








生物技术通报 ›› 2024, Vol. 40 ›› Issue (7): 307-313.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0136
蔡逸安1( ), 张轶群1, 杨子璇1, 刘业学1, 刘文龙2, 路福平1(
), 张轶群1, 杨子璇1, 刘业学1, 刘文龙2, 路福平1( ), 李玉1(
), 李玉1( )
)
收稿日期:2024-02-03
出版日期:2024-07-26
发布日期:2024-07-30
通讯作者:
李玉,女,博士,教授,研究方向:应用微生物与酶工程;E-mail: liyu@tust.edu.cn;作者简介:蔡逸安,男,硕士研究生,研究方向:应用微生物与酶工程;E-mail: ianchoi97@126.com
基金资助:
CAI Yi-an1( ), ZHANG Yi-qun1, YANG Zi-xuan1, LIU Ye-xue1, LIU Wen-long2, LU Fu-ping1(
), ZHANG Yi-qun1, YANG Zi-xuan1, LIU Ye-xue1, LIU Wen-long2, LU Fu-ping1( ), LI Yu1(
), LI Yu1( )
)
Received:2024-02-03
Published:2024-07-26
Online:2024-07-30
摘要:
【目的】 通过分子伴侣共表达增强毕赤酵母中异源蛋白酶K的分泌水平,并对其的羊毛无氯剥鳞作用机制进行解析,旨在提高蛋白酶K的表达量并为高效酶法剥鳞技术的应用奠定基础。【方法】 利用毕赤酵母表达系统对tprK基因进行异源表达,首次分析了影响蛋白质折叠和质量控制的分子伴侣Ssa1、Erj5、Sil1、Hac1、Kar2、Lhs1和Ydj1分别过表达对TPRK的表达量和酶活力的作用,并对TPRK处理的羊毛纤维效果进行分析。【结果】 TPRK在毕赤酵母GS115中表达,其最适反应条件为65℃、pH 9.0,且具有良好的热稳定性和pH稳定性。过表达ssa1、hac1、erj5和sil1基因的重组菌株酶活分别提升了36.8%、20.0%、17.7%和14.8%。5 L发酵罐进行高密度发酵,诱导72 h后,TPRK的酶活达到77 471.99 U/mL。在羊毛水解应用中TPRK可水解羊毛鳞片内层使鳞片逐渐剥落,起到剥鳞效果,并且最适水解条件为:TPRK添加量为300 U/mL、反应温度55℃、pH 9.0和反应时间2 h。【结论】 过表达分子伴侣Ssa1能够有效提升TPRK的表达量,利用该TPRK处理羊毛纤维,可有效去除羊毛鳞片层,而对羊毛核心皮质层造成的损伤较小。
蔡逸安, 张轶群, 杨子璇, 刘业学, 刘文龙, 路福平, 李玉. 分子伴侣增强蛋白酶K在毕赤酵母中的表达及对羊毛鳞片层的作用分析[J]. 生物技术通报, 2024, 40(7): 307-313.
CAI Yi-an, ZHANG Yi-qun, YANG Zi-xuan, LIU Ye-xue, LIU Wen-long, LU Fu-ping, LI Yu. Enhanced Expression of Protease K in Pichia pastoris through Molecular Chaperones and Analysis of Its Effect on Wool Scale Layer[J]. Biotechnology Bulletin, 2024, 40(7): 307-313.

图1 TPRK在毕赤酵母中的表达 A:高拷贝重组转化子的筛选;B:培养物上清液中的TPRK的SDS-PAGE分析
Fig. 1 Expression of TPRK in P. pastoris A: High copies screening of recombinant strains; B: SDS-PAGE analysis of TPRK in culture supernatant
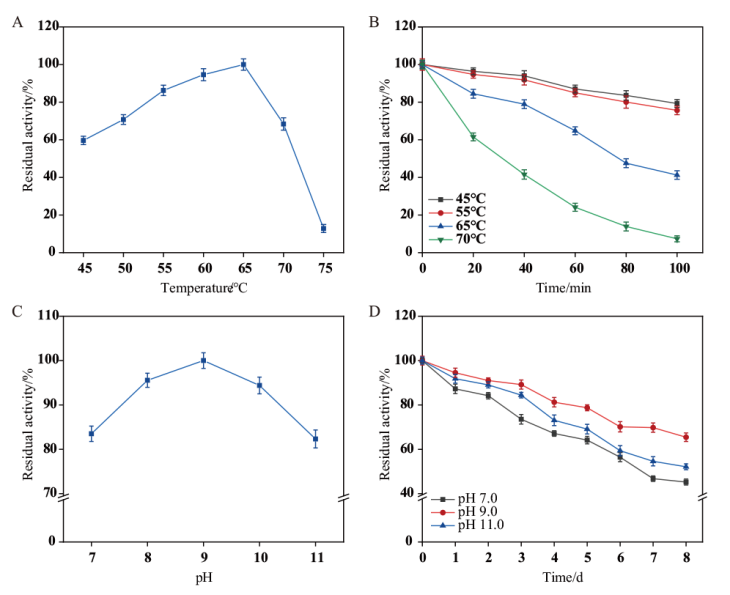
图2 TPRK的酶学性质 A:TPRK的最适作用温度;B:TPRK的热稳定性;C:TPRK的最适pH;D:TPRK的pH稳定性
Fig. 2 Enzymatic properties of TPRK A: Optimal temperature of TPRK; B: thermal stability of TPRK; C: optimal pH of TPRK; D: pH stability of TPRK
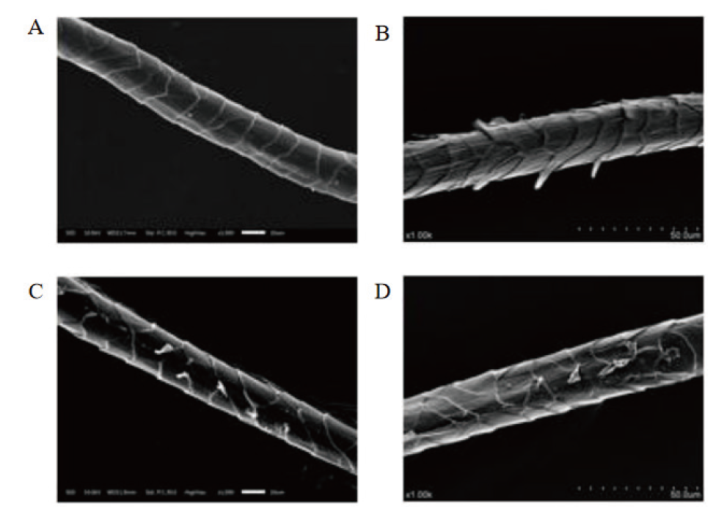
图5 不同蛋白酶水解羊毛鳞片的电镜图 A:未处理羊毛样品;B:TPRK处理;C:碱性蛋白酶处理;D:角蛋白酶处理
Fig. 5 Electron microscopy of wool scales hydrolyzed by different proteases A: Untreated wool sample; B: TPRK treatment; C: alkaline protease treatment; D: keratinase treatment
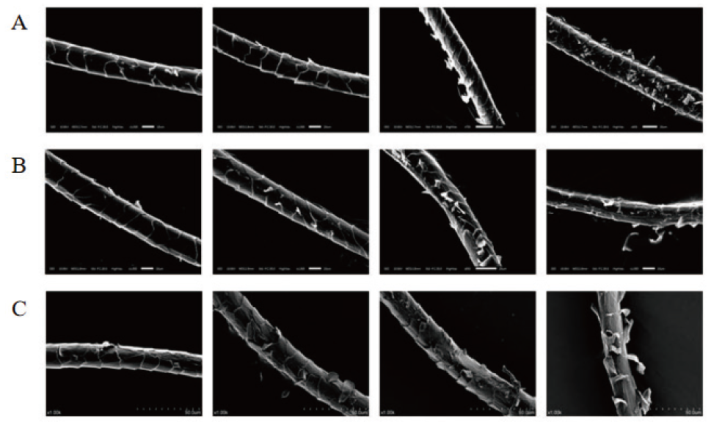
图6 TPRK水解羊毛鳞片的电镜图 A:100 U/mL TPRK依次水解羊毛0.5 h、1 h、1.5 h、2 h电镜图;B:300 U/mL TPRK依次水解羊毛0.5 h、1 h、1.5 h、2 h电镜图;C:500 U/mL TPRK依次水解羊毛0.5 h、1 h、1.5 h、2 h电镜图
Fig. 6 Electron microscopy of TPRK hydrolyzed wool scales A: 100 U/mL TPRK hydrolyzed wool for 0.5 h, 1 h, 1.5 h, and 2 h; B: 300 U/mL TPRK hydrolyzed wool for 0.5 h, 1 h, 1.5 h, and 2 h; C: 500 U/mL TPRK hydrolyzed wool for 0.5 h, 1 h, 1.5 h, and 2 h
| [1] |
Rani S, Kadam V, Rose NM, et al. Wheat starch, gum arabic and chitosan biopolymer treatment of wool fabric for improved shrink resistance finishing[J]. Int J Biol Macromol, 2020, 163: 1044-1052.
doi: S0141-8130(20)33811-3 pmid: 32673714 |
| [2] | 张腾飞. 基于蛋白酶K的羊毛剥鳞技术研究[D]. 上海: 东华大学, 2023. |
| Zhang TF. Reaserch on wool scale-peeling technology based on proteinase K[D]. Shanghai: Donghua University, 2023. | |
| [3] | Li B, Li JY, Shen YQ, et al. Development of environmentally friendly wool shrink-proof finishing technology based on L-cysteine/protease treatment solution system[J]. Int J Mol Sci, 2022, 23(21): 13553. |
| [4] |
Bhari R, Kaur M, Sarup Singh R. Chicken feather waste hydrolysate as a superior biofertilizer in agroindustry[J]. Curr Microbiol, 2021, 78(6): 2212-2230.
doi: 10.1007/s00284-021-02491-z pmid: 33903939 |
| [5] |
An FF, Fang KJ, Liu XM, et al. Protease and sodium alginate combined treatment of wool fabric for enhancing inkjet printing performance of reactive dyes[J]. Int J Biol Macromol, 2020, 146: 959-964.
doi: S0141-8130(19)34207-2 pmid: 31726143 |
| [6] | Wang L, Yao JB, Niu JR, et al. Eco-friendly and highly efficient enzyme-based wool shrinkproofing finishing by multiple padding techniques[J]. Polymers, 2018, 10(11): 1213. |
| [7] | Ren YX, Luo HY, Huang HQ, et al. Improving the catalytic performance of proteinase K from Parengyodontium album for use in feather degradation[J]. Int J Biol Macromol, 2020, 154: 1586-1595. |
| [8] | Li R, Liu Z, Jiang F, et al. Enhancement of thermal stability of proteinase K by biocompatible cholinium-based ionic liquids[J]. Phys Chem Chem Phys, 2022, 24(21): 13057-13065. |
| [9] | Yadollahi E, Shareghi B, Eslami farsani R. Molecular aspects of the interaction of organic solvents and proteinase K: kinetics and docking studies[J]. Iran J Sci Technol Trans A Sci, 2019, 43(1): 57-62. |
| [10] | Skowron PM, Krefft D, Brodzik R, et al. An alternative for proteinase K-heat-sensitive protease from fungus Onygena corvina for biotechnology: cloning, engineering, expression, characterization and special application for protein sequencing[J]. Microb Cell Fact, 2020, 19(1): 135. |
| [11] | Yang JX, Chu N, Chen XW. Preparation of polyoxometalate-based composite by solidification of highly active cobalt-containing polytungstate on polymeric ionic liquid for the efficient isolation of proteinase K[J]. Molecules, 2023, 28(8): 3307. |
| [12] | Yang H, Zhai C, Yu XH, et al. High-level expression of proteinase K from Tritirachium album Limber in Pichia pastoris using multi-copy expression strains[J]. Protein Expr Purif, 2016, 122: 38-44. |
| [13] | Cai P, Duan XP, Wu XY, et al. Recombination machinery engineering facilitates metabolic engineering of the industrial yeast Pichia pastoris[J]. Nucleic Acids Res, 2021, 49(13): 7791-7805. |
| [14] | 李登科, 王欣驰, 陈雪佳, 等. 不同β-葡萄糖苷酶性能分析及对罗汉果苷的转化[J]. 食品科学技术学报, 2019, 37(3): 48-54. |
| Li DK, Wang XC, Chen XJ, et al. Properties analysis and transformation ability to mogroside of β-glucosidase[J]. J Food Sci Technol, 2019, 37(3): 48-54. | |
| [15] |
Raschmanová H, Weninger A, Knejzlík Z, et al. Engineering of the unfolded protein response pathway in Pichia pastoris: enhancing production of secreted recombinant proteins[J]. Appl Microbiol Biotechnol, 2021, 105(11): 4397-4414.
doi: 10.1007/s00253-021-11336-5 pmid: 34037840 |
| [16] | Fauzee YNBM, Taniguchi N, Ishiwata-Kimata Y, et al. The unfolded protein response in Pichia pastoris without external stressing stimuli[J]. FEMS Yeast Res, 2020, 20(7): foaa053. |
| [17] |
Herrera-Estala AL, Fuentes-Garibay JA, Guerrero-Olazarán M, et al. Low specific growth rate and temperature in fed-batch cultures of a beta-propeller phytase producing Pichia pastoris strain under GAP promoter trigger increased KAR2 and PSA1-1 gene expression yielding enhanced extracellular productivity[J]. J Biotechnol, 2022, 352: 59-67.
doi: 10.1016/j.jbiotec.2022.05.010 pmid: 35618082 |
| [18] |
Tyson JR, Stirling CJ. LHS1 and SIL1 provide a lumenal function that is essential for protein translocation into the endoplasmic reticulum[J]. EMBO J, 2000, 19(23): 6440-6452.
doi: 10.1093/emboj/19.23.6440 pmid: 11101517 |
| [19] | Wang YX, Luo X, Zhao YQ, et al. Integrated strategies for enhancing the expression of the AqCoA chitosanase in Pichia pastoris by combined optimization of molecular chaperones combinations and copy numbers via a novel plasmid pMC-GAP[J]. Appl Biochem Biotechnol, 2021, 193(12): 4035-4051. |
| [20] | Li Y, Hu XY, Sang JC, et al. An acid-stable β-glucosidase from Aspergillus aculeatus: gene expression, biochemical characterization and molecular dynamics simulation[J]. Int J Biol Macromol, 2018, 119: 462-469. |
| [21] | 罗漫杰, 徐灿, 王梁, 等. 一种蛋白酶K高表达工程菌株的构建及应用: CN113481225A>[P]. 2021-10-08. |
| Luo MJ, Xu C, Wang L, et al. Construction and application of an engineering strain with high expression of protease K: CN113481225A[P]. 2021-10-08. | |
| [22] | 杨琥, 陈莹, 谭华菊. 一种蛋白酶K多拷贝菌种构建方法: CN112359035A[P]. 2021-02-12. |
| Yang H, Chen Y, Tan HJ. A method for constructing multi copy strains of proteinase K: CN112359035A[P]. 2021-02-12. | |
| [23] | 王华明, 林艳梅. 一种高产蛋白酶K的黑曲霉菌株及其应用: CN104480027A[P]. 2015-04-01. |
| Wang HM, Lin YM. A high-yield proteinase K strain of Aspergillus niger and its application: CN104480027B[P]. 2016-09-21. | |
| [24] |
Li WH, Singer RH. Detecting the non-conventional mRNA splicing and translational activation of HAC1 in budding yeast[J]. Methods Mol Biol, 2022, 2378: 113-120.
doi: 10.1007/978-1-0716-1732-8_8 pmid: 34985697 |
| [25] |
Zahrl RJ, Prielhofer R, Ata Ö, et al. Pushing and pulling proteins into the yeast secretory pathway enhances recombinant protein secretion[J]. Metab Eng, 2022, 74: 36-48.
doi: 10.1016/j.ymben.2022.08.010 pmid: 36057427 |
| [26] | Li C, Lin Y, Zheng XY, et al. Combined strategies for improving expression of Citrobacter amalonaticus phytase in Pichia pastoris[J]. BMC Biotechnol, 2015, 15: 88. |
| [27] | Killian AN, Miller SC, Hines JK. Impact of amyloid polymorphism on prion-chaperone interactions in yeast[J]. Viruses, 2019, 11(4): 349. |
| [28] |
Fürsch J, Voormann C, Kammer KM, et al. Structural probing of Hsp26 activation and client binding by quantitative cross-linking mass spectrometry[J]. Anal Chem, 2021, 93(39): 13226-13234.
doi: 10.1021/acs.analchem.1c02282 pmid: 34542282 |
| [29] | Deng JB, Li JQ, Ma MP, et al. Co-expressing GroEL-GroES, Ssa1-Sis1 and Bip-PDI chaperones for enhanced intracellular production and partial-wall breaking improved stability of porcine growth hormone[J]. Microb Cell Fact, 2020, 19(1): 35. |
| [30] |
de Jesus JR, Aragão AZB, Arruda MAZ, et al. Optimization of a methodology for quantification and removal of zinc gives insights into the effect of this metal on the stability and function of the zinc-binding co-chaperone Ydj1[J]. Front Chem, 2019, 7: 416.
doi: 10.3389/fchem.2019.00416 pmid: 31263692 |
| [31] |
Gaur D, Singh P, Guleria J, et al. The yeast Hsp70 cochaperone Ydj1 regulates functional distinction of Ssa Hsp70s in the Hsp90 chaperoning pathway[J]. Genetics, 2020, 215(3): 683-698.
doi: 10.1534/genetics.120.303190 pmid: 32299842 |
| [32] | Gaur D, Kumar N, Ghosh A, et al. Ydj1 interaction at nucleotide-binding-domain of yeast Ssa1 impacts Hsp90 collaboration and client maturation[J]. PLoS Genet, 2022, 18(11): e1010442. |
| [33] | Li WJ, Zhang N, Wang Q, et al. A sustainable and effective bioprocessing approach for improving anti-felting, anti-pilling and dyeing properties of wool fabric[J]. Fibres Polym, 2021, 22(11): 3045-3054. |
| [1] | 茹扎·也里扎提, 杨宇. 毕赤酵母中外源蛋白表达量的提升策略[J]. 生物技术通报, 2024, 40(3): 118-134. |
| [2] | 赵思佳, 王晓璐, 孙纪录, 田健, 张杰. 代谢工程改造毕赤酵母生产赤藓糖醇[J]. 生物技术通报, 2023, 39(8): 137-147. |
| [3] | 梅欢, 李玥, 刘可蒙, 刘吉华. 小檗碱桥酶高效原核表达及生物合成l-SLR的研究[J]. 生物技术通报, 2023, 39(7): 277-287. |
| [4] | 董聪, 高庆华, 王玥, 罗同阳, 王庆庆. 基于联合策略提高FAD依赖的葡萄糖脱氢酶的酵母表达[J]. 生物技术通报, 2023, 39(6): 316-324. |
| [5] | 赵昕, 杜玉瑶, 殷子扬, 毛淑红. 胆固醇7α-羟化酶在毕赤酵母中的异源表达[J]. 生物技术通报, 2023, 39(10): 304-310. |
| [6] | 王玥, 高庆华, 董聪, 罗同阳, 王庆庆. 密码子优化的吡喃糖氧化酶基因在毕赤酵母中的表达[J]. 生物技术通报, 2022, 38(4): 269-277. |
| [7] | 赵宝顶, 吕佳, 申玉玉, 桂玲, 陈钟秀, 陈杰, 路福平, 黎明. 基于信号肽和分子伴侣策略促进大肠杆菌高效转化尿苷[J]. 生物技术通报, 2022, 38(11): 238-249. |
| [8] | 杨威, 伍茜, 程建国, 罗燕, 王印, 杨泽晓, 姚学萍. 林麝干扰素α基因克隆、表达及转录调控分析[J]. 生物技术通报, 2022, 38(1): 194-204. |
| [9] | 段绪果, 张玉华, 黄婷婷, 丁乾, 栾舒越, 朱秋雨. 化学分子伴侣及诱导条件协同强化Thermotoga maritima α-葡聚糖磷酸化酶可溶性表达[J]. 生物技术通报, 2021, 37(8): 233-242. |
| [10] | 贺小丽, 郭磊周, 韩佳慧, 唐殷, 袁媛, 代其林, 平淑珍, 江世杰. 细菌周质分子伴侣LolA研究进展[J]. 生物技术通报, 2021, 37(8): 275-283. |
| [11] | 廖兆民, 蔡俊, 林建国, 杜馨, 王常高. 黑曲霉葡萄糖氧化酶基因在毕赤酵母中的表达及产酶条件的优化[J]. 生物技术通报, 2021, 37(6): 97-107. |
| [12] | 杨悦, 陶妍, 谢晶, 钱韻芳. 基于重组毕赤酵母的草鱼C型溶菌酶生物合成及其抑菌活性[J]. 生物技术通报, 2021, 37(12): 169-179. |
| [13] | 赵震, 王莎莎, 吕星星, 陶妍, 谢晶, 钱韻芳. 重组毕赤酵母产青蛤Mytimacin抗菌肽的表达研究[J]. 生物技术通报, 2020, 36(5): 150-158. |
| [14] | 闵琪, 高子涵, 姚银, 张华山, 熊海容, 张莉. 共表达HAC1和分子伴侣基因对甘露聚糖酶在毕赤酵母中表达的影响[J]. 生物技术通报, 2020, 36(5): 159-168. |
| [15] | 李雅楠, 余利红, 陈新美, 杨浩萌, 黄火清. 来源于Penicillium sp.C1的水产用中性植酸酶基因在毕赤酵母中的表达及性质研究[J]. 生物技术通报, 2020, 36(2): 134-141. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||