








生物技术通报 ›› 2022, Vol. 38 ›› Issue (4): 79-85.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1226
• 作物品质遗传与改良专题(专题主编: 刘巧泉 教授) • 上一篇 下一篇
收稿日期:2021-09-27
出版日期:2022-04-26
发布日期:2022-05-06
通讯作者:
姜凌,女,博士,副研究员,研究方向:植物分子生物学与基因工程;E-mail: jiangling@caas.cn作者简介:任莹,女,硕士研究生,研究方向:植物分子生物学与基因工程;E-mail: ryry0612@163.com
基金资助:
REN Ying LIAN Tong ZHANG Chun-yi JIANG Ling( )
)
Received:2021-09-27
Published:2022-04-26
Online:2022-05-06
摘要:
在植物中,5-甲基四氢叶酸和同型半胱氨酸在甲硫氨酸合酶(methionine synthase,METS)的作用下生成甲硫氨酸,甲硫氨酸合酶是连接甲基代谢和一碳代谢的枢纽,克隆玉米甲硫氨酸合酶基因METS并分析其表达特性可为解析玉米籽粒的甲硫氨酸和叶酸的积累机制奠定基础。通过逆转录聚合酶链反应克隆得到玉米METS的编码序列,通过生物信息学方法分析其蛋白的特点,同时运用实时荧光定量技术分析了基因的组织表达特性及在萌发过程中的表达模式。结果发现玉米包含3个编码甲硫氨酸合酶的同源基因ZmMETS1、ZmMETS2、ZmMETS3,基因长度分别为2 301、2 298和2 298 bp,编码蛋白的氨基酸数量分别为766、765和765个,蛋白质分子质量约为84.5 kD,3个蛋白均含有N端和C端2个结构域,每个结构域由α螺旋环绕平行的β片层构成立体结构。系统发育树分析3个蛋白与高粱SbMETS的相似程度最高。表达分析发现它们在玉米各组织中均表达,各组织中转录水平最高的是ZmMETS1;相对来说雌穗中3个基因的转录水平较其他组织高;另外,在萌发过程中,ZmMETS1和ZmMETS3的转录水平随着萌发的时间迅速增加,之后迅速下降。玉米中存在3个具有组成型表达特征的玉米甲硫氨酸合酶基因,ZmMETS1可能在雌穗发育和萌发过程中起主要作用。
任莹, 连通, 张春义, 姜凌. 玉米甲硫氨酸合酶基因METS的克隆及表达特性[J]. 生物技术通报, 2022, 38(4): 79-85.
REN Ying LIAN Tong ZHANG Chun-yi JIANG Ling. Gene Cloning and Expression Characteristics of Methionine Synthase METS in Maize[J]. Biotechnology Bulletin, 2022, 38(4): 79-85.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 用途 Function |
|---|---|---|
| ZmMETS1-F1 | CAGAAAGATGGCGTCCCACATTG | 基因克隆 Gene cloning |
| ZmMETS1-R1 | GACGACGCCCTCCTCGGAAACG | |
| ZmMETS2-F1 | GGGAGCAACACATGCAGTATGA- ATTC | |
| ZmMETS2-R1 | CACATACCCCCAGAAACATAGCCC | |
| ZmMETS3-F2 | CCTCAGACAGTCAGACGTCCCGTG | |
| ZmMETS3-R2 | CTGTCCTGTACATACGTTGAGC- CTGG | |
| METS1-QRT-F1 | GATCCAGGACACCACCCAGATC | qRT-PCR |
| METS1-QRT-R1 | CTCCTCGGAAACGAAAAAGGC | |
| METS2-QRT-F1 | TAAGATTTTACTGTTATATAGAA- AGATGGC | |
| METS2-QRT-R1 | GGGACAATAAAGTGGTAGTTTG- TATC | |
| METS3-QRT-F1 | CTCTACAAATGAAACCCCTCAGAC | |
| METS3-QRT-R1 | GTATCCAACAATATGGGACGCC | |
| ZmGAPDH-F | CCCTTCATCACCACGGACTAC | Internal primers for qRT-PCR |
| ZmGAPDH-R | AACCTTCTTGGCACCACCCT |
表1 试验所用引物
Table 1 Primers used in the experiment
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 用途 Function |
|---|---|---|
| ZmMETS1-F1 | CAGAAAGATGGCGTCCCACATTG | 基因克隆 Gene cloning |
| ZmMETS1-R1 | GACGACGCCCTCCTCGGAAACG | |
| ZmMETS2-F1 | GGGAGCAACACATGCAGTATGA- ATTC | |
| ZmMETS2-R1 | CACATACCCCCAGAAACATAGCCC | |
| ZmMETS3-F2 | CCTCAGACAGTCAGACGTCCCGTG | |
| ZmMETS3-R2 | CTGTCCTGTACATACGTTGAGC- CTGG | |
| METS1-QRT-F1 | GATCCAGGACACCACCCAGATC | qRT-PCR |
| METS1-QRT-R1 | CTCCTCGGAAACGAAAAAGGC | |
| METS2-QRT-F1 | TAAGATTTTACTGTTATATAGAA- AGATGGC | |
| METS2-QRT-R1 | GGGACAATAAAGTGGTAGTTTG- TATC | |
| METS3-QRT-F1 | CTCTACAAATGAAACCCCTCAGAC | |
| METS3-QRT-R1 | GTATCCAACAATATGGGACGCC | |
| ZmGAPDH-F | CCCTTCATCACCACGGACTAC | Internal primers for qRT-PCR |
| ZmGAPDH-R | AACCTTCTTGGCACCACCCT |
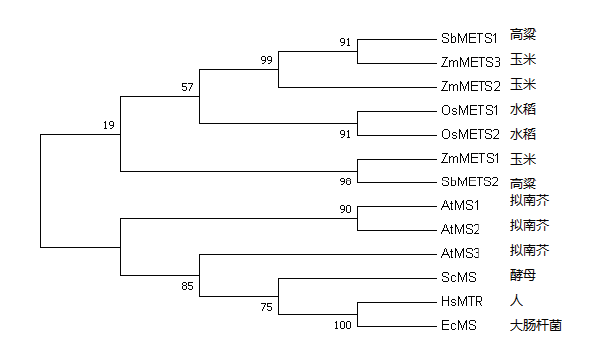
图3 METS同源基因的进化分析 SbMETS1:高粱Sorghum bicolor,A0A1B6PEC9;SbMETS2:高粱Sorghum bicolor,Q8W0Q7;ZmMETS1:玉米Zea mays,C0P5Y3;ZmMETS2:玉米Zea mays,B8A1R8;ZmMETS3:玉米Zea mays,A0A1D6KZ65;OsMETS1:水稻Oryza sativa L.,Q2QLY5;OsMETS2:水稻Oryza sativa L.,Q2QLY4;AtMS1:拟南芥Arabidopsis thaliana,O50008;AtMS2:拟南芥Arabidopsis thaliana,Q9SRV5;AtMS3:拟南芥Arabidopsis thaliana,Q0WNZ5;ScMS:酵母Saccharomyces cerevisiae,P05694;HsMTR:人Homo sapiens,Q99707;EcMS:大肠杆菌Escherichia coli,P13009。所有蛋白序列来自Uniprot数据库,All information of proteins is from www.uniprot.org
Fig.3 Evolutionary analysis of METS homologous proteins

图4 ZmMETS1/ZmMETS2/ZmMETS3在不同组织中的相对表达量 误差线表示标准偏差,n=3;星号代表与根中表达水平相比显著差异(*P<0.05;**P<0.01;***P<0.001;****P<0.0001)。下同
Fig. 4 Relative expressions of ZmMETS1/ZmMETS2/ZmMETS3 in different tissues The error line refers to the standard deviation,n=3;significant difference compared with root(*P<0.05;**P<0.01;***P<0.001;****P<0.0001). The same below
| [1] | 周峰. 植物种子萌发及活力的调控机制[J]. 湖北农业科学, 2014, 53(3):497-499. |
| Zhou F. Regulation mechanism of plants seed germination and vigor[J]. Hubei Agric Sci, 2014, 53(3):497-499. | |
| [2] | 姜凌, 张春义. 植物维生素生物强化进展[J]. 生物技术进展, 2016, 6(6):381-388. |
| Jiang L, Zhang CY. Progress on vitamins fortification in plants[J]. Curr Biotechnol, 2016, 6(6):381-388. | |
| [3] |
Cossins EA, Chen L. Folates and one-carbon metabolism in plants and fungi[J]. Phytochemistry, 1997, 45(3):437-452.
pmid: 9190084 |
| [4] |
Fukagawa NK. Sparing of methionine requirements:evaluation of human data takes sulfur amino acids beyond protein[J]. J Nutr, 2006, 136(6):1676S-1681S.
doi: 10.1093/jn/136.6.1676S URL |
| [5] |
Galili G, et al. Improving the levels of essential amino acids and sulfur metabolites in plants[J]. Biol Chem, 2005(9):817-831.
pmid: 16164407 |
| [6] |
Amir R. Current understanding of the factors regulating methionine content in vegetative tissues of higher plants[J]. Amino Acids, 2010, 39(4):917-931.
doi: 10.1007/s00726-010-0482-x URL |
| [7] | Liang Q, Wang K, Shariful I, et al. Folate content and retention in wheat grains and wheat-based foods:Effects of storage, processing, and cooking methods[J]. Food Chem, 2020, 333:127459. |
| [8] | 陈印军, 王琦琪, 向雁. 我国玉米生产地位、优势与自给率分析[J]. 中国农业资源与区划, 2019, 40(1):7-16. |
| Chen YJ, Wang QQ, Xiang Y. Analysis on the status, superiority and self-sufficiency ratio of maize in China[J]. Chin J Agric Resour Reg Plan, 2019, 40(1):7-16. | |
| [9] | Amir R, Cohen H. Revisiting the attempts to fortify methionine content in plant seeds[J]. J Exp Bot, 2019(16):4105-4114. |
| [10] |
Ranocha P, et al. The S-methylmethionine cycle in angiosperms:ub-iquity, antiquity and activity[J]. Plant J, 2001(5):575-584.
pmid: 11309147 |
| [11] |
Marsolais F, Pajak A, Yin F, et al. Proteomic analysis of common bean seed with storage protein deficiency reveals up-regulation of sulfur-rich proteins and starch and raffinose metabolic enzymes, and down-regulation of the secretory pathway[J]. J Proteomics, 2010, 73(8):1587-1600.
doi: 10.1016/j.jprot.2010.03.013 pmid: 20353836 |
| [12] |
Zhu X, Galili G. Increased lysine synjournal coupled with a knockout of its catabolism synergistically boosts lysine content and also transregulates the metabolism of other amino acids in Arabidopsis seeds[J]. Plant Cell, 2003, 15(4):845-853.
doi: 10.1105/tpc.009647 URL |
| [13] | Giovanelli J. Sulfur amino acids of plants:an overview[J]. Methods Enzymol, 1987, 143:419-426. |
| [14] |
Avraham T, Amir R. The expression level of threonine synthase and cystathionine-γ-synthase is influenced by the level of both threonine and methionine in Arabidopsis plants[J]. Transgenic Res, 2005, 14(3):299-311.
pmid: 16145838 |
| [15] | Wirtz M, Hell R. Functional analysis of the cysteine synthase protein complex from plants:structural, biochemical and regulatory properties[J]. J Plant Physiol, 2006(3):273-286. |
| [16] |
Mudd SH, Datko AH. The S-methylmethionine cycle in Lemna paucicostata[J]. Plant Physiol, 1990, 93(2):623-630.
doi: 10.1104/pp.93.2.623 pmid: 16667513 |
| [17] |
Kumar P, Jander G. Concurrent overexpression of Arabidopsis thaliana cystathionine γ-synthase and silencing of endogenous methionine γ-lyase enhance tuber methionine content in Solanum tuberosum[J]. J Agric Food Chem, 2017, 65(13):2737-2742.
doi: 10.1021/acs.jafc.7b00272 URL |
| [18] |
Nguyen HC, Hoefgen R, Hesse H. Improving the nutritive value of rice seeds:elevation of cysteine and methionine contents in rice plants by ectopic expression of a bacterial serine acetyltransferase[J]. J Exp Bot, 2012, 63(16):5991-6001.
doi: 10.1093/jxb/ers253 pmid: 23048130 |
| [19] |
Zhang Y, Schernthaner J, Labbé N, et al. Improved protein quality in transgenic soybean expressing a de novo synthetic protein, MB-16[J]. Transgenic Res, 2014, 23(3):455-467.
doi: 10.1007/s11248-013-9777-5 URL |
| [20] |
Hesse H, Hoefgen R. Molecular aspects of methionine biosynjournal[J]. Trends Plant Sci, 2003, 8(6):259-262.
doi: 10.1016/S1360-1385(03)00107-9 URL |
| [21] |
Ravanel S, Block MA, Rippert P, et al. Methionine metabolism in plants:chloroplasts are autonomous for de novo methionine synjournal and can import S-adenosylmethionine from the cytosol[J]. J Biol Chem, 2004, 279(21):22548-22557.
doi: 10.1074/jbc.M313250200 URL |
| [22] |
Roeder S, Dreschler K, Wirtz M, et al. SAM levels, gene expression of SAM synthetase, methionine synthase and ACC oxidase, and ethylene emission from N. suaveolens flowers[J]. Plant Mol Biol, 2009, 70(5):535-546.
doi: 10.1007/s11103-009-9490-1 URL |
| [23] |
Gallardo K, Job C, Groot SP, et al. Importance of methionine biosynjournal for Arabidopsis seed germination and seedling growth[J]. Physiol Plant, 2002, 116(2):238-247.
pmid: 12354201 |
| [24] |
Rajjou L, Duval M, Gallardo K, et al. Seed germination and vigor[J]. Annu Rev Plant Biol, 2012, 63:507-533.
doi: 10.1146/annurev-arplant-042811-105550 pmid: 22136565 |
| [25] | Ju C, Kong D, et al. Methionine synthase 1 provides methionine for activation of the GLR3. 5 Ca2+ channel and regulation of germination in Arabidopsis[J]. J Exp Bot, 2020(1):178-187. |
| [26] |
Ferrer JL, Ravanel S, Robert M, et al. Crystal structures of cobalamin-independent methionine synthase complexed with zinc, homocysteine, and methyltetrahydrofolate[J]. J Biol Chem, 2004, 279(43):44235-44238.
doi: 10.1074/jbc.C400325200 URL |
| [27] |
Whitcomb SJ, Rakpenthai A, Brückner F, et al. Cysteine and methionine biosynthetic enzymes have distinct effects on seed nutritional quality and on molecular phenotypes associated with accumulation of a methionine-rich seed storage protein in rice[J]. Front Plant Sci, 2020, 11:1118.
doi: 10.3389/fpls.2020.01118 pmid: 32793268 |
| [28] |
Jabrin S, Ravanel S, et al. One-carbon metabolism in plants. regulation of tetrahydrofolate synjournal during germination and seedling development[J]. Plant Physiol, 2003, 131(3):1431-1439.
doi: 10.1104/pp.016915 URL |
| [29] |
Wan X, Han LD, Yang M, et al. Simultaneous extraction and determination of mono-/polyglutamyl folates using high-performance liquid chromatography-tandem mass spectrometry and its applications in starchy crops[J]. Anal Bioanal Chem, 2019, 411(13):2891-2904.
doi: 10.1007/s00216-019-01742-0 URL |
| [1] | 王宝宝, 王海洋. 理想株型塑造之于玉米耐密改良[J]. 生物技术通报, 2023, 39(8): 11-30. |
| [2] | 张道磊, 甘雨军, 乐亮, 普莉. 玉米产量性状的表观遗传调控机制和育种应用[J]. 生物技术通报, 2023, 39(8): 31-42. |
| [3] | 冷燕, 马晓薇, 陈光, 任鹤, 李翔. 玉米高产竞赛助力中国玉米种业振兴[J]. 生物技术通报, 2023, 39(8): 4-10. |
| [4] | 王天依, 王荣焕, 王夏青, 张如养, 徐瑞斌, 焦炎炎, 孙轩, 王继东, 宋伟, 赵久然. 玉米矮秆基因与矮秆育种研究[J]. 生物技术通报, 2023, 39(8): 43-51. |
| [5] | 刘月娥, 徐田军, 蔡万涛, 吕天放, 张勇, 薛洪贺, 王荣焕, 赵久然. 我国玉米超高产研究现状与展望[J]. 生物技术通报, 2023, 39(8): 52-61. |
| [6] | 张勇, 徐田军, 吕天放, 邢锦丰, 刘宏伟, 蔡万涛, 刘月娥, 赵久然, 王荣焕. 种植密度对夏播玉米茎秆质量和根系表型性状的影响[J]. 生物技术通报, 2023, 39(8): 70-79. |
| [7] | 朱少喜, 金肇阳, 葛建镕, 王蕊, 王凤格, 路运才. 基于KASP平台的转基因玉米高通量特异性检测方法[J]. 生物技术通报, 2023, 39(6): 133-140. |
| [8] | 罗义, 张丽娟, 黄伟, 王宁, 吾尔丽卡·买提哈斯木, 施宠, 王玮. 一株耐铀菌株的鉴定及其促生特性研究[J]. 生物技术通报, 2023, 39(5): 286-296. |
| [9] | 陈楠楠, 王春来, 蒋振忠, 焦鹏, 关淑艳, 马义勇. 玉米ZmDHN15基因在烟草中的遗传转化及抗冷性分析[J]. 生物技术通报, 2023, 39(4): 259-267. |
| [10] | 徐扬, 丁红, 张冠初, 郭庆, 张智猛, 戴良香. 盐胁迫下花生种子萌发期代谢组学分析[J]. 生物技术通报, 2023, 39(1): 199-213. |
| [11] | 李圣彦, 李香银, 李鹏程, 张明俊, 张杰, 郎志宏. 转基因玉米2HVB5的性状鉴定及遗传稳定性分析[J]. 生物技术通报, 2023, 39(1): 21-30. |
| [12] | 李东阳, 肖冰, 王晨尧, 杨现明, 梁晋刚, 吴孔明. 转基因抗虫耐除草剂玉米瑞丰125 Cry1Ab/Cry2Aj杀虫蛋白的时空表达分析[J]. 生物技术通报, 2023, 39(1): 31-39. |
| [13] | 李鹏程, 张明俊, 王银晓, 李香银, 李圣彦, 郎志宏. 转基因玉米HGK60在不同遗传背景下抗虫性鉴定及农艺性状分析[J]. 生物技术通报, 2023, 39(1): 40-47. |
| [14] | 金云倩, 王彬, 郭书磊, 赵霖熙, 韩赞平. 赤霉素调控玉米种子活力的研究进展[J]. 生物技术通报, 2023, 39(1): 84-94. |
| [15] | 王松, 简晓平, 潘婉舒, 张永光, 王涛, 游玲. 玉米小曲酒糟发酵饲料对育肥猪肠道菌群的影响[J]. 生物技术通报, 2022, 38(9): 248-257. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||