








生物技术通报 ›› 2024, Vol. 40 ›› Issue (9): 74-81.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0441
• 薯类作物生物技术专题(专题主编:徐建飞,尚轶) • 上一篇 下一篇
赵平娟1,2( ), 林晨俞1, 王梦月1, 张秀春1,2, 李淑霞1,2, 阮孟斌1,2(
), 林晨俞1, 王梦月1, 张秀春1,2, 李淑霞1,2, 阮孟斌1,2( )
)
收稿日期:2024-05-11
出版日期:2024-09-26
发布日期:2024-10-12
通讯作者:
阮孟斌,男,博士,研究员,研究方向:作物抗逆机理;E-mail: ruanmmmmmengbin@itbb.org.cn作者简介:赵平娟,女,博士,副研究员,研究方向:作物抗逆机理;E-mail: zhaopingjuan@itbb.org.cn林晨俞同为本文第一作者
基金资助:
ZHAO Ping-juan1,2( ), LIN Chen-yu1, WANG Meng-yue1, ZHANG Xiu-chun1,2, LI Shu-xia1,2, RUAN Meng-bin1,2(
), LIN Chen-yu1, WANG Meng-yue1, ZHANG Xiu-chun1,2, LI Shu-xia1,2, RUAN Meng-bin1,2( )
)
Received:2024-05-11
Published:2024-09-26
Online:2024-10-12
摘要:
【目的】山梨糖醇脱氢酶(SDH)在调控蔷薇科植物果糖和山梨醇转化中起重要作用,也参与植物对逆境的应答过程,木薯SDH功能的研究可以为培育优质木薯种质提供理论基础。【方法】以‘SC124’的cDNA为模板克隆木薯SDH基因,并利用实时荧光定量PCR分析木薯SDH基因的组织特异性及其对干旱、低温、PEG和ABA的响应模式。通过筛选木薯干旱和低温混合的酵母cDNA文库,并利用Y2H点对点及其双分子荧光互补(BiFC)实验确认与目标蛋白的关系。【结果】木薯SDH基因CDS全长1 092 bp,编码364个氨基酸,与数据库中的序列无差异。MeSDH蛋白含有催化锌结合位点, NADP结合位点,结构锌结合位点,属于MDR超家族。烟草叶片表皮细胞瞬时表达显示MeSDH蛋白定位于细胞核。MeSDH基因的表达量在功能叶、幼嫩叶、须根和茎中依次降低。MeSDH基因受干旱、低温和PEG胁迫诱导在木薯叶片上调表达,在ABA处理后的木薯叶片和根系中都显著上调表达。文库筛选、Y2H点对点和BiFC实验证实MeH1.2与MeSDH互作。【结论】MeSDH基因可以响应多种胁迫上调表达,并可能在蛋白水平和MeH1.2共同作用。
赵平娟, 林晨俞, 王梦月, 张秀春, 李淑霞, 阮孟斌. 木薯SDH蛋白的序列分析及其与MeH1.2关系的研究[J]. 生物技术通报, 2024, 40(9): 74-81.
ZHAO Ping-juan, LIN Chen-yu, WANG Meng-yue, ZHANG Xiu-chun, LI Shu-xia, RUAN Meng-bin. Sequence Analysis of MeSDH Protein and Its Relationship with MeH1.2 in Cassava[J]. Biotechnology Bulletin, 2024, 40(9): 74-81.
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 目的Purpose |
|---|---|---|
| MeSDH-F | atgggtaaaggagggatgtctc | 基因克隆 Gene cloning |
| MeSDH-R | cagattaaacatgaccttaatg | |
| 1300-MeSDH-F | tgatacatatgcccgtcgac atgggtaaaggagggatgtctc | 植物表达载体 Plant expression vector |
| 1300-MeSDH-R | ctcaccatggatccggtacc cagattaaacatgaccttaatg | |
| Actin1-F | tggattctggtgatggtgtgagt | 内参基因 Reference gene |
| Actin1-R | ccgttcagcagtggtggtga | |
| MeSDH-Q-F | gtgaaggagccgatggtga | MeSDH定量PCR MeSDH RT-qPCR |
| MeSDH-Q-R | ccacacggtctccaggtaaaa | |
| H1.2-PGBKT7-X | tctagaatggccgactctgaagttcaggct | 酵母文库筛选 Yeast library screening |
| H1.2-PGBKT7-B | ggatcctttcttcgccttcttcgctgtc | |
| MeSDH-AD-F | ccatggaggccagtgaattcatgggtaaaggagggatgtc | Y2H载体 Y2H vector |
| MeSDH-AD-R | agctcgagctcgatggatccttacagattaaacatgacctt | |
| MeSDH-C-F | tctagaatggccgactctgaagttcaggct | BiFC载体 BiFC vector |
| MeSDH-C-R | ggatcctttcttcgccttcttcgctgtc |
表1 实验用引物序列
Table 1 Primers’ sequences used in the study
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 目的Purpose |
|---|---|---|
| MeSDH-F | atgggtaaaggagggatgtctc | 基因克隆 Gene cloning |
| MeSDH-R | cagattaaacatgaccttaatg | |
| 1300-MeSDH-F | tgatacatatgcccgtcgac atgggtaaaggagggatgtctc | 植物表达载体 Plant expression vector |
| 1300-MeSDH-R | ctcaccatggatccggtacc cagattaaacatgaccttaatg | |
| Actin1-F | tggattctggtgatggtgtgagt | 内参基因 Reference gene |
| Actin1-R | ccgttcagcagtggtggtga | |
| MeSDH-Q-F | gtgaaggagccgatggtga | MeSDH定量PCR MeSDH RT-qPCR |
| MeSDH-Q-R | ccacacggtctccaggtaaaa | |
| H1.2-PGBKT7-X | tctagaatggccgactctgaagttcaggct | 酵母文库筛选 Yeast library screening |
| H1.2-PGBKT7-B | ggatcctttcttcgccttcttcgctgtc | |
| MeSDH-AD-F | ccatggaggccagtgaattcatgggtaaaggagggatgtc | Y2H载体 Y2H vector |
| MeSDH-AD-R | agctcgagctcgatggatccttacagattaaacatgacctt | |
| MeSDH-C-F | tctagaatggccgactctgaagttcaggct | BiFC载体 BiFC vector |
| MeSDH-C-R | ggatcctttcttcgccttcttcgctgtc |
| A 实验组1 | B 实验组2 | C 实验组3 | D 实验组4 |
|---|---|---|---|
| pGADT7-T | pGADT7-T | pGADT7-MeSDH | pGADT7 |
| pGBKT7-53 | pGBKT7-lam | pGBKT7-MeH1.2 | pGBKT7-MeH1.2 |
表2 点样设计
Table 2 Sample design
| A 实验组1 | B 实验组2 | C 实验组3 | D 实验组4 |
|---|---|---|---|
| pGADT7-T | pGADT7-T | pGADT7-MeSDH | pGADT7 |
| pGBKT7-53 | pGBKT7-lam | pGBKT7-MeH1.2 | pGBKT7-MeH1.2 |
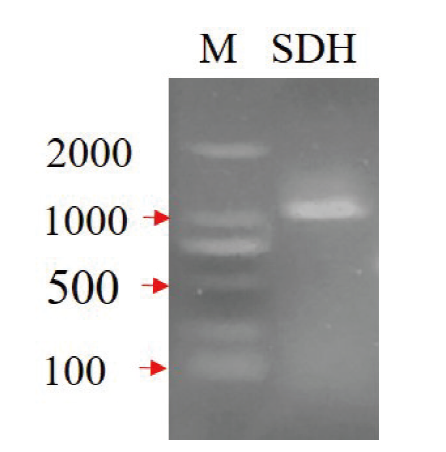
图1 MeSDH基因PCR扩增 M: DL2000 DNA marker; SDH: MeSDH基因PCR扩增产物
Fig. 1 PCR amplification of MeSDH gene M: DL2000 DNA marker; SDH: PCR amplification product of MeSDH gene
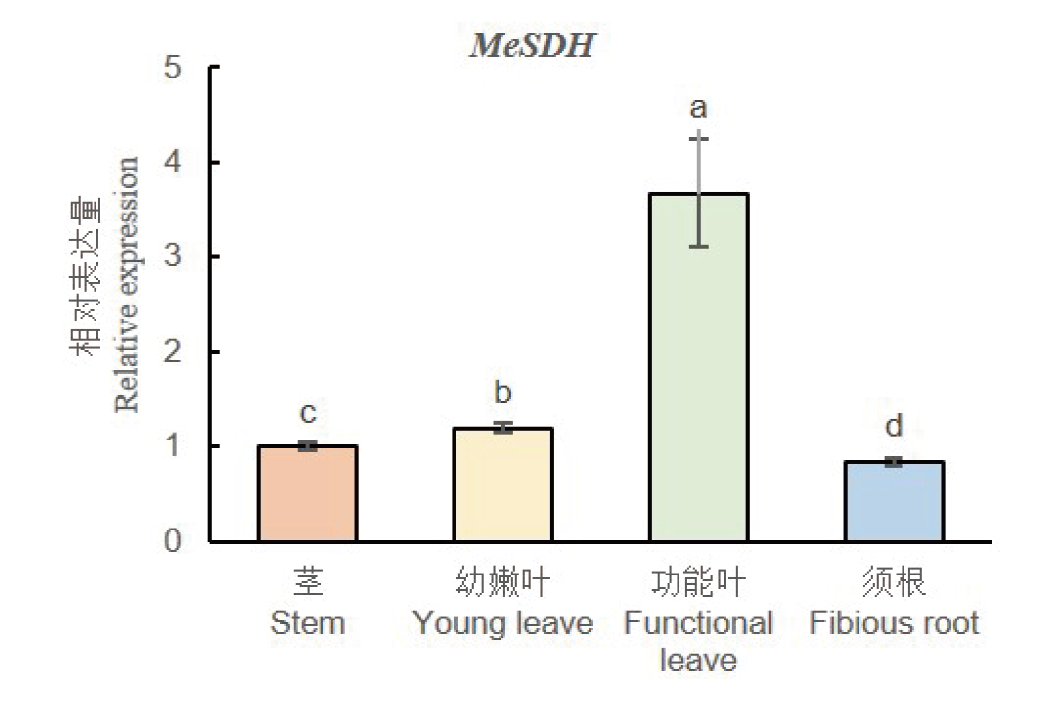
图3 木薯MeSDH基因的组织特异性表达 不同字母表示基因在不同组织中的表达量在P<0.05水平有显著性差异
Fig. 3 Tissue-specific expressions of MeSDH gene in cassava Different letters show that gene expression is significant difference among other tissues on P<0.05
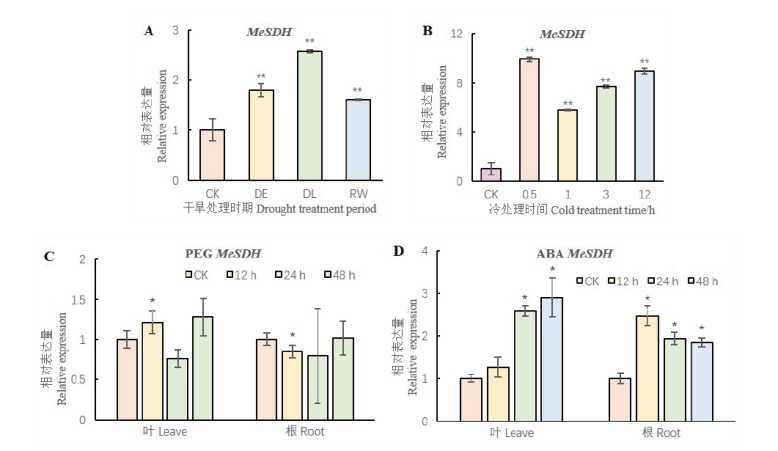
图5 叶片和根系MeSDH基因对不同胁迫的响应模式 *,**表示基因在处理和对照中的表达量在P<0.05 和P<0.01水平有显著性差异
Fig. 5 Expression patterns of MeSDH in the leaves and roots under different treatment *, ** indicate gene expression is significant difference between treatment and control groups in P<0.05 and P<0.01 level
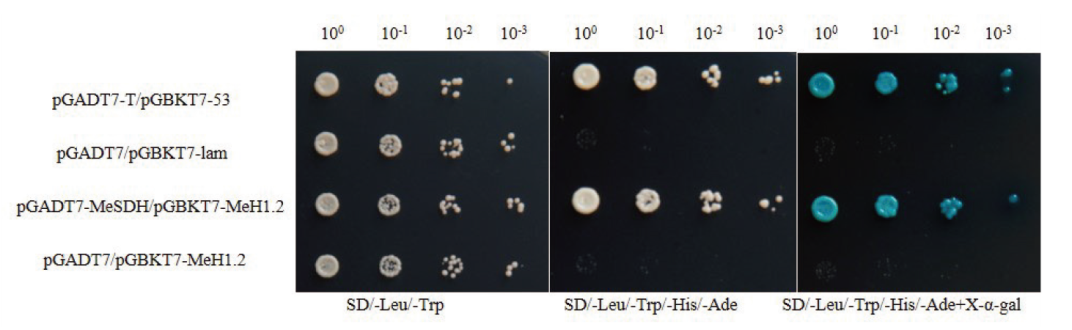
图6 Y2H验证MeSDH与MeH1.2蛋白间的互作关系 pGADT7-T/pGBKT7-53 为阳性对照;pGADT7-T/pGBKT7-Lam和pGADT7-T/pGBKT7-MeH1.2 为阴性对照;100、10-1、10-2、10-3 表示菌液的稀释梯度
Fig. 6 Relationship between MeSDH and MeH1.2 proteins verified by Y2H pGADT7-T/pGBKT7-53 is positive control; pGADT7-T/pGBKT7-Lam and pGADT7-T/pGBKT7-MeH1.2 are negative control; 100, 10-1, 10-2, and 10-3 refers to the dilution gradients of bacterial solution

图7 BiFC验证MeSDH与MeH1.2蛋白间的互作关系 MeTGA2-nYFP/MeHistone3-cYFP为阳性对照;MeH12-nYFP/cYFP和-nYFP/MeSDH-cYFP为阴性对照
Fig. 7 Relationship between MeSDH and MeH1.2 proteins verified by BiFC MeTGA2-nYFP/MeHistone3-cYFP is positive control; MeH12-nYFP/cYFP和-nYFP/MeSDH-cYFP are negative control
| [1] | Khumaida N, Ardie S, Dianasari M, et al. Cassava(Manihot esculenta crantz.) improvement through gamma irradiation[J]. Procedia Food Sci, 2015, 3: 27-34. |
| [2] | 付丹丹, 韩昕儒, 问锦尚, 等. 基于Rotterdam模型的中国热带农产品进口市场格局研究[J]. 中国农业资源与区划, 2022, 43(11):168-177. |
| Fu DD, Han XR, Wen JS, et al. China's tropical agricultural product imports: A rotterdam model anlysis[J]. Chinese Journal of Agricultural Resources and Rrgional Planning, 2022, 43(11):168-177. | |
| [3] | Muiruri SK, Ntui VO, Tripathi L, et al. Mechanisms and approaches towards enhanced drought tolerance in cassava(Manihot esculenta)[J]. Curr Plant Biol, 2021, 28: 100227. |
| [4] |
Jia Y, Wong DCJ, Sweetman C, et al. New insights into the evolutionary history of plant sorbitol dehydrogenase[J]. BMC Plant Biol, 2015, 15: 101.
doi: 10.1186/s12870-015-0478-5 pmid: 25879735 |
| [5] |
Chen T, Zhang ZQ, Li BQ, et al. Molecular basis for optimizing sugar metabolism and transport during fruit development[J]. aBIOTECH, 2021, 2(3): 330-340.
doi: 10.1007/s42994-021-00061-2 pmid: 36303881 |
| [6] | Dai MS, Shi ZB, Xu CJ. Genome-wide analysis of sorbitol dehydrogenase(SDH)genes and their differential expression in two sand pear(Pyrus pyrifolia)fruits[J]. Int J Mol Sci, 2015, 16(6): 13065-13083. |
| [7] |
Nosarzewski M, Archbold DD. Tissue-specific expression of SORBITOL DEHYDROGENASE in apple fruit during early development[J]. J Exp Bot, 2007, 58(7): 1863-1872.
pmid: 17404378 |
| [8] | Wanek W, Richter A. L-Iditol: NAD+5-oxidoreductase in Viscum album: utilization of host-derived sorbitol[J]. Plant Physiol Biochem, 1993, 31: 205-211. |
| [9] |
Kuo TM, Doehlert DC, Crawford CG. Sugar metabolism in germinating soybean seeds: evidence for the sorbitol pathway in soybean axes[J]. Plant Physiol, 1990, 93(4): 1514-1520.
doi: 10.1104/pp.93.4.1514 pmid: 16667649 |
| [10] |
Nosarzewski M, Downie AB, Wu BH, et al. The role of SORBITOL DEHYDROGENASE in Arabidopsis thaliana[J]. Funct Plant Biol, 2012, 39(6): 462-470.
doi: 10.1071/FP12008 pmid: 32480797 |
| [11] |
Ohta K, Moriguchi R, Kanahama K, et al. Molecular evidence of sorbitol dehydrogenase in tomato, a Non-Rosaceae plant[J]. Phytochemistry, 2005, 66(24): 2822-2828.
doi: 10.1016/j.phytochem.2005.09.033 pmid: 16289145 |
| [12] |
Nadwodnik J, Lohaus G. Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens[J]. Planta, 2008, 227(5): 1079-1089.
doi: 10.1007/s00425-007-0682-0 pmid: 18188589 |
| [13] | 刘政, 安莉园, 林世华, 等. 梨树山梨醇代谢及其调控因子研究进展[J]. 中国南方果树, 2018, 47(4): 165-168. |
| Liu Z, An LY, Lin SH, et al. Research progress on sorbitol metabolism and its regulatory factors in pear trees[J]. South China Fruits, 2018, 47(4): 165-168. | |
| [14] |
聂佩显, 王来平, 韩雪平, 等. 杏山梨醇脱氢酶基因克隆及其在果实发育过程中的表达与酶活性分析[J]. 核农学报, 2021, 35(2): 291-297.
doi: 10.11869/j.issn.100-8551.2021.02.0291 |
|
Nie PX, Wang LP, Han XP, et al. Cloning of NAD-dependent sorbitol dehydrogenase gene from apricot fruit and analysis of its expression and enzyme activity[J]. J Nucl Agric Sci, 2021, 35(2): 291-297.
doi: 10.11869/j.issn.100-8551.2021.02.0291 |
|
| [15] |
Yamada K, Oura Y, Mori H, et al. Cloning of NAD-dependent sorbitol dehydrogenase from apple fruit and gene expression[J]. Plant Cell Physiol, 1998, 39(12): 1375-1379.
pmid: 10050321 |
| [16] | 任秋萍, 周淑梅, 王秀玲. 苹果山梨醇脱氢酶的基因表达、组织分布和活性调控[J]. 中国细胞生物学学报, 2010, 32(4): 668-672. |
| Ren QP, Zhou SM, Wang XL. Gene expression, tissue distribution and activity regulation of NAD+-dependent sorbitol dehydrogenase in apple[J]. Chin J Cell Biol, 2010, 32(4): 668-672. | |
| [17] | Bantog NA, Yamada K, Niwa N, et al. Gene expression of NAD+-dependent sorbitol dehydrogenase and NADP+-dependent sorbitol-6-phosphate dehydrogenase during development of loquat(Eriobotrya japonica lindl.) fruit[J]. Engei Gakkai Zasshi, 2000, 69(3): 231-236. |
| [18] |
Kelker NE, Anderson RL. Sorbitol metabolism in Aerobacter aerogenes[J]. J Bacteriol, 1971, 105(1): 160-164.
doi: 10.1128/jb.105.1.160-164.1971 pmid: 5541002 |
| [19] |
Sarthy AV, Schopp C, Idler KB. Cloning and sequence determination of the gene encoding sorbitol dehydrogenase from Saccharomyces cerevisiae[J]. Gene, 1994, 140(1): 121-126.
pmid: 8125328 |
| [20] | Yamaki S, Ishikawa K. Roles of four sorbitol related enzymes and invertase in the seasonal alteration of sugar metabolism in apple tissue[J]. J Amer Soc Hort Sci, 1986, 111(1): 134-137. |
| [21] | Loescher WH. Physiology and metabolism of sugar alcohols in higher plants[J]. Physiol Plant, 1987, 70(3): 553-557. |
| [22] | Wu BH, Li SH, Nosarzewski M, et al. Sorbitol dehydrogenase gene expression and enzyme activity in apple: tissue specificity during bud development and response to rootstock vigor and growth manipulation[J]. J Amer Soc Hort Sci, 2010, 135(4): 379-387. |
| [23] |
Brown PH, Bellaloui N, Hu H, et al. Transgenically enhanced sorbitol synthesis facilitates phloem boron transport and increases tolerance of tobacco to boron deficiency[J]. Plant Physiol, 1999, 119(1): 17-20.
doi: 10.1104/pp.119.1.17 pmid: 9880341 |
| [24] |
Bellaloui N, Brown PH, Dandekar AM. Manipulation of in vivo sorbitol production alters boron uptake and transport in tobacco[J]. Plant Physiol, 1999, 119(2): 735-742.
doi: 10.1104/pp.119.2.735 pmid: 9952470 |
| [25] | Wang T, Hou M, Zhao N, et al. Cloning and expression of the sorbitol dehydrogenase gene during embryonic development and temperature stress in Artemia sinica[J]. Gene, 2013, 521(2): 296-302. |
| [26] |
El-Kabbani O, Darmanin C, Chung RPT. Sorbitol dehydrogenase: structure, function and ligand design[J]. Curr Med Chem, 2004, 11(4): 465-476.
pmid: 14965227 |
| [27] | Wu T, Wang Y, Zheng Y, et al. Suppressing sorbitol synthesis substantially alters the global expression profile of stress response genes in apple(Malus domestica)leaves[J]. Plant Cell Physiol, 2015, 56(9): 1748-1761. |
| [28] | Aguayo MF, Ampuero D, Mandujano P, et al. Sorbitol dehydrogenase is a cytosolic protein required for sorbitol metabolism in Arabidopsis thaliana[J]. Plant Sci, 2013, 205-206: 63-75. |
| [29] | Bellaloui N, Yadavc RC, Chern MS, et al. Transgenically enhanced sorbitol synthesis facilitates phloem-boron mobility in rice[J]. Physiol Plant, 2003, 117(1): 79-84. |
| [30] |
Hergeth SP, Schneider R. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle[J]. EMBO Rep, 2015, 16(11): 1439-1453.
doi: 10.15252/embr.201540749 pmid: 26474902 |
| [31] | Andrés M, García-Gomis D, Ponte I, et al. Histone H1 post-translational modifications: update and future perspectives[J]. Int J Mol Sci, 2020, 21(16): 5941. |
| [32] | Wu X, Xu JN, Meng XN, et al. Linker histone variant HIS1-3 and WRKY1 oppositely regulate salt stress tolerance in Arabidopsis[J]. Plant Physiol, 2022, 189(3): 1833-1847. |
| [33] | Zhao PJ, Guo X, Wang B, et al. Overexpression of MeH1.2 gene inhibited plant growth and increased branch root differentiation in transgenic cassava[J]. Crop Sci, 2021, 61(4): 2639-2650. |
| [34] |
Zhao PJ, Liu P, Shao JF, et al. Analysis of different strategies adapted by two cassava cultivars in response to drought stress: ensuring survival or continuing growth[J]. J Exp Bot, 2015, 66(5): 1477-1488.
doi: 10.1093/jxb/eru507 pmid: 25547914 |
| [35] |
薛满德, 赵峰月, 李洁, 等. 组蛋白变体在植物表观遗传调控中的研究进展[J]. 生物技术通报, 2022, 38(7): 1-12.
doi: 10.13560/j.cnki.biotech.bull.1985.2022-0071 |
| Xue MD, Zhao FY, Li J, et al. Advances in histone variants in plant epigenetic regulation[J]. Biotechnol Bull, 2022, 38(7): 1-12. | |
| [36] |
Kalashnikova AA, Rogge RA, Hansen JC. Linker histone H1 and protein-protein interactions[J]. Biochimica et Biophysica Acta 2016, 1859(3):455-461.
doi: 10.1016/j.bbagrm.2015.10.004 pmid: 26455956 |
| [37] | 凡慧明. 砂梨山梨醇脱氢酶基因PpSDH3和PpSDH9调控水心病发生的分子机制研究[D]. 扬州: 扬州大学, 2021. |
| Fan HM. Study on the molecular mechanism of sorbitol dehydrogenase genes PpSDH3 and PpSDH9 regulating the occurrence of water heart disease in Pyrus pyrifolia[D]. Yangzhou: Yangzhou University, 2021. |
| [1] | 童玮婧, 罗数, 陆新露, 沈建福, 陆柏益, 李开绵, 马秋香, 张鹏. CRISPR/Cas9编辑MeHNL基因创制低生氰糖苷木薯[J]. 生物技术通报, 2024, 40(9): 11-19. |
| [2] | 马博涛, 伍国强, 魏明. bZIP转录因子在植物逆境胁迫响应和生长发育中的作用[J]. 生物技术通报, 2024, 40(9): 148-160. |
| [3] | 谭博文, 张懿, 张鹏, 王振宇, 马秋香. 木薯镁离子转运蛋白家族基因的鉴定及生物信息学分析[J]. 生物技术通报, 2024, 40(9): 20-32. |
| [4] | 杨巍, 赵丽芬, 唐兵, 周麟笔, 杨娟, 莫传园, 张宝会, 李飞, 阮松林, 邓英. 芥菜SRO基因家族全基因组鉴定与表达分析[J]. 生物技术通报, 2024, 40(8): 129-141. |
| [5] | 胡雅丹, 伍国强, 刘晨, 魏明. MYB转录因子在调控植物响应逆境胁迫中的作用[J]. 生物技术通报, 2024, 40(6): 5-22. |
| [6] | 李昊, 伍国强, 魏明, 韩悦欣. 甜菜BvBADH基因家族全基因组鉴定及其高盐胁迫下的表达分析[J]. 生物技术通报, 2024, 40(2): 233-244. |
| [7] | 肖亮, 吴正丹, 陆柳英, 施平丽, 尚小红, 曹升, 曾文丹, 严华兵. 木薯重要性状基因的研究进展[J]. 生物技术通报, 2023, 39(6): 31-48. |
| [8] | 姚晓文, 梁晓, 陈青, 伍春玲, 刘迎, 刘小强, 税军, 乔阳, 毛奕茗, 陈银华, 张银东. 二斑叶螨抗性木薯木质素合成途径基因表达特性研究[J]. 生物技术通报, 2023, 39(2): 161-171. |
| [9] | 于晓玲, 李文彬, 李智博, 阮孟斌. 木薯MeMYC2.2基因耐低温功能研究[J]. 生物技术通报, 2023, 39(1): 224-231. |
| [10] | 韩志玲, 陈青, 梁晓, 伍春玲, 刘迎, 伍牧锋, 徐雪莲. 二斑叶螨取食抗、感螨木薯品种对茉莉酸信号途径基因表达的影响[J]. 生物技术通报, 2022, 38(6): 211-220. |
| [11] | 张斌, 杨昕霞. 水稻响应盐胁迫关键转录因子的鉴定[J]. 生物技术通报, 2022, 38(3): 9-15. |
| [12] | 邹良平, 郭鑫, 起登凤, 翟敏, 李壮, 赵平娟, 彭明, 牛兴奎. 低氮胁迫诱导木薯幼苗花青素积累及其基因表达[J]. 生物技术通报, 2022, 38(2): 75-82. |
| [13] | 罗维, 牟琼, 舒健虹, 吴佳海, 王小利. 高羊茅FaFT基因表达、蛋白互作及生物学功能分析[J]. 生物技术通报, 2021, 37(4): 8-17. |
| [14] | 许涛, 夏冬健, 万菁, 姜书涵, 宋江华. F-box蛋白参与植物逆境胁迫研究进展[J]. 生物技术通报, 2021, 37(12): 205-211. |
| [15] | 杨树萍, 张琳, 徐继林. 藻类中添加剂的应用研究进展[J]. 生物技术通报, 2020, 36(2): 178-187. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||