结果显示,吡喃糖氧化酶密码子优化后,其在毕赤酵母中实现了高效表达。在10 L发酵罐经过132 h诱导表达后,酶活达220 U/mL。酶学性质研究发现:重组吡喃糖氧化酶最适作用温度为55℃,在不超过60℃条件下热稳定性良好。该酶作用的最适pH为6.5,在pH 5-9条件下,其相对酶活高于50%。P2O在较广的pH范围内均表现出较高的稳定性,尤其是对碱性条件有较强的耐性。金属离子Cu2+ 对酶活的抑制作用较大。底物特异性检测结果显示,该重组酶的最适底物为D-葡萄糖。该研究成功构建了密码子优化的吡喃糖氧化酶的重组表达质粒pPIC9K-P2O,并在毕赤酵母中实现了高效表达。
王玥, 高庆华, 董聪, 罗同阳, 王庆庆. 密码子优化的吡喃糖氧化酶基因在毕赤酵母中的表达[J]. 生物技术通报, 2022, 38(4): 269-277.
WANG Yue, GAO Qing-hua, DONG Cong, LUO Tong-yang, WANG Qing-qing. Expression of Pyranose Oxidase with Optimized Codon in Pichia pastoris[J]. Biotechnology Bulletin, 2022, 38(4): 269-277.

图1 密码子优化后的基因序列 方框内序列分别为 EcoR I 和 Not I的酶切位点;划线序列为6个组氨酸标签
Fig. 1 Gene sequence after the optimization of codons The boxed sequences indicate restriction sites EcoR Iand Not I. The underlined sequences show 6×His-tag
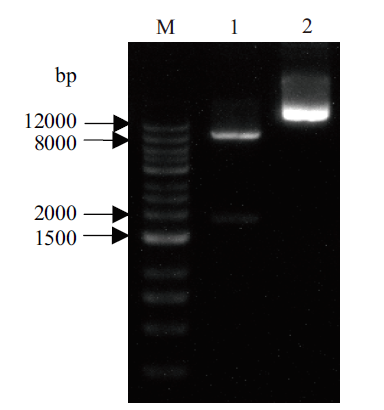
图2 pPIC9K-P2O质粒双酶切验证 M:DNA marker;1:pPIC9K-P2O质粒经EcoR I和Not I双酶切;2:pPIC9K-P2O质粒
Fig. 2 Verification of pPIC9K-P2O digested by double enzymes M:DNA marker. 1:Digestion of pPIC9K-P2O by EcoR I and Not I.2:pPIC9K-P2O plasmid
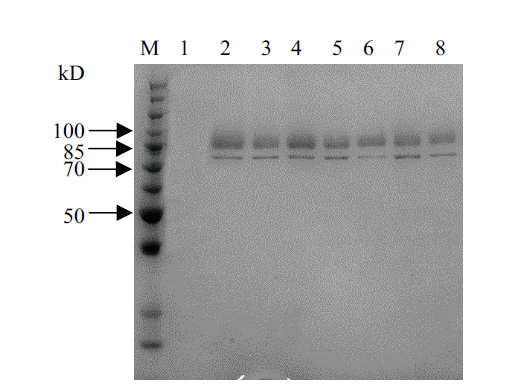
图5 重组蛋白的SDS-PAGE结果 M:蛋白 marker;1:对照发酵上清液;2-8:阳性转化子发酵上清液
Fig. 5 SDS-PAGE result of recombinant protein M:Protein marker. 1:Supernatant of negative control. 2-8:Supernatant of positive transformants

图7 10 L 发酵罐高密度发酵生产 P2O A:P2O酶活生长曲线;B:SDS-PAGE 结果;M:蛋白 marker;1-12:0、12、24、36、48、60、72、84、96、108、120、132 h后的重组蛋白积累量
Fig. 7 Production of P2O by high-density fermentation in 10 L bioreactor A:Growth curve of P2O activity. B:SDS-PAGE result. M:Protein marker. 1-12:Accumulation of recombinant protein after 0,12,24,36,48,60,72,84,96,108,120 and 132 h

图8 最适pH及pH稳定性 A:30℃不同pH条件下P2O相对酶活;B:30℃不同pH条件下保温24 h后P2O相对酶活;缓冲液分别是100 mmol/L 柠檬酸-柠檬酸钠(■,黑线),磷酸钾(●,红线),Tris-HCl(▲,蓝线),甘氨酸-NaOH(▼,粉线)
Fig. 8 Optimal pH and pH stability A:Relative activity at different pH conditions at 30℃. B:The relative enzyme activity of P2O kept in the same temperature for 24 h at different pH conditions at 30℃. The used buffers were 100 mmol/L sodium citrate(■,black line),potassium phosphate(●,red line),Tris-HCl(▲,blue line)and glycine-NaOH(▼,pink line)
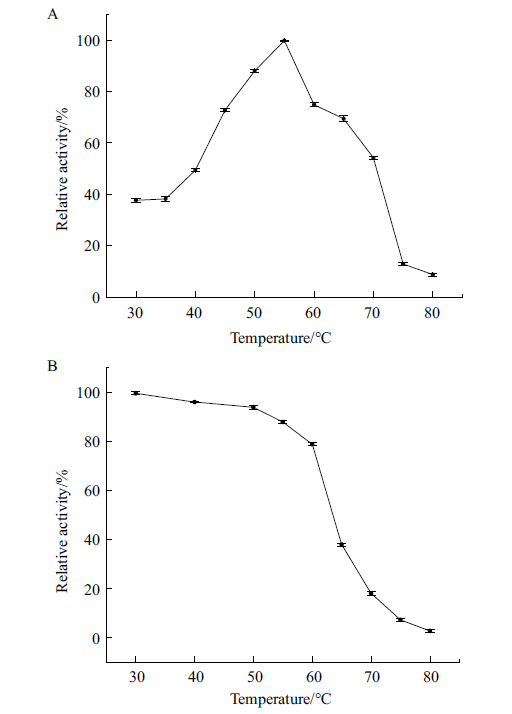
图9 最适温度及温度稳定性 A:在pH 6.5,不同温度下P2O相对酶活;B:在pH 6.5,不同温度下保温30 min后P2O相对酶活
Fig. 9 Optimal temperature and temperature stability A:Relative activity of P2O at different temperatures at pH 6.5. B:The relative enzyme activity of P2O kept in the different temperatures for 30 min at pH 6.5
| [1] |
Ai MQ, Wang FF, Zhang YZ, et al. Purification of pyranose oxidase from the white rot fungus Irpex lacteus and its cooperation with laccase in lignin degradation[J]. Process Biochem, 2014, 49(12):2191-2198.
doi: 10.1016/j.procbio.2014.10.001 URL |
| [2] |
Wongnate T, Chaiyen P. The substrate oxidation mechanism of pyranose 2-oxidase and other related enzymes in the glucose-methanol-choline superfamily[J]. FEBS J, 2013, 280(13):3009-3027.
doi: 10.1111/febs.12280 URL |
| [3] |
Albrecht M, Lengauer T. Pyranose oxidase identified as a member of the GMC oxidoreductase family[J]. Bioinformatics, 2003, 19(10):1216-1220.
pmid: 12835264 |
| [4] |
Leitner C, Volc J, Haltrich D. Purification and characterization of pyranose oxidase from the white rot fungus Trametes multicolor[J]. Appl Environ Microbiol, 2001, 67(8):3636-3644.
doi: 10.1128/AEM.67.8.3636-3644.2001 URL |
| [5] |
Giffhorn F. Fungal pyranose oxidases:occurrence, properties and biotechnical applications in carbohydrate chemistry[J]. Appl Microbiol Biotechnol, 2000, 54(6):727-740.
pmid: 11152063 |
| [6] | 刘敏, 王彦. 1, 5-脱水葡萄糖醇与糖尿病及其并发症[J]. 国际内分泌代谢杂志, 2018, 38(2):106-109. |
| Liu M, Wang Y. 1,5-anhydroglucitol in diabetes and its compllications[J]. Int J Endocrinol Metab, 2018, 38(2):106-109. | |
| [7] |
Bannwarth M, Bastian S, Heckmann-Pohl D, et al. Crystal structure of pyranose 2-oxidase from the white-rot fungus Peniophora sp[J]. Biochemistry, 2004, 43(37):11683-11690.
pmid: 15362852 |
| [8] |
Vecerek B, Maresová H, Kocanová M, et al. Molecular cloning and expression of the pyranose 2-oxidase cDNA from Trametes ochracea MB49 in Escherichia coli[J]. Appl Microbiol Biotechnol, 2004, 64(4):525-530.
pmid: 14689250 |
| [9] |
de Koker TH, Mozuch MD, Cullen D, et al. Isolation and purification of pyranose 2-oxidase from Phanerochaete chrysosporium and characterization of gene structure and regulation[J]. Appl Environ Microbiol, 2004, 70(10):5794-5800.
doi: 10.1128/AEM.70.10.5794-5800.2004 URL |
| [10] |
Nishimura I, Okada K, Koyama Y. Cloning and expression of pyranose oxidase cDNA from Coriolus versicolor in Escherichia coli[J]. J Biotechnol, 1996, 52(1):11-20.
pmid: 9025322 |
| [11] |
Dietrich D, Crooks C. Gene cloning and heterologous expression of pyranose 2-oxidase from the brown-rot fungus, Gloeophyllum trabeum[J]. Biotechnol Lett, 2009, 31(8):1223-1228.
doi: 10.1007/s10529-009-9983-7 pmid: 19343506 |
| [12] |
Takakura Y, Kuwata S. Purification, characterization, and molecular cloning of a pyranose oxidase from the fruit body of the basidiomycete, Tricholoma matsutake[J]. Biosci Biotechnol Biochem, 2003, 67(12):2598-2607.
doi: 10.1271/bbb.67.2598 URL |
| [13] | 杨运桂, 童芹, 郑卫东, 等. 分子伴侣过量表达对蛋白质分泌及可溶性的影响[J]. 中国生物化学与分子生物学报, 2000, 16(3):382-387. |
| Yang YG, Tong Q, Zheng WD, et al. The effect of over-expression of chaperones on the secretion and the solubility of proteins[J]. Chin J Biochem Mol Biol, 2000, 16(3):382-387. | |
| [14] | 陆海, 吴薇, 曾庆银, 等. 大肠杆菌BL21(DE3)中表达重组蛋白的研究[J]. 北京林业大学学报, 2001, 23(6):1-4. |
| Lu H, Wu W, Zeng QY, et al. The expression analysis of recombinant protein in Escherichia coli BL21(DE3)[J]. J Beijing For Univ, 2001, 23(6):1-4. | |
| [15] | 彭凌, 朱必凤, 刘主. 包涵体复性研究[J]. 韶关学院学报, 2007, 28(9):89-93. |
| Peng L, Zhu BF, Liu Z. Refolding of genetic engineering protein expressed as inclusion bodies[J]. J Shaoguan Univ, 2007, 28(9):89-93. | |
| [16] | Palmer I, Wingfield PT. Preparation and extraction of insoluble(inclusion-body)proteins from Escherichia coli[J]. Curr Protoc Protein Sci, 2012, Chapter 6: Unit6. 3. |
| [17] | 郭井利, 彭永刚, 刘鹏, 等. 重组蛋白在大肠杆菌中包涵体蛋白的表达及其复性[J]. 畜牧兽医科技信息, 2005(8):66-68. |
| Guo JL, Peng YG, Liu P, et al. Expression and renaturation of recombinant inclusion body protein in Escherichia coli[J]. Sci Inf Animal Husbamdry Vet Mecicine, 2005(8):66-68. | |
| [18] | 邝爱丽, 陈圆圆, 彭志峰, 等. 包涵体的形成原因及其处理方法[J]. 上海畜牧兽医通讯, 2009(1):62-63. |
| Kuang AL, Chen YY, Peng ZF, et al. The formation of inclusion body and its treatment[J]. Shanghai J Animal Husb Vet Med, 2009(1):62-63. | |
| [19] |
Wu PC, Chien MS, Tseng YY, et al. Expression of the porcine Circovirus type 2 capsid protein subunits and application to an indirect ELISA[J]. J Biotechnol, 2008, 133(1):58-64.
doi: 10.1016/j.jbiotec.2007.09.015 URL |
| [20] | Selleck W, Tan S. Recombinant protein complex expression in E. coli[J]. Curr Protoc Protein Sci, 2008, Chapter 5:Unit 5. 21. |
| [21] | 郜赵伟. 葡萄糖氧化酶基因密码子优化及其在毕赤酵母中的高效表达[D]. 重庆:西南大学, 2010. |
| Gao ZW. Codon optimization and high level expression in Pichia pastoris of glucose oxidase[D]. Chongqing:Southwest University, 2010. | |
| [22] | 孙风敏, 韩焱, 李文利. 基于密码子优化的蛋白酶K在毕赤酵母中的表达及分离纯化[J]. 微生物学通报, 2014, 41(11):2198-2207. |
| Sun FM, Han Y, Li WL. Expression and purification of Codon optimized proteinase K in Pichia pastoris[J]. Microbiol China, 2014, 41(11):2198-2207. | |
| [23] |
高庆华, 胡美荣, 吴芳彤, 等. 点青霉葡萄糖氧化酶基因的克隆及其酶学性质研究[J]. 生物技术通报, 2016, 32(7):152-159.
doi: 10.13560/j.cnki.biotech.bull.1985.2016.07.023 |
| Gao QH, Hu MR, Wu FT, et al. Cloning of gene for a glucose oxidase from Penicillium notatum and its enzymatic properties[J]. Biotechnol Bull, 2016, 32(7):152-159. | |
| [24] | 张可可. 一种新的细菌吡喃糖氧化酶的表达及其催化性质研究[D]. 长沙:湖南大学, 2017. |
| Zhang KK. Expression and catalytic characterization of a new bacterial pyranose oxidase[D]. Changsha:Hunan University, 2017. | |
| [25] |
Hallberg BM, Leitner C, Haltrich D, et al. Crystal structure of the 270 kDa homotetrameric lignin-degrading enzyme pyranose 2-oxidase[J]. J Mol Biol, 2004, 341(3):781-796.
pmid: 15288786 |
| [26] | 周亚萍, 于海燕, 朱钰峰, 等. 吡喃糖氧化酶及其表达纯化方法和应用:CN105861456A[P]. 2016-08-17. |
| Zhou YP, Yu HY, Shu YF. et al. Pyranose oxidase and expression and purification method and application thereof:CN105861456A[P]. 2016-08-17. | |
| [27] |
Danneel HJ, Rössner E, Zeeck A, et al. Purification and characterization of a pyranose oxidase from the basidiomycete Peniophora gigantea and chemical analyses of its reaction products[J]. Eur J Biochem, 1993, 214(3):795-802.
pmid: 8319689 |
| [28] |
Schneider K, Dorscheid S, Witte K, et al. Controlled feeding of hydrogen peroxide as oxygen source improves production of 5-ketofructose from L-sorbose using engineered pyranose 2-oxidase from Peniophora gigantea[J]. Biotechnol Bioeng, 2012, 109(11):2941-2945.
doi: 10.1002/bit.24572 URL |
| [29] |
Tamaki T, Ito T, Yamaguchi T. Immobilization of hydroquinone through a spacer to polymer grafted on carbon black for a high-surface-area biofuel cell electrode[J]. J Phys Chem B, 2007, 111(34):10312-10319.
doi: 10.1021/jp074334n URL |
| [30] | 陈颖, 李莎, 胡婷, 等. 吡喃糖氧化酶法检测1, 5-脱水葡萄糖醇的空白限、检出限和功能灵敏度的建立及评价[J]. 检验医学与临床, 2019, 16(15):2131-2133. |
| Chen Y, Li S, Hu T, et al. Establishment and evaluation of blank limit, detection limit and function sensitivity of pyranose oxidase method for detecting 1, 5-anhydroglucitol[J]. Lab Med Clin, 2019, 16(15):2131-2133. | |
| [31] | 王海生, 周亚萍, 于海燕, 等. 吡喃糖氧化酶的原核表达及初步实验应用[J]. 国际检验医学杂志, 2019, 40(7):774-777. |
| Wang HS, Zhou YP, Yu HY, et al. Prokaryotic expression and initial experimental application of pyranose oxidase[J]. Int J Lab Med, 2019, 40(7):774-777. |
| [1] | 赵思佳, 王晓璐, 孙纪录, 田健, 张杰. 代谢工程改造毕赤酵母生产赤藓糖醇[J]. 生物技术通报, 2023, 39(8): 137-147. |
| [2] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [3] | 董聪, 高庆华, 王玥, 罗同阳, 王庆庆. 基于联合策略提高FAD依赖的葡萄糖脱氢酶的酵母表达[J]. 生物技术通报, 2023, 39(6): 316-324. |
| [4] | 马玉倩, 孙东辉, 岳浩峰, 辛佳瑜, 刘宁, 曹志艳. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135. |
| [5] | 陈楠楠, 王春来, 蒋振忠, 焦鹏, 关淑艳, 马义勇. 玉米ZmDHN15基因在烟草中的遗传转化及抗冷性分析[J]. 生物技术通报, 2023, 39(4): 259-267. |
| [6] | 赵昕, 杜玉瑶, 殷子扬, 毛淑红. 胆固醇7α-羟化酶在毕赤酵母中的异源表达[J]. 生物技术通报, 2023, 39(10): 304-310. |
| [7] | 牛馨, 张莹, 王茂军, 刘文龙, 路福平, 李玉. 解淀粉芽胞杆菌不同整合位点对外源碱性蛋白酶表达的影响[J]. 生物技术通报, 2022, 38(4): 253-260. |
| [8] | 王博雅, 姜勇, 黄艳, 曹颖, 胡尚连. 慈竹纤维素合酶BeCesA4的克隆及功能分析[J]. 生物技术通报, 2022, 38(11): 185-193. |
| [9] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [10] | 张彤彤, 郑登俞, 吴忠义, 张中保, 于荣. 玉米NF-Y转录因子基因ZmNF-YB13响应干旱和盐胁迫的功能分析[J]. 生物技术通报, 2022, 38(10): 115-123. |
| [11] | 杨威, 伍茜, 程建国, 罗燕, 王印, 杨泽晓, 姚学萍. 林麝干扰素α基因克隆、表达及转录调控分析[J]. 生物技术通报, 2022, 38(1): 194-204. |
| [12] | 廖兆民, 蔡俊, 林建国, 杜馨, 王常高. 黑曲霉葡萄糖氧化酶基因在毕赤酵母中的表达及产酶条件的优化[J]. 生物技术通报, 2021, 37(6): 97-107. |
| [13] | 杨悦, 陶妍, 谢晶, 钱韻芳. 基于重组毕赤酵母的草鱼C型溶菌酶生物合成及其抑菌活性[J]. 生物技术通报, 2021, 37(12): 169-179. |
| [14] | 刘珊, 叶伟, 朱牧孜, 李赛妮, 邓张双, 章卫民. 一种新型酰基转移酶GPAT的克隆、表达与酶学性质研究[J]. 生物技术通报, 2021, 37(11): 257-266. |
| [15] | 赵海燕, 宋晨斌, 刘正亚, 马兴荣, 尚会会, 李安华, 关现军, 王建设. 来源于Laceyella sp.的α-淀粉酶基因克隆、重组表达及酶学性质研究[J]. 生物技术通报, 2020, 36(8): 23-33. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||