








生物技术通报 ›› 2022, Vol. 38 ›› Issue (5): 84-92.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1305
收稿日期:2021-10-15
出版日期:2022-05-26
发布日期:2022-06-10
作者简介:魏倩,女,硕士研究生;研究方向:昆虫分子生物学;E-mail: 基金资助:
WEI Qian1( ), LIU Xiao-ning1(
), LIU Xiao-ning1( ), ZHAO Jie2(
), ZHAO Jie2( )
)
Received:2021-10-15
Published:2022-05-26
Online:2022-06-10
摘要:
旨在研究2-十三烷酮胁迫对棉铃虫Helicoverpa armigera叉头框A类似蛋白(forkhead box A-like protein,FoxAl)基因表达水平的影响,及FoxAl蛋白是如何调控解毒酶基因CYP6B6表达,为进一步明确FoxAl参与棉铃虫解毒代谢和生长发育过程提供依据。通过酵母自激活检测FoxAl蛋白的转录激活能力;通过凝胶阻滞检测FoxAl蛋白与CYP6B6启动子的结合能力;通过RNAi沉默棉铃虫5龄幼虫中肠内FoxAl基因,检测不同时间(24,48,72和 96 h)后FoxAl和CYP6B6的表达变化情况;最后通过qPCR检测不同浓度(5,10和20 mg/g)2-十三烷酮处理不同时间(6,12,20,30和48 h)后棉铃虫6龄幼虫中肠内FoxAl和CYP6B6的表达谱,并分析FoxAl和CYP6B6表达谱的相关性。FoxAl蛋白具有激活酵母MEL1报告基因转录的能力,且该蛋白能与CYP6B6启动子中响应植物次生物质应答的核心片段结合。利用dsFoxAl沉默棉铃虫5龄幼虫中肠内FoxAl的表达量后,CYP6B6的表达量也显著降低。2-十三烷酮处理棉铃虫6龄幼虫后,中肠内FoxAl和CYP6B6的表达量变化情况相似,基本都呈抛物线趋势,在短时间12 h内两者的表达量迅速上升后,随胁迫时间的延长两者的表达量逐渐降低;此外,FoxAl和CYP6B6的表达量变化基本呈正相关,甚至在20 mg/g的胁迫浓度以及12 h和48 h的胁迫时间下两者都是高度正相关(r=0.819,P=0.045;r=0.987,P=0.007;r=0.978,P=0.011)。棉铃虫FoxAl蛋白可能是CYP6B6的转录激活因子,在植物次生物质2-十三烷酮短期胁迫下,FoxAl的表达量增加,进一步上调CYP6B6的表达,从而参与棉铃虫对2-十三烷酮的解毒作用。
魏倩, 刘小宁, 赵洁. 2-十三烷酮胁迫下棉铃虫FoxAl调控CYP6B6的表达[J]. 生物技术通报, 2022, 38(5): 84-92.
WEI Qian, LIU Xiao-ning, ZHAO Jie. FoxAl Regulating CYP6B6 Expression Under 2-tridecanone Stress in Helicoverpa armigera[J]. Biotechnology Bulletin, 2022, 38(5): 84-92.

图1 酵母Y2HGold(pGBKT7-FoxAl)菌株的转录激活功能检测 A:pGBKT7-FoxAl质粒的酶切鉴定(M:DL15000 DNA marker;1:pGBKT7-FoxAl质粒;2:pGBKT7-FoxAl质粒的EcoR I和BamH I酶切产物);B:Y2HGold(pGBKT7-FoxAl)菌株的菌落PCR鉴定(M:DL2000 DNA marker;-:阴性对照;1和2:两个转化后菌株);C:Y2HGold(pGBKT7-FoxAl)菌株在X-α-gal诱导下的自激活试验
Fig.1 Transcriptional activation test of transformed yeast Y2HGold(pGBKT7-FoxAl)strain A:Restriction digestion of pGBKT7-FoxAl plasmid(M:DL15000 DNA marker;1:pGBKT7-FoxAl plasmid;2:digestion products of pGBKT7-FoxAl plasmid by EcoRI and BamH I). B:Colony PCR identification of Y2HGold(pGBKT7-FoxAl)strain(M:DL2000 DNA marker;-:negative control;1 and 2:two transformed strains). C:Self-activation test of Y2HGold(pGBKT7-FoxAl)strain under the induction of X-α-gal
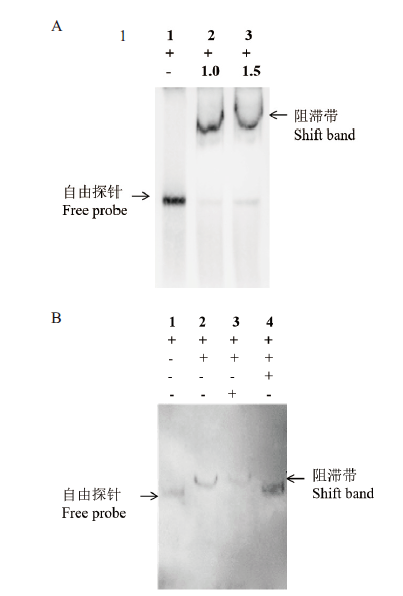
图2 棉铃虫FoxAl蛋白与CYP6B6启动子HE1片段的结合验证 A:凝胶迁移率检测FoxAl蛋白与HE1片段的相互作用 (泳道1: 1.0 ng HE1探针;泳道2: 1.0 ng HE1探针和1 μg FoxAl蛋白;泳道3: 1.0 ng HE1探针和1.5 μg FoxAl蛋白). B:凝胶阻滞试验证实FoxAl蛋白与HE1片段的结合 (泳道1: 1.0 ng HE1探针; 泳道2: 1.0 ng HE1探针和1 μg FoxAl蛋白; 泳道3: 1.0 ng HE1探针、1 μg FoxAl蛋白和标记探针100倍量的无关PGRP-B片段; 泳道4: 1.0 ng HE1探针、1 μg FoxAl蛋白和100倍量的未标记HE1片段)
Fig. 2 Verification of FoxAl in H. armigera binding to the HE1 fragment of CYP6B6 promotor A: Gel-shift assay of interaction between FoxAl and HE1 fragment (Lane 1: 1.0 ng HE1 probe; Lane 2: 1.0 ng HE1 probe and 1 μg FoxAl protein; Lane 3: 1.0 ng HE1 probe and 1.5 μg FoxAl protein). B: EMSA test of FoxAl combination with HE1 fragment (Lane 1: 1.0 ng HE1 probe; Lane 2: 1.0 ng HE1 probe and 1 μg FoxAl protein; Lane 3: 1.0 ng HE1 probe, 1 μg FoxAl protein and 100-fold labelled probe independent PGRP-B fragment; Lane 4: 1.0 ng HE1 probe, 1 μg FoxAl protein and 100-fold unlabelled HE1 fragment)
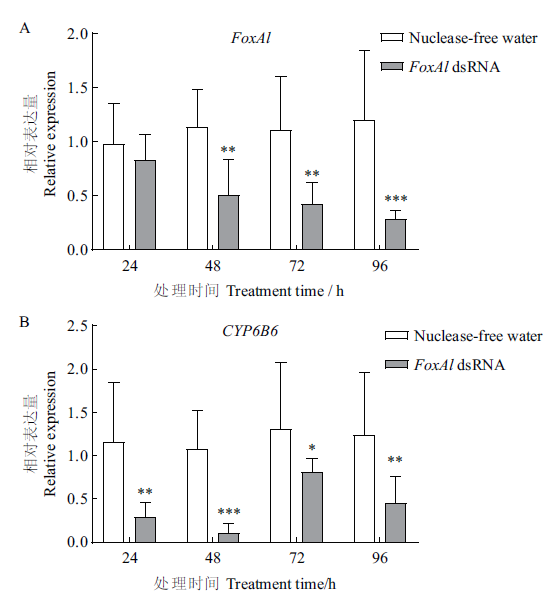
图3 FoxAl dsRNA 注射后棉铃虫5龄幼虫中肠内FoxAl和CYP6B6的相对表达量 在同一时间下用不同星号表示基因的相对表达量与对照组差异显著(*P<0.05,** P<0.01,*** P<0.001)
Fig. 3 Relative expression of FoxAl and CYP6B6 in the midgut of 5th instar larvae after FoxAl dsRNA injected Different asterisks indicate that the gene relative expression is of significant difference compared to control group under the same time(* P<0.05,** P<0.01,*** P<0.001)
| 2-TD处理2-TD treatment | r | P | |
|---|---|---|---|
| 胁迫浓度 Stress concentration/(mg·g-1) | 5 | 0.532 | 0.178 |
| 10 | 0.653 | 0.116 | |
| 20 | 0.819 | 0.045 | |
| 胁迫时间 Stress time/h | 6 | 0.614 | 0.193 |
| 12 | 0.987 | 0.007 | |
| 20 | -0.326 | 0.337 | |
| 30 | 0.863 | 0.069 | |
| 48 | 0.978 | 0.011 | |
表1 FoxAl和CYP6B6表达量的相关性分析
Table 1 Correlation analysis of FoxAl and CYP6B6 expre-ssion
| 2-TD处理2-TD treatment | r | P | |
|---|---|---|---|
| 胁迫浓度 Stress concentration/(mg·g-1) | 5 | 0.532 | 0.178 |
| 10 | 0.653 | 0.116 | |
| 20 | 0.819 | 0.045 | |
| 胁迫时间 Stress time/h | 6 | 0.614 | 0.193 |
| 12 | 0.987 | 0.007 | |
| 20 | -0.326 | 0.337 | |
| 30 | 0.863 | 0.069 | |
| 48 | 0.978 | 0.011 | |
| [1] |
Weigel D, Jürgens G, Küttner F, et al. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo[J]. Cell, 1989, 57(4):645-658.
pmid: 2566386 |
| [2] |
Clark KL, Halay ED, Lai E, et al. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5[J]. Nature, 1993, 364(6436):412-420.
doi: 10.1038/364412a0 URL |
| [3] |
Cardinaux JR, Chapel S, Wahli W. Complex organization of CTF/NF-I, C/EBP, and HNF3 binding sites within the promoter of the liver-specific vitellogenin gene[J]. J Biol Chem, 1994, 269(52):32947-32956.
pmid: 7806524 |
| [4] |
Rastegar S, Albert S, et al. A floor plate enhancer of the zebrafish netrin1 gene requires Cyclops(nodal)signalling and the winged helix transcription factor FoxA2[J]. Dev Biol, 2002, 252(1):1-14.
pmid: 12453456 |
| [5] |
Xu L, Li DZ, Luo YY, et al. Identification of the 2-tridecanone cis-acting element in the promoter of cytochrome P450 CYP6B7 in Helicoverpa armigera[J]. Insect Sci, 2018, 25(6):959-968.
doi: 10.1111/1744-7917.12479 pmid: 28497882 |
| [6] |
Yu JK, Mazet F, Chen YT, et al. The fox genes of Branchiostoma floridae[J]. Dev Genes Evol, 2008, 218(11/12):629-638.
doi: 10.1007/s00427-008-0229-9 URL |
| [7] |
Song JB, Li ZQ, Tong XL, et al. Genome-wide identification and characterization of Fox genes in the silkworm, Bombyx mori[J]. Funct Integr Genomics, 2015, 15(5):511-522.
doi: 10.1007/s10142-015-0440-5 URL |
| [8] |
Matsuno K, Takiya S, et al. Transcriptional stimulation via SC site of Bombyx sericin-1 gene through an interaction with a DNA binding protein SGF-3[J]. Nucleic Acids Res, 1990(7):1853-1858.
pmid: 2336359 |
| [9] |
Mattila J, Bremer A, Ahonen L, et al. Drosophila FoxO regulates organism size and stress resistance through an adenylate cyclase[J]. Mol Cell Biol, 2009, 29(19):5357-5365.
doi: 10.1128/MCB.00302-09 pmid: 19651894 |
| [10] |
Varma D, Bülow MH, Pesch YY, et al. Forkhead, a new cross regulator of metabolism and innate immunity downstream of TOR in Drosophila[J]. J Insect Physiol, 2014, 69:80-88.
doi: 10.1016/j.jinsphys.2014.04.006 URL |
| [11] |
Le Bourg É, Massou I. Fasting increases survival to cold in FOXO, DIF, autophagy mutants and in other genotypes of Drosophila melanogaster[J]. Biogerontology, 2015, 16(4):411-421.
doi: 10.1007/s10522-015-9557-0 pmid: 25663303 |
| [12] | 孟竹, 文茂羽, 康晓丽, 等. 饥饿胁迫对家蚕糖脂代谢的影响及BmFoxO的作用[J]. 昆虫学报, 2018, 61(8):895-904. |
| Meng Z, Wen MY, Kang XL, et al. Effects of starvation stress on the glucose and lipid metabolism and the role of BmFoxO in Bombyx mori[J]. Acta Entomol Sin, 2018, 61(8):895-904. | |
| [13] |
Bao B, Hong B, et al. Transcription factor fork head regulates the promoter of diapause hormone gene in the cotton bollworm, Helicoverpa armigera, and the modification of SUMOylation[J]. Insect Biochem Mol Biol, 2011, 41(9):670-679.
doi: 10.1016/j.ibmb.2011.04.009 URL |
| [14] | Cai MJ, Zhao WL, Jing YP, et al, 20-Hydroxyecdysone activates Forkhead box O to promote proteolysis during Helicoverpa armigera molting[J]. Development, 2016, 143(6):1005-1015. |
| [15] |
Li JH, Ma YM, Yuan WL, et al. FOXA transcriptional factor modulates insect susceptibility to Bacillus thuringiensis Cry1Ac toxin by regulating the expression of toxin-receptor ABCC2 and ABCC3 genes[J]. Insect Biochem Mol Biol, 2017, 88:1-11.
doi: 10.1016/j.ibmb.2017.07.004 URL |
| [16] | 于彩虹, 高希武, 郑炳宗. 2-十三烷酮对棉铃虫细胞色素P450的诱导作用[J]. 昆虫学报, 2002, 45(1):1-7. |
| Yu CH, Gao XW, Zheng BZ. Induction of the cytochrome P450 by 2-tridecanone in Helicoverpa armigera[J]. Acta Entomol Sin, 2002, 45(1):1-7. | |
| [17] | Liu XN, Liang P, et al. Induction of the cytochrome P 450 activity by plant allelochemicals in the cotton bollworm, Helicoverpa armigera(Hübner)[J]. Pestic Biochem Physiol, 2006(2):127-134. |
| [18] |
Zhao J, et al. Effect of silencing CYP6B6 of Helicoverpa armigera(Lepidoptera:Noctuidae)on its growth, development, and insecticide tolerance[J]. J Econ Entomol, 2016(6):2506-2516.
doi: 10.1093/jee/tow181 pmid: 27591286 |
| [19] | 刘小宁, 李芬, 张学涛, 等. 2-十三烷酮诱导棉铃虫(Helicoverpa armigera)细胞色素P450 CYP6B6时空表达规律的研究[J]. 干旱区研究, 2014, 31(5):978-983. |
| Liu XN, Li F, Zhang XT, et al. Spatiotemporal expression profile of the cytochrome P450 CYP6B6 from Helicoverpa armigera(Lepidoptera:Noctuidae)treated with 2-tridecanone[J]. Arid Zone Res, 2014, 31(5):978-983. | |
| [20] |
Zhang L, Lu Y, et al. The retardant effect of 2-Tridecanone, mediated by cytochrome P450, on the development of cotton bollworm, Helicoverpa armigera[J]. BMC Genomics, 2016, 17(1):954.
pmid: 27875986 |
| [21] |
Li F, Liu XN, Zhu Y, et al. Identification of the 2-tridecanone responsive region in the promoter of cytochrome P450 CYP6B6 of the cotton bollworm, Helicoverpa armigera(Lepidoptera:Noctuidae)[J]. Bull Entomol Res, 2014, 104(6):801-808.
doi: 10.1017/S0007485314000698 URL |
| [22] | 赵洁, 魏倩, 等. 棉铃虫叉头框蛋白A类似蛋白基因Harm-FoxAl的克隆及表达谱分析[J]. 昆虫学报, 2019(6):672-684. |
| Zhao J, Wei Q, Ren SW, et al. Cloning and expression profiling of the forkhead box protein A-like protein gene HarmFoxAl in Helicoverpa armigera(Lepidoptera:Noctuidae)[J]. Acta Entomol Sin, 2019, 62(6):672-684. | |
| [23] | 申光茂, 王晓娜, 黄勇, 等. 橘小实蝇幼虫解毒酶系基因应对高效氯氰菊酯胁迫的组织特异性表达[J]. 中国农业科学, 2015, 48(19):3857-3865. |
| Shen GM, Wang XN, Huang Y, et al. Tissue specific expression of genes encoding detoxification enzymes in the larvae of Bactrocera dorsalis under β-cypermethrin stress[J]. Sci Agric Sin, 2015, 48(19):3857-3865. | |
| [24] | 龙再浩, 马思杰, 罗洁. 昆虫发生菊酯抗性细胞色素P450s研究进展[J]. 检验检疫学刊, 2019, 29(6):120-123. |
| Long ZH, Ma SJ, Luo J. Advances in study of cytochrome P450s in pyrethroid-resistant insect[J]. J Insp Quar, 2019, 29(6):120-123. | |
| [25] |
Yang YH, Chen S, Wu SW, et al. Constitutive overexpression of multiple cytochrome P450 genes associated with pyrethroid resistance in Helicoverpa armigera[J]. J Econ Entomol, 2006, 99(5):1784-1789.
doi: 10.1093/jee/99.5.1784 URL |
| [26] | Xu YL, Peng ZQ, et al. Characterization of fork head transcription factor 1 gene FOXO1 and its role in sugar and lipid metabolism in the summer prediapause pupae of Delia antiqua(Diptera:Anthomyiidae)[J]. Acta Entomol Sin, 2018(12):1384-1392. |
| [27] |
Liu Y, Lehmann M. Genes and biological processes controlled by the Drosophila FOXA orthologue Fork head[J]. Insect Mol Biol, 2008, 17(2):91-101.
doi: 10.1111/j.1365-2583.2007.00785.x pmid: 18353099 |
| [1] | 黄小龙, 孙贵连, 马丹丹, 闫慧清. 水稻幼苗酵母单杂文库构建及LAZY1上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 126-135. |
| [2] | 韩浩章, 张丽华, 李素华, 赵荣, 王芳, 王晓立. 盐碱胁迫诱导的猴樟酵母cDNA文库构建及CbP5CS上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 236-245. |
| [3] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [4] | 徐靖, 朱红林, 林延慧, 唐力琼, 唐清杰, 王效宁. 甘薯IbHQT1启动子的克隆及上游调控因子的鉴定[J]. 生物技术通报, 2023, 39(8): 213-219. |
| [5] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [6] | 陈晓, 于茗兰, 吴隆坤, 郑晓明, 逄洪波. 植物lncRNA及其对低温胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(7): 1-12. |
| [7] | 郭怡婷, 赵文菊, 任延靖, 赵孟良. 菊芋NAC转录因子家族基因的鉴定及分析[J]. 生物技术通报, 2023, 39(6): 217-232. |
| [8] | 冯珊珊, 王璐, 周益, 王幼平, 方玉洁. WOX家族基因调控植物生长发育和非生物胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(5): 1-13. |
| [9] | 王兵, 赵会纳, 余婧, 余世洲, 雷波. 植物侧枝发育的调控研究进展[J]. 生物技术通报, 2023, 39(5): 14-22. |
| [10] | 张岩峰, 叶丽丹, 于洪巍. 氧化还原伴侣工程:P450低效问题的解决方案之一[J]. 生物技术通报, 2023, 39(4): 10-23. |
| [11] | 张新博, 崔浩亮, 史佩华, 高锦春, 赵顺然, 陶晨雨. 低起始量的免疫共沉淀技术研究进展[J]. 生物技术通报, 2023, 39(4): 227-235. |
| [12] | 郁慧丽, 李爱涛. 细胞色素P450酶在香精香料绿色生物合成中的应用[J]. 生物技术通报, 2023, 39(4): 24-37. |
| [13] | 葛颜锐, 赵冉, 徐静, 李若凡, 胡云涛, 李瑞丽. 植物维管形成层发育及其调控的研究进展[J]. 生物技术通报, 2023, 39(3): 13-25. |
| [14] | 刘铖霞, 孙宗艳, 罗云波, 朱鸿亮, 曲桂芹. bHLH转录因子的磷酸化调控植物生理功能的研究进展[J]. 生物技术通报, 2023, 39(3): 26-34. |
| [15] | 赵孟良, 郭怡婷, 任延靖. 菊芋WRKY转录因子家族基因的鉴定及分析[J]. 生物技术通报, 2023, 39(2): 116-125. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||