








生物技术通报 ›› 2024, Vol. 40 ›› Issue (6): 68-80.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1196
收稿日期:2023-12-19
出版日期:2024-06-26
发布日期:2024-05-14
通讯作者:
何勇锦,男,研究员,研究方向:微藻生物工程、油脂改性、生物质能源、功能活性物质;E-mail: yongjinhe@fjnu.edu.cn作者简介:蔡楠,女,硕士研究生,研究方向:微藻固碳及微藻生物质转化;E-mail: cainan327@163.com
基金资助:
CAI Nan1( ), FANG Jing-ping1, CHEN Bi-lian1,2, HE Yong-jin1,2(
), FANG Jing-ping1, CHEN Bi-lian1,2, HE Yong-jin1,2( )
)
Received:2023-12-19
Published:2024-06-26
Online:2024-05-14
摘要:
工业生产和人类活动释放的大量CO2是造成全球性气候变暖的主要诱因。气候变暖往往伴随着极端恶劣天气的发生,对人类的生活、财产和基础设施构成严重威胁。为了减轻由此产生的负面影响和应对全球变暖,各国纷纷设定了碳达峰和碳减排目标,并致力于对CO2进行固定和资源化利用。海洋等鞭金藻(Isochrysis galbana)具有生长速度快和固碳效率高的特点,集成废/污水处理和生物固碳,转化合成蛋白质、多不饱和脂肪酸等多种高值生物活性物质的等鞭金藻固碳技术,被认为是最有前途的碳捕获和资源高值化利用的技术之一。本文首先介绍和比较了常用的CO2捕获技术的优缺点,强调基于等鞭金藻的碳捕获技术的适用范围和固碳效率的优势。其次阐明了海洋等鞭金藻光合固碳机制及其与卡尔文循环、三羧酸循环等代谢通路的联系;探讨光和CO2对微藻固碳能力和胞内碳流分布的影响,探究培养条件、光生物反应器、基因工程/合成生物学技术改造藻株等影响等鞭金藻固碳效率的因素。最后,概述了等鞭金藻光合固碳与岩藻黄素、多不饱和脂肪酸、蛋白质等高值生物活性物质合成的关系,为精深加工、开发高值化等鞭金藻提供理论和实践依据,推动等鞭金藻固碳技术的发展和应用,协同推进节能减排,为助力实现“双碳”目标提供一条经济可行的新策略。
蔡楠, 方静平, 陈必链, 何勇锦. 高值化等鞭金藻固碳研究进展[J]. 生物技术通报, 2024, 40(6): 68-80.
CAI Nan, FANG Jing-ping, CHEN Bi-lian, HE Yong-jin. Research Progress in Carbon Sequestration by High-valued Isochrysis Strain[J]. Biotechnology Bulletin, 2024, 40(6): 68-80.
| 藻株 Microalgae strain | 生物量产量 Biomass yield/(g·L-1·d-1) | CO2来源 CO2 source | CO2固定效率 Fixed efficiency/(gCO2·L-1·d-1) | 参考文献 Reference |
|---|---|---|---|---|
| 四爿藻 Tetraselmis sp. | 0.42 | 燃煤电厂;CO2(10%-15%, V/V) | 0.19 | [ |
| 微拟球藻 Nannochloropsis sp. | 0.27 | 燃煤电厂:CO2(13%, V/V) | 0.51 | [ |
| 小球藻Chlorella vulgaris | 0.28 | 钢铁厂;CO2(10%-15%, V/V) | 0.45 | [ |
| 螺旋藻Spirulina | 0.22 | 煤化工烟气:CO2(10%, V/V) | 0.32 | [ |
| 杜氏盐藻 NIES-2257 Dunaliella salina NIES-2257 | 0.23 | 5% CO2 | 0.11 | [ |
| 等鞭金藻 Isochrysis sp. | 0.32 | 燃煤电厂;CO2(10%-15%, V/V) | 0.61 | [ |
表1 微藻固碳效率
Table 1 Microalgae carbon-fixing efficiency
| 藻株 Microalgae strain | 生物量产量 Biomass yield/(g·L-1·d-1) | CO2来源 CO2 source | CO2固定效率 Fixed efficiency/(gCO2·L-1·d-1) | 参考文献 Reference |
|---|---|---|---|---|
| 四爿藻 Tetraselmis sp. | 0.42 | 燃煤电厂;CO2(10%-15%, V/V) | 0.19 | [ |
| 微拟球藻 Nannochloropsis sp. | 0.27 | 燃煤电厂:CO2(13%, V/V) | 0.51 | [ |
| 小球藻Chlorella vulgaris | 0.28 | 钢铁厂;CO2(10%-15%, V/V) | 0.45 | [ |
| 螺旋藻Spirulina | 0.22 | 煤化工烟气:CO2(10%, V/V) | 0.32 | [ |
| 杜氏盐藻 NIES-2257 Dunaliella salina NIES-2257 | 0.23 | 5% CO2 | 0.11 | [ |
| 等鞭金藻 Isochrysis sp. | 0.32 | 燃煤电厂;CO2(10%-15%, V/V) | 0.61 | [ |
| CO2捕获技术 CO2 capture technology | 优点 Advantage | 缺点 Disadvantage | 参考文献 Reference |
|---|---|---|---|
| 地质封存 Geological sequestration | 1. CO2性质稳定,封存长; 2. CO2注入油田或气田可提高采收率 | 1. 地质运动会导致CO2泄露,形成毁灭性的窒息区域,引发土壤、大气环境变化; 2. 地质封层需要改造蒸汽储存、运输、在线地震勘探设备,机械造价和运行成本高; 3. 目前我国CO2地质封存技术仍处于示范阶段,亟待深入研究 | [ |
| 物理化学吸附 Physicochemical adsorption | 1. CO2封存安全、稳定; 2. CO2封存的同时还可将浓缩的CO2用于生产水泥、生物塑料等产品,经济效益高; 3. 固碳工艺相对成熟 | 1. 吸附剂材料消耗大,成本高昂; 2. 反应器运行成本高; 3. 许多化学试剂有毒且挥发性强,对操作要求高且易造成环境问题 | [ |
| 植物固碳 Carbon sequestration in plants | 1. 固碳效率高; 2. 无需使用化学品,固碳过程安全、无毒; 3. 固碳成本低 | 1. 耗时长、占用土地面积大; 2. 固碳效果受外部条件影响大 | [ |
| 微藻生物固碳 Microalgae biocarbon sequestration | 1. 固碳效率高,是陆生植物的10-50倍; 2. 微藻生长速度快,易于培养; 3. 微藻对极端环境耐受性高,适用于多种环境下培养; 4. 微藻能在固碳同时合成生物质,并应用于能源、食品和饲料等行业 | 1. 微藻生物固碳起步较晚,某些工艺/技术仍处于起步阶段; 2. 微藻培养需安装光生物反应器,耗费高; 3. 微藻固碳目前市场规模小,还有广大待挖掘区域 | [ |
表2 CO2捕获技术及其优缺点
Table 2 CO2 capture technology and its advantages and disadvantages
| CO2捕获技术 CO2 capture technology | 优点 Advantage | 缺点 Disadvantage | 参考文献 Reference |
|---|---|---|---|
| 地质封存 Geological sequestration | 1. CO2性质稳定,封存长; 2. CO2注入油田或气田可提高采收率 | 1. 地质运动会导致CO2泄露,形成毁灭性的窒息区域,引发土壤、大气环境变化; 2. 地质封层需要改造蒸汽储存、运输、在线地震勘探设备,机械造价和运行成本高; 3. 目前我国CO2地质封存技术仍处于示范阶段,亟待深入研究 | [ |
| 物理化学吸附 Physicochemical adsorption | 1. CO2封存安全、稳定; 2. CO2封存的同时还可将浓缩的CO2用于生产水泥、生物塑料等产品,经济效益高; 3. 固碳工艺相对成熟 | 1. 吸附剂材料消耗大,成本高昂; 2. 反应器运行成本高; 3. 许多化学试剂有毒且挥发性强,对操作要求高且易造成环境问题 | [ |
| 植物固碳 Carbon sequestration in plants | 1. 固碳效率高; 2. 无需使用化学品,固碳过程安全、无毒; 3. 固碳成本低 | 1. 耗时长、占用土地面积大; 2. 固碳效果受外部条件影响大 | [ |
| 微藻生物固碳 Microalgae biocarbon sequestration | 1. 固碳效率高,是陆生植物的10-50倍; 2. 微藻生长速度快,易于培养; 3. 微藻对极端环境耐受性高,适用于多种环境下培养; 4. 微藻能在固碳同时合成生物质,并应用于能源、食品和饲料等行业 | 1. 微藻生物固碳起步较晚,某些工艺/技术仍处于起步阶段; 2. 微藻培养需安装光生物反应器,耗费高; 3. 微藻固碳目前市场规模小,还有广大待挖掘区域 | [ |
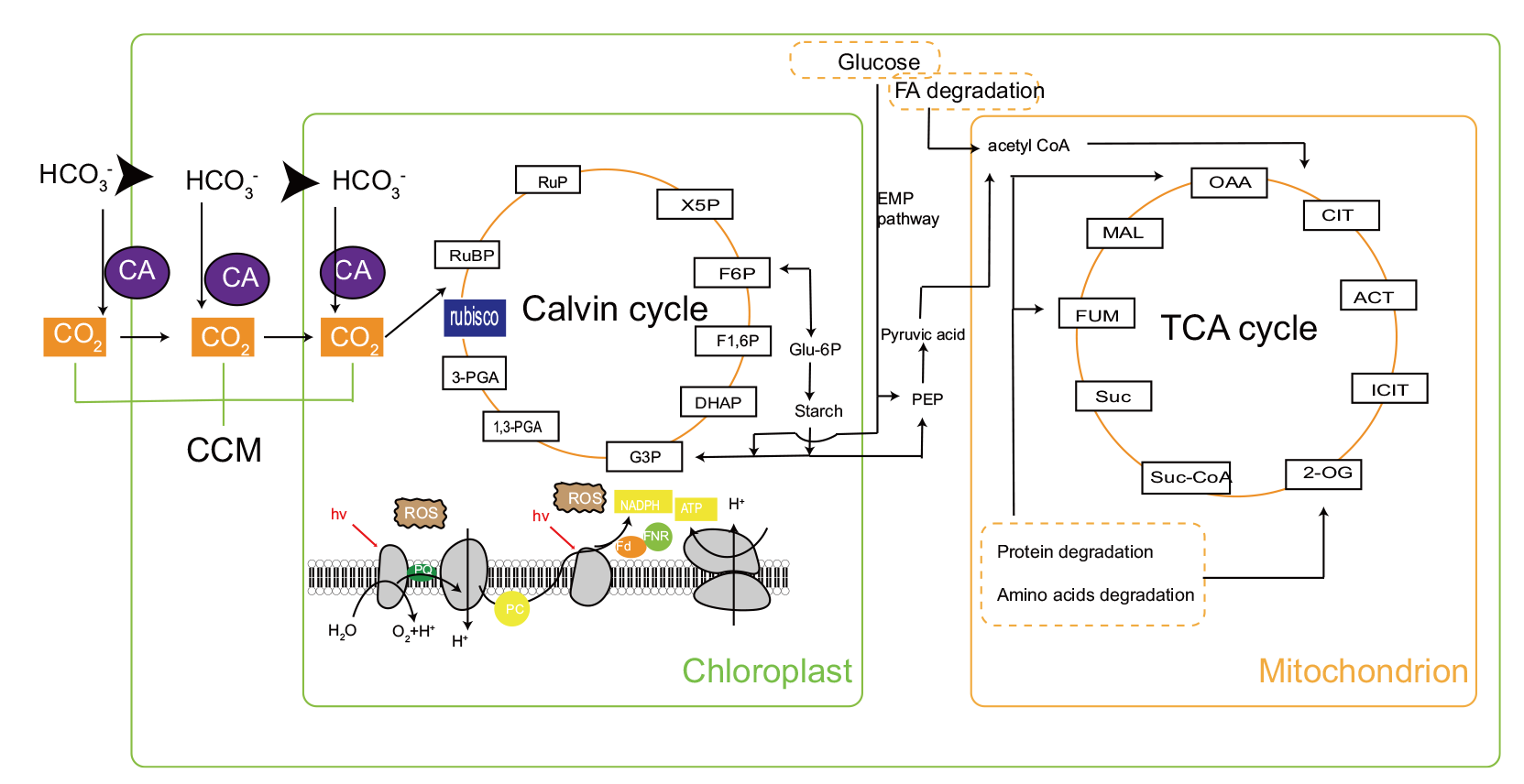
图2 等鞭金藻中的碳流代谢通路 CA:碳酸酐酶;CCM:CO2富集机制;RuBP: 二磷酸核酮糖;rubisco: 二磷酸核酮糖羧化酶;3-PGA :3-磷酸甘油酸;1, 3-PGA:1, 3-二磷酸甘油酸; G3P:甘油醛-3-磷酸; DHAP: 二羟丙酮磷酸; F-1,6P:果糖-1, 6-二磷酸;F-6P:果糖-6-磷酸;X5P:木酮糖-5-磷酸;RuP:核酮糖-5-磷酸;Glu-6P:葡萄糖-6-磷酸;Starch:淀粉;ROS:活性氧;NADPH:烟酰胺腺嘌呤二核苷酸磷酸;FNR:铁氧化蛋白NADP +还原酶;PQ:质体醌;PC:质体蓝素;ATP:三磷酸腺苷;Fd:铁氧还蛋白;OAA:草酰乙酸;MAL:苹果酸;FUM:延胡索酸;Suc:琥珀酸;Suc-CoA:琥珀酰辅酶A;2-OG:α-酮戊二酸;ICIT:异柠檬酸;ACT:顺乌头酸;CIT:柠檬酸;FA:脂肪酸;Acetyl-CoA;乙酰辅酶A
Fig. 2 Carbon flux metabolic pathway in Isochrysis CA: Carbonic anhydrase. CCM: CO2-concentrating mechanism. RuBP: Ribulose bisphosphate. Rubisco: Ribulose bisphosphate carboxylase. 3-PGA: 3-phosphoglycerate. 1,3-PGA: 1,3-disphosphoglycerate. G3P: Glyceraldehyde 3-phosphate. DHAP: Dihydroxyacetone phosphate. F-1,6P: Fructose-1,6bisphoshate. F-6P: Fructose6-phosphate. X5P: Xylulose 5-phosphate. RuP: Ribulose 5-phosphate. Glu-6P: Glucose-6-phosphate. ROS: Reactive oxygen species. NADPH: Nicotinamide adenine dinucleotide phosphate. FNR: Ferredoxin-NADP+-oxidoreductase. PQ: Plastoquinone. PC: Plastocyanin. ATP: Adenosine triphosphate. Fd: Ferredoxin. OAA: Oxaloacetic acid. MAL: Malic acid. FUM: Fumaric acid. Suc: Succinic acid. Suc-CoA: Succinyl-CoA-synthetase. 2-OG: α-ketoglutaric acid. ICIT: Isocitricacid. ACT: Cis-aconitic acid. CIT: Citric acid. FA: Fatty acid. Acetyl-CoA: Acetyl-coenzyme A
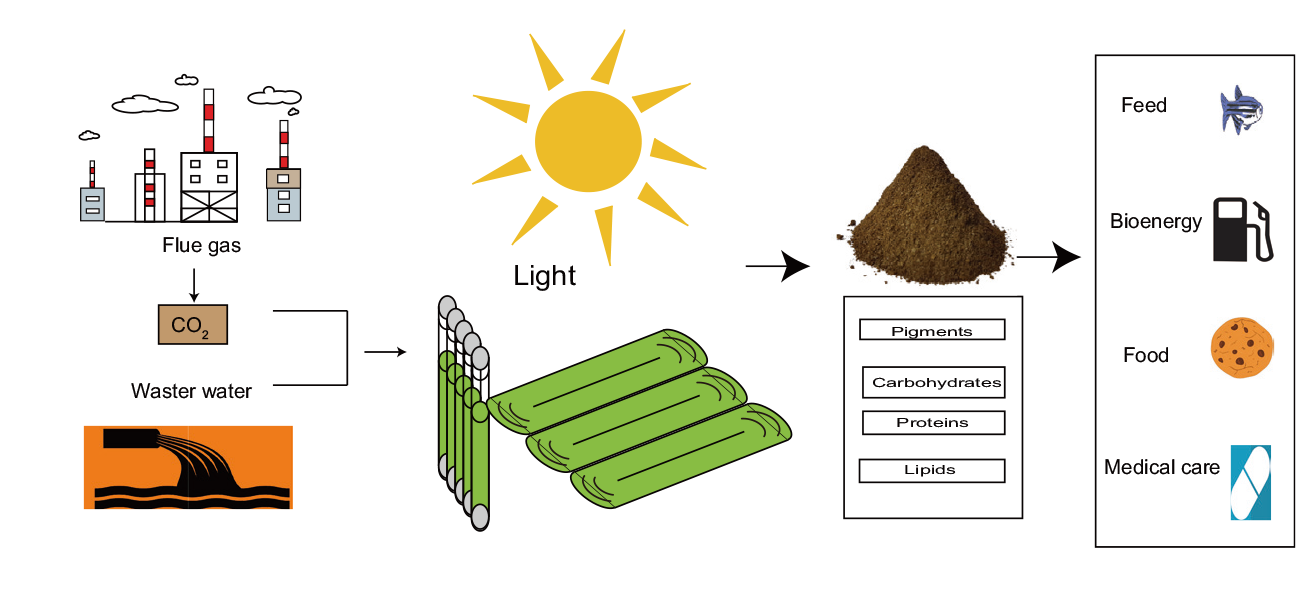
图3 利用微藻固碳合成高值活性成分并应用于各行业的概念展望
Fig. 3 Concept prospect of using microalgae carbon fixation to synthesize high-value bioactive ingredients for applications in various industries
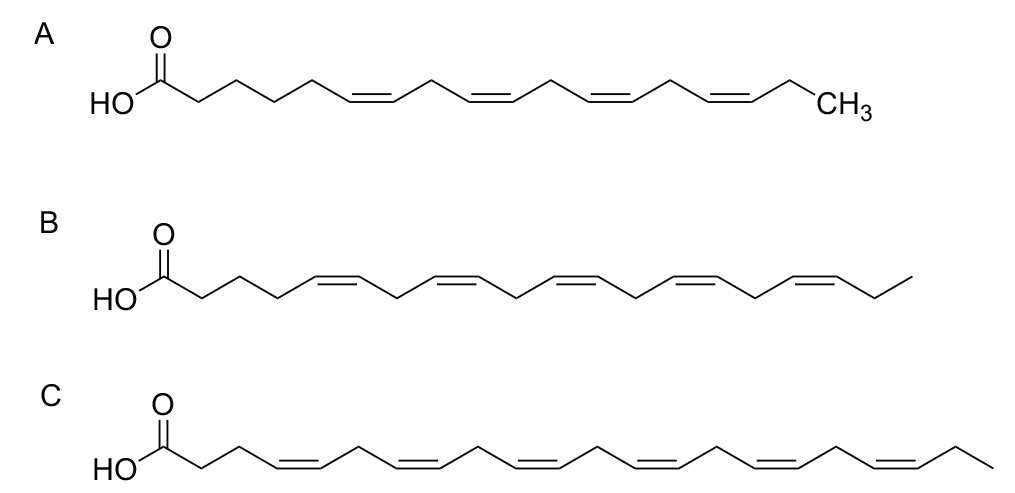
图5 n-3多不饱和脂肪酸结构式 A:SDA结构式;B:EPA结构式;C:DHA结构式
Fig. 5 Structures of n-3 polyunsaturated fatty acids A: SDA structural formula. B: EPA structural formula. C: DHA structural formula
| 藻株 Microalgae strain | n-3 多不饱和脂肪酸 n-3 polyunsaturated acids/(mg·g-1 oil) | |||||
|---|---|---|---|---|---|---|
| ALA(C18:3n-3) | SDA(C18:4n-3) | EPA(C20:5n-3) | DPA(C22:5n-3) | DHA(C22:6n-3) | ||
| 等鞭金藻Isochrysis T-Iso | 29.00 ± 4.00 | 43.00 ± 10.00 | 2.80 ± 0.70 | - | 46.00 ± 14.00 | |
| 微拟球藻Nannochloropsis oculata | 0.30 ± 0.03 | 0.30 ± 0.10 | 175.00 ± 12.00 | - | - | |
| 巴夫藻Pavlova Lutheri | 10.00 ± 0.30 | 17.00 ± 0.50 | 92.00 ± 2.00 | - | 40.90 ± 0.90 | |
| 硅藻Thalassiosira Preudonana | 1.90 ± 0.10 | 20.40 ± 0.80 | 81.00 ± 2.00 | 1.82 ± 0.01 | 20.90 ± 0.80 | |
| 鱼油Fish oil | 7.70 ± 0.20 | 29.00 ± 1.00 | 184.00 ± 5.00 | 16.80 ± 0.30 | 105.20 ± 0.70 | |
表3 几种微藻和鱼油中n-3多不饱和脂肪酸含量
Table 3 n-3 polyunsaturated fatty acid content in several microalgae and fish oil
| 藻株 Microalgae strain | n-3 多不饱和脂肪酸 n-3 polyunsaturated acids/(mg·g-1 oil) | |||||
|---|---|---|---|---|---|---|
| ALA(C18:3n-3) | SDA(C18:4n-3) | EPA(C20:5n-3) | DPA(C22:5n-3) | DHA(C22:6n-3) | ||
| 等鞭金藻Isochrysis T-Iso | 29.00 ± 4.00 | 43.00 ± 10.00 | 2.80 ± 0.70 | - | 46.00 ± 14.00 | |
| 微拟球藻Nannochloropsis oculata | 0.30 ± 0.03 | 0.30 ± 0.10 | 175.00 ± 12.00 | - | - | |
| 巴夫藻Pavlova Lutheri | 10.00 ± 0.30 | 17.00 ± 0.50 | 92.00 ± 2.00 | - | 40.90 ± 0.90 | |
| 硅藻Thalassiosira Preudonana | 1.90 ± 0.10 | 20.40 ± 0.80 | 81.00 ± 2.00 | 1.82 ± 0.01 | 20.90 ± 0.80 | |
| 鱼油Fish oil | 7.70 ± 0.20 | 29.00 ± 1.00 | 184.00 ± 5.00 | 16.80 ± 0.30 | 105.20 ± 0.70 | |
| [1] | 潘家华, 陈梦玫, 刘保留. “双碳” 战略助推中国落实联合国可持续发展目标的作用机理[J]. 阅江学刊, 2024, 16(1): 60-70, 172-173. |
| Pan JH, Chen MM, Liu BL. The mechanisms of facilitating China's implementation of the united nations sustainable development goals through the “dual carbon” strategy[J]. Yuejiang Acad J, 2024, 16(1): 60-70, 172-173. | |
| [2] | Tripathi S, Choudhary S, Meena A, et al. Carbon capture, storage, and usage with microalgae: a review[J]. Environ Chem Lett, 2023, 21(4): 2085-2128. |
| [3] | 康佳宁, 张云龙, 彭凇, 等. 实现碳中和目标的CCUS产业发展展望[J]. 北京理工大学学报:社会科学版, 2024, 26(2):68-75. |
| Kang JN, Zhang YL, Peng S, et al. Prospect of CCUS industry development to achieve carbon neutrality[J]. J Beijing Inst Technol Soc Sci Ed, 2024, 26(2):68-75. | |
| [4] | Li P, Pan SY, Pei SL, et al. Challenges and perspectives on carbon fixation and utilization technologies: an overview[J]. Aerosol Air Qual Res, 2016, 16(6): 1327-1344. |
| [5] | 莫壮洪, 朱俊英, 荣峻峰, 等. 微藻生物固碳技术在碳中和中的应用及潜在价值[J]. 石油炼制与化工, 2024, 55(1): 98-111, 4. |
| Mo ZH, Zhu JY, Rong JF, et al. Application and potential value of microalgae bio-carbon fixation technology in carbon neutrality[J]. Petrol Process Petrochem, 2024, 55(1): 98-111, 4. | |
| [6] |
崔金玉, 张爱娣, 栾国栋, 等. 微藻光驱固碳合成技术的发展现状与未来展望[J]. 合成生物学, 2022, 3(5): 884-900.
doi: 10.12211/2096-8280.2022-005 |
| Cui JY, Zhang AD, Luan GD, et al. Engineering microalgae for photosynthetic biosynthesis: progress and prospect[J]. Synth Biol J, 2022, 3(5): 884-900. | |
| [7] | Xu XZ, Gu XG, Wang ZY, et al. Progress, challenges and solutions of research on photosynthetic carbon sequestration efficiency of microalgae[J]. Renew Sustain Energy Rev, 2019, 110: 65-82. |
| [8] |
Matos J, Cardoso C, Gomes A, et al. Bioprospection of Isochrysis galbana and its potential as a nutraceutical[J]. Food Funct, 2019, 10(11): 7333-7342.
doi: 10.1039/c9fo01364d pmid: 31646314 |
| [9] | Yahya L, Harun R, Abdullah LC. Screening of native microalgae species for carbon fixation at the vicinity of Malaysian coal-fired power plant[J]. Sci Rep, 2020, 10(1): 22355. |
| [10] | Zheng MM, Ji XW, He YJ, et al. Simultaneous fixation of carbon dioxide and purification of undiluted swine slurry by culturing Chlorella vulgaris MBFJNU-1[J]. Algal Res, 2020, 47: 101866. |
| [11] | Fan JH, Xu H, Luo YC, et al. Impacts of CO2 concentration on growth, lipid accumulation, and carbon-concentrating-mechanism-related gene expression in oleaginous Chlorella[J]. Appl Microbiol Biotechnol, 2015, 99(5): 2451-2462. |
| [12] | Sydney EB, Sturm W, de Carvalho JC, et al. Potential carbon dioxide fixation by industrially important microalgae[J]. Bioresour Technol, 2010, 101(15): 5892-5896. |
| [13] | de Morais MG, Costa JAV. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor[J]. J Biotechnol, 2007, 129(3): 439-445. |
| [14] | Kishi M, Toda T. Carbon fixation properties of three alkalihalophilic microalgal strains under high alkalinity[J]. J Appl Phycol, 2018, 30(1): 401-410. |
| [15] |
Kumar A, Ergas S, Yuan X, et al. Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions[J]. Trends Biotechnol, 2010, 28(7): 371-380.
doi: 10.1016/j.tibtech.2010.04.004 pmid: 20541270 |
| [16] | Nguyen LN, Vu MT, Vu HP, et al. Microalgae-based carbon capture and utilization: a critical review on current system developments and biomass utilization[J]. Crit Rev Environ Sci Technol, 2023, 53(2): 216-238. |
| [17] | Zhao BT, Su YX, Zhang YX, et al. Carbon dioxide fixation and biomass production from combustion flue gas using energy microalgae[J]. Energy, 2015, 89: 347-357. |
| [18] | Realmonte G, Drouet L, Gambhir A, et al. An inter-model assessment of the role of direct air capture in deep mitigation pathways[J]. Nat Commun, 2019, 10(1): 3277. |
| [19] | Wang XX, Song CS. Carbon capture from flue gas and the atmosphere: a perspective[J]. Front Energy Res, 2020, 8: 560849. |
| [20] | Ali M, Jha NK, Pal N, et al. Recent advances in carbon dioxide geological storage, experimental procedures, influencing parameters, and future outlook[J]. Earth Sci Rev, 2022, 225: 103895. |
| [21] | Bui M, Adjiman CS, Bardow A, et al. Carbon capture and storage(CCS): the way forward[J]. Energy Environ Sci, 2018, 11(5): 1062-1176. |
| [22] | Varshney P, Mikulic P, Vonshak A, et al. Extremophilic micro-algae and their potential contribution in biotechnology[J]. Bioresour Technol, 2015, 184: 363-372. |
| [23] |
Spalding MH. Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters[J]. J Exp Bot, 2008, 59(7): 1463-1473.
pmid: 17597098 |
| [24] | Sayre R. Microalgae: the potential for carbon capture[J]. BioScience, 2010, 60(9): 722-727. |
| [25] | Li SN, Li X, Ho SH. How to enhance carbon capture by evolution of microalgal photosynthesis?[J]. Sep Purif Technol, 2022, 291: 120951. |
| [26] | 程亚田, 汤皓, 孙丽丽, 等. 植物源二萜类化合物微生物合成研究进展[J]. 生物工程学报, 2023, 39(6): 2265-2283. |
| Cheng YT, Tang H, Sun LL, et al. Advances on the microbial synthesis of plant-derived diterpenoids[J]. Chin J Biotechnol, 2023, 39(6): 2265-2283. | |
| [27] | Li YL, Sun H, Wang YN, et al. Integrated metabolic tools reveal carbon alternative in Isochrysis zhangjiangensis for fucoxanthin improvement[J]. Bioresour Technol, 2022, 347: 126401. |
| [28] | Kapoore RV, Padmaperuma G, Maneein S, et al. Co-culturing microbial consortia: approaches for applications in biomanufacturing and bioprocessing[J]. Crit Rev Biotechnol, 2022, 42(1): 46-72. |
| [29] | Kao CY, Chen TY, Chang YB, et al. Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp[J]. Bioresour Technol, 2014, 166: 485-493. |
| [30] | Zhou YC, He YJ, Guo X, et al. Pilot-scale remediation of rare earth elements ammonium wastewater by Chlamydomonas sp. YC in summer under outdoor conditions[J]. Bioresour Technol, 2023, 372: 128674. |
| [31] | Zheng MM, Dai JX, Ji XW, et al. An integrated semi-continuous culture to treat original swine wastewater and fix carbon dioxide by an indigenous Chlorella vulgaris MBFJNU-1 in an outdoor photobioreactor[J]. Bioresour Technol, 2021, 340: 125703. |
| [32] | Xia YJ, Sekine M, Hirahara M, et al. Effects of concentration and frequency of CO2 supply on productivity of marine microalga Isochrysis galbana[J]. Algal Res, 2023, 70: 102985. |
| [33] | Liu JY, Song YM, Qiu W. Oleaginous microalgae Nannochloropsis as a new model for biofuel production: review & analysis[J]. Renew Sustain Energy Rev, 2017, 72: 154-162. |
| [34] | Baer S, Heining M, Schwerna P, et al. Optimization of spectral light quality for growth and product formation in different microalgae using a continuous photobioreactor[J]. Algal Res, 2016, 14: 109-115. |
| [35] | Ramanna L, Rawat I, Bux F. Light enhancement strategies improve microalgal biomass productivity[J]. Renew Sustain Energy Rev, 2017, 80: 765-773. |
| [36] | Michael C, Del Ninno M, Gross M, et al. Use of wavelength-selective optical light filters for enhanced microalgal growth in different algal cultivation systems[J]. Bioresour Technol, 2015, 179: 473-482. |
| [37] | Seo YH, Cho C, Lee JY, et al. Enhancement of growth and lipid production from microalgae using fluorescent paint under the solar radiation[J]. Bioresour Technol, 2014, 173: 193-197. |
| [38] |
Zehentbauer FM, Moretto C, Stephen R, et al. Fluorescence spectroscopy of Rhodamine 6G: concentration and solvent effects[J]. Spectrochim Acta A Mol Biomol Spectrosc, 2014, 121: 147-151.
doi: 10.1016/j.saa.2013.10.062 pmid: 24239710 |
| [39] | 方静平, 陈钦常, 黄鹭强. 岩藻黄素生物合成途径及其对光照响应研究进展[J]. 福建师范大学学报: 自然科学版, 2021, 37(5): 96-108. |
| Fang JP, Chen QC, Huang LQ. A review of biosynthesis pathway of fucoxanthin and fucoxanthin production in response to light[J]. J Fujian Norm Univ Nat Sci Ed, 2021, 37(5): 96-108. | |
| [40] | Wang SK, Stiles AR, Guo C, et al. Microalgae cultivation in photobioreactors: an overview of light characteristics[J]. Eng Life Sci, 2014, 14(6): 550-559. |
| [41] | Xu PL, Li J, Qian J, et al. Recent advances in CO2 fixation by microalgae and its potential contribution to carbon neutrality[J]. Chemosphere, 2023, 319: 137987. |
| [42] | Guardini Z, Dall’Osto L, Barera S, et al. High carotenoid mutants of Chlorella vulgaris show enhanced biomass yield under high irradiance[J]. Plants, 2021, 10(5): 911. |
| [43] | Sirohi R, Kumar Pandey A, Ranganathan P, et al. Design and applications of photobioreactors- a review[J]. Bioresour Technol, 2022, 349: 126858. |
| [44] |
Pulz O. Photobioreactors: production systems for phototrophic microorganisms[J]. Appl Microbiol Biotechnol, 2001, 57(3): 287-293.
doi: 10.1007/s002530100702 pmid: 11759675 |
| [45] | Thomas DM, Mechery J, Paulose SV. Carbon dioxide capture strategies from flue gas using microalgae: a review[J]. Environ Sci Pollut Res Int, 2016, 23(17): 16926-16940. |
| [46] | Wang LL, Zhao RQ, Wang Q, et al. Novel bioreactor with inclined baffles in cost-efficiently increasing algal biomass and carbon fixation[J]. Energy, 2022, 247: 123453. |
| [47] | Ye Q, Cheng J, Guo WB, et al. Serial lantern-shaped draft tube enhanced flashing light effect for improving CO2 fixation with microalgae in a gas-lift circumflux column photobioreactor[J]. Bioresour Technol, 2018, 255: 156-162. |
| [48] | Ali Kubar A, Cheng J, Kumar S, et al. Developing a Zigzag-baffled column photobioreactor to increase mass-transfer, CO2 fixation and biomass yield during A. platensis cultivation[J]. J CO2 Util, 2022, 63: 102126. |
| [49] |
Genkov T, Meyer M, Griffiths H, et al. Functional hybrid rubisco enzymes with plant small subunits and algal large subunits: engineered rbcS cDNA for expression in chlamydomonas[J]. J Biol Chem, 2010, 285(26): 19833-19841.
doi: 10.1074/jbc.M110.124230 pmid: 20424165 |
| [50] | Mueller-Cajar O, Stotz M, Wendler P, et al. Structure and function of the AAA+ protein CbbX, a red-type Rubisco activase[J]. Nature, 2011, 479(7372): 194-199. |
| [51] | Wei L, Wang QT, Xin Y, et al. Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCO activase[J]. Algal Res, 2017, 27: 366-375. |
| [52] | Xie D, Ji XW, Zhou YC, et al. Chlorella vulgaris cultivation in pilot-scale to treat real swine wastewater and mitigate carbon dioxide for sustainable biodiesel production by direct enzymatic transesterification[J]. Bioresour Technol, 2022, 349: 126886. |
| [53] |
Nomura T, Kikuchi M, Kubodera A, et al. Proton-donative antioxidant activity of fucoxanthin with 1, 1-diphenyl-2-picrylhydrazyl(DPPH)[J]. Biochem Mol Biol Int, 1997, 42(2): 361-370.
pmid: 9238535 |
| [54] | Sabia A, Clavero E, Pancaldi S, et al. Effect of different CO2 concentrations on biomass, pigment content, and lipid production of the marine diatom Thalassiosira pseudonana[J]. Appl Microbiol Biotechnol, 2018, 102(4): 1945-1954. |
| [55] | Li JW, Zhao XQ, Chang JS, et al. A two-stage culture strategy for Scenedesmus sp. FSP3 for CO2 fixation and the simultaneous production of lutein under light and salt stress[J]. Molecules, 2022, 27(21): 7497. |
| [56] | Lin JY, Effendi SSW, Ng IS. Enhanced carbon capture and utilization(CCU)using heterologous carbonic anhydrase in Chlamydomonas reinhardtii for lutein and lipid production[J]. Bioresour Technol, 2022, 351: 127009. |
| [57] | Katiyar R, Arora A. Health promoting functional lipids from microalgae pool: a review[J]. Algal Res, 2020, 46: 101800. |
| [58] | Bonfanti C, Cardoso C, Afonso C, et al. Potential of microalga Isochrysis galbana: bioactivity and bioaccessibility[J]. Algal Res, 2018, 29: 242-248. |
| [59] | Balakrishnan J, Dhavamani S, Sadasivam SG, et al. Omega-3-rich Isochrysis sp. biomass enhances brain docosahexaenoic acid levels and improves serum lipid profile and antioxidant status in Wistar rats[J]. J Sci Food Agric, 2019, 99(13): 6066-6075. |
| [60] |
Ryckebosch E, Bruneel C, Termote-Verhalle R, et al. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil[J]. Food Chem, 2014, 160: 393-400.
doi: 10.1016/j.foodchem.2014.03.087 pmid: 24799253 |
| [61] | He YJ, Wu T, Sun H, et al. Comparison of fatty acid composition and positional distribution of microalgae triacylglycerols for human milk fat substitutes[J]. Algal Res, 2019, 37: 40-50. |
| [62] | He YJ, Lin G, Rao XZ, et al. Microalga Isochrysis galbana in feed for Trachinotus ovatus: effect on growth performance and fatty acid composition of fish fillet and liver[J]. Aquac Int, 2018, 26(5): 1261-1280. |
| [63] | 农业农村部. 中华人民共和国农业农村部公告第692号[J]. 广东饲料, 2023, 32(7): 5-6. |
| Ministry of Agriculture and Rural Affairs. Announcement No.692 of the ministry of agriculture and rural affairs of the People's republic of China[J]. Guangdong Feed, 2023, 32(7): 5-6. | |
| [64] | 晁红娟, 雷占兰, 刘爱琴, 等. Omega-3多不饱和脂肪酸性质、功能及主要应用[J]. 中国食品添加剂, 2019, 30(10): 122-130. |
| Chao HJ, Lei ZL, Liu AQ, et al. Properties, functions and main applications of Omega-3 polyunsaturated fatty acids[J]. China Food Addit, 2019, 30(10): 122-130. | |
| [65] | Huang AY, Wu SC, Gu WH, et al. Provision of carbon skeleton for lipid synthesis from the breakdown of intracellular protein and soluble sugar in Phaeodactylum tricornutum under high CO2[J]. BMC Biotechnol, 2019, 19(1): 53. |
| [66] | Yun HS, Ji MK, Park YT, et al. Microalga, Acutodesmus obliquus KGE 30 as a potential candidate for CO2 mitigation and biodiesel production[J]. Environ Sci Pollut Res Int, 2016, 23(17): 17831-17839. |
| [67] | Kumar K, Banerjee D, Das D. Carbon dioxide sequestration from industrial flue gas by Chlorella sorokiniana[J]. Bioresour Technol, 2014, 152: 225-233. |
| [68] | Gao FZ, Sá M, Cabanelas ITD, et al. Improved fucoxanthin and docosahexaenoic acid productivities of a sorted self-settling Tisochrysis lutea phenotype at pilot scale[J]. Bioresour Technol, 2021, 325: 124725. |
| [69] | Li DG, Chen N, Zhou ZH, et al. Isochrysis sp. cultivation in pilot-scale to concurrently produce sustainable triacylglycerols for human milk fat substitutes and fucoxanthin[J]. Algal Res, 2023, 69: 102937. |
| [70] | Zhang PY, Sun Q, Dong Y, et al. Effects of different bicarbonate on spirulina in CO2 absorption and microalgae conversion hybrid system[J]. Front Bioeng Biotechnol, 2023, 10: 1119111. |
| [71] | Liu Y, Wei D, Chen WN. Oleaginous microalga Coccomyxa subellipsoidea as a highly effective cell factory for CO2 fixation and high-protein biomass production by optimal supply of inorganic carbon and nitrogen[J]. Front Bioeng Biotechnol, 2022, 10: 921024. |
| [72] | 赵震宇, 姚舜, 杨朔鹏, 等. “双碳” 目标下:中国CCUS发展现状、存在问题及建议[J]. 环境科学, 2023, 44(2): 1128-1138. |
| Zhao ZY, Yao S, Yang SP, et al. Under goals of carbon peaking and carbon neutrality: status, problems, and suggestions of CCUS in China[J]. Environ Sci, 2023, 44(2): 1128-1138. |
| [1] | 陈中元, 王玉红, 代为俊, 张艳敏, 叶倩, 刘旭平, 谭文松, 赵亮. 柠檬酸铁铵对悬浮HEK293细胞转染的影响机制探究[J]. 生物技术通报, 2023, 39(9): 311-318. |
| [2] | 张龙喜, 吕琳, 张欢欢, 周金成, 车午男, 董辉. RNAi技术在寄生蜂中的应用研究进展[J]. 生物技术通报, 2023, 39(12): 99-108. |
| [3] | 李昕悦, 周明海, 樊亚超, 廖莎, 张风丽, 刘晨光, 孙悦, 张霖, 赵心清. 基于转运蛋白工程提升微生物菌株耐受性和生物制造效率的研究进展[J]. 生物技术通报, 2023, 39(11): 123-136. |
| [4] | 山琦, 贾惠舒, 姚文博, 刘伟灿, 李海燕. 植物miR396-GRF模块的生物学功能及其潜在应用价值[J]. 生物技术通报, 2022, 38(10): 34-44. |
| [5] | 张雪, 谭玉萌, 蒋海霞, 杨广宇. 基于单细胞超高通量筛选的α-1,2-岩藻糖基转移酶定向进化[J]. 生物技术通报, 2022, 38(1): 289-298. |
| [6] | 郝俊尧, 马富强, 杨广宇. 产碱杆菌Alcaligenes sp.KS-85来源肌酸酶活性中心的关键氨基酸功能研究[J]. 生物技术通报, 2021, 37(3): 75-83. |
| [7] | 徐美慧, 张耀杰, 唐克轩, 苗志奇. GoldenBraid酶切连接反应体系的优化[J]. 生物技术通报, 2020, 36(9): 266-274. |
| [8] | 韩梅, 袁超, 郭婷婷, 吴怡, 岳耀敬, 杨博辉. 哺乳动物手工克隆的研究进展[J]. 生物技术通报, 2020, 36(3): 54-61. |
| [9] | 吴家劲, 朱森林, 周密, 孙会增. 奶牛瘤胃微生物研究进展和趋势[J]. 生物技术通报, 2020, 36(2): 27-38. |
| [10] | 邱锦, 黄火清, 姚斌, 罗会颖. 解淀粉芽孢杆菌淀粉酶催化活力改良及其在枯草芽孢杆菌中的高效表达[J]. 生物技术通报, 2019, 35(9): 134-143. |
| [11] | 邓晓芬, 杨晓佳, 易天红, 冯英, 柯潇, 赖维莉. 融合蛋白基因与抗体基因电转染CHO-S细胞的条件摸索优化[J]. 生物技术通报, 2019, 35(4): 223-228. |
| [12] | 许祥, 董维鹏, 张少华, 冯晨毅, 刘田福, 燕炯. 围脂滴蛋白基因CRISPR/Cas9载体的活性分析[J]. 生物技术通报, 2019, 35(11): 89-95. |
| [13] | 郭珺, 樊芳芳, 王立革, 武爱莲, 郑军. 固碳微生物菌株的分离鉴定及其固碳能力测定[J]. 生物技术通报, 2019, 35(1): 90-97. |
| [14] | 任天雷, 杨海泉, 许菲. 基于分子结构与生物信息学等多维度特征的定向进化改造甲基对硫磷水解酶[J]. 生物技术通报, 2018, 34(10): 194-200. |
| [15] | 安飞飞, 陈霆, 杨龙, 陈松笔. 木薯叶片显微结构及叶绿素荧光参数比较[J]. 生物技术通报, 2018, 34(1): 104-109. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||