








生物技术通报 ›› 2023, Vol. 39 ›› Issue (1): 224-231.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0461
于晓玲1,2( ), 李文彬1,2, 李智博1, 阮孟斌1,2(
), 李文彬1,2, 李智博1, 阮孟斌1,2( )
)
收稿日期:2022-04-15
出版日期:2023-01-26
发布日期:2023-02-02
作者简介:于晓玲,女,博士,研究方向:农业生物技术;E-mail: 基金资助:
YU Xiao-ling1,2( ), LI Wen-bin1,2, LI Zhi-bo1, RUAN Meng-bin1,2(
), LI Wen-bin1,2, LI Zhi-bo1, RUAN Meng-bin1,2( )
)
Received:2022-04-15
Published:2023-01-26
Online:2023-02-02
摘要:
MYC2(MYeloCytomatosis)转录因子是植物应对逆境胁迫过程中茉莉酸信号传导相关的核心转录因子。本研究旨在初步分析木薯MeMYC2.2基因在低温胁迫响应中的功能。利用生物信息学分析木薯MeMYC2.1和MeMYC2.2基因的结构及其编码蛋白的理化性质;通过定量PCR分析了上述2个基因在木薯组培苗叶片中对低温胁迫的响应;通过转基因拟南芥研究MeMYC2.2的抗冻功能。木薯组培苗叶片中2个MeMYC2基因的表达均在低温胁迫早期被诱导,其中,与MeMYC2.1相比,MeMYC2.2差异表达更显著。MeMYC2.2蛋白主要定位于细胞核中,且在酵母中具有明显转录自激活功能,表明该蛋白具有转录因子特性。与野生型相比,过表达MeMYC2.2的转基因拟南芥抗冻能力显著提高。在低温处理下,CBF3基因在转基因拟南芥中的表达量要明显高于其在野生型的表达量,但另外3个CBF基因在转基因拟南芥中的表达量明显下降。木薯MeMYC2.2的表达受低温和茉莉酸调控,可以提高植物的抗冻性,且可能影响CBF基因对低温的响应。本研究为进一步利用MeMYC2基因改良木薯的低温耐受性奠定了理论基础。
于晓玲, 李文彬, 李智博, 阮孟斌. 木薯MeMYC2.2基因耐低温功能研究[J]. 生物技术通报, 2023, 39(1): 224-231.
YU Xiao-ling, LI Wen-bin, LI Zhi-bo, RUAN Meng-bin. Cold Resistance Function Analysis of Cassava MeMYC2.2[J]. Biotechnology Bulletin, 2023, 39(1): 224-231.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 引物用途 Primer usage |
|---|---|---|
| MeMYC 2.2-F | GCTCTAGAATGAATCTGT- GGACGGACGA | 基因克隆 Gene cloning |
| MeMYC 2.2-R | GTGGATCCGGAGTCACCA- ACTTTGGTTG | |
| MeActin-qF | TGATGAGTCTGGTCCATCCA | 内参基因 |
| MeActin-qR | CCTCCTACGACCCAATCTCA | Reference gene |
| MeMYC 2.2-qF | CCCTGACCAGGGTGAGAATGAT | 实时荧光定量 RT-qPCR |
| MeMYC 2.2-qR | CCCTTTCGACACATGCTGAT | |
| AtActin1-qF | GGGCAAGTGATCACCATTGG | 转基因拟南芥基因表达分析 Gene expression analysis of transgenic Arabidopsis |
| AtActin1-qR | TGGAGCCAAAGCAGTGATCTC | |
| AtCBF1-qF | ATTATTGTCCGACGTTGGCCA | |
| AtCBF1-qR | GCGAAGTTGAGACATGCTGAT | |
| AtCBF2-qF | TGCCTGTCTCAATTTCGCTGA | |
| AtCBF2-qR | CGGCCATGTTATCCAACAAAC | |
| AtCBF3-qF | CAAGGATTTGGCTCGGAACAT | |
| AtCBF3-qR | TCCACCAACGTCTCCTCCATG | |
| AtCBF4-qF | AAGCTGCAATGGCGTTTCAGA | |
| AtCBF4-qR | CGTCACCCACTCCGTCAAAGT |
表1 本研究所用引物
Table 1 Primers used in this study
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 引物用途 Primer usage |
|---|---|---|
| MeMYC 2.2-F | GCTCTAGAATGAATCTGT- GGACGGACGA | 基因克隆 Gene cloning |
| MeMYC 2.2-R | GTGGATCCGGAGTCACCA- ACTTTGGTTG | |
| MeActin-qF | TGATGAGTCTGGTCCATCCA | 内参基因 |
| MeActin-qR | CCTCCTACGACCCAATCTCA | Reference gene |
| MeMYC 2.2-qF | CCCTGACCAGGGTGAGAATGAT | 实时荧光定量 RT-qPCR |
| MeMYC 2.2-qR | CCCTTTCGACACATGCTGAT | |
| AtActin1-qF | GGGCAAGTGATCACCATTGG | 转基因拟南芥基因表达分析 Gene expression analysis of transgenic Arabidopsis |
| AtActin1-qR | TGGAGCCAAAGCAGTGATCTC | |
| AtCBF1-qF | ATTATTGTCCGACGTTGGCCA | |
| AtCBF1-qR | GCGAAGTTGAGACATGCTGAT | |
| AtCBF2-qF | TGCCTGTCTCAATTTCGCTGA | |
| AtCBF2-qR | CGGCCATGTTATCCAACAAAC | |
| AtCBF3-qF | CAAGGATTTGGCTCGGAACAT | |
| AtCBF3-qR | TCCACCAACGTCTCCTCCATG | |
| AtCBF4-qF | AAGCTGCAATGGCGTTTCAGA | |
| AtCBF4-qR | CGTCACCCACTCCGTCAAAGT |
| 分析内容 Item | MeMYC2.1 | MeMYC2.2 |
|---|---|---|
| 开放阅读框Open read frame(ORF) | 2 055 bp | 2 019 bp |
| 内含子 Intron | - | - |
| 蛋白质的分子量Molecular weight(Mw) | 75 043.89 | 74 015.17 |
| 等电点 Isoelectric point(pI) | 5.49 | 5.14 |
| Plant-mPLoc软件预测 Plant-mPLoc software prediction | 定位于细胞核 | 定位于细胞核 |
| SignalP | 非分泌蛋白 | 非分泌蛋白 |
| 保守功能结构域Conservative functional domains | bHLH-MYC保守结构域(N69-20),及HLH DNA结合域(N505-556) | bHLH-MYC保守结构域(N5-243),及HLH DNA结合域(N218-239) |
表2 MeMYC2生物信息学分析
Table 2 Bioinformatics analysis of MeMYC2
| 分析内容 Item | MeMYC2.1 | MeMYC2.2 |
|---|---|---|
| 开放阅读框Open read frame(ORF) | 2 055 bp | 2 019 bp |
| 内含子 Intron | - | - |
| 蛋白质的分子量Molecular weight(Mw) | 75 043.89 | 74 015.17 |
| 等电点 Isoelectric point(pI) | 5.49 | 5.14 |
| Plant-mPLoc软件预测 Plant-mPLoc software prediction | 定位于细胞核 | 定位于细胞核 |
| SignalP | 非分泌蛋白 | 非分泌蛋白 |
| 保守功能结构域Conservative functional domains | bHLH-MYC保守结构域(N69-20),及HLH DNA结合域(N505-556) | bHLH-MYC保守结构域(N5-243),及HLH DNA结合域(N218-239) |
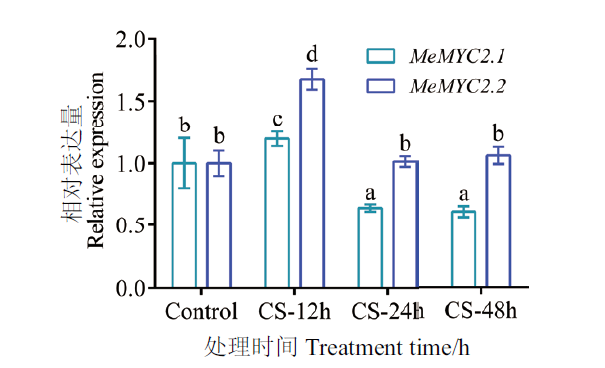
图1 不同冷胁迫时间下MeMYC2的表达分析 冷胁迫温度为10℃,样品为木薯种苗新生叶片;荧光定量PCR分析,内参基因为MeActin1;显著差异用不同字母表示(P≤0.05);误差线为每组处理的标准误差(n=3),下同
Fig. 1 Expression patterns of MeMYC2 under different cold stress(CS)time In vitro cassava seedling leaves were treated by low temperature at 10℃. New expanding leaves of stressed or unstressed seedlings were used in qPCR analysis. The MeActin1 was used as housekeep gene. Error bars are ± SD(n=3), different letters indicate significant differences with P≤0.05(ANOVA test), the same below

图2 MeMYC2.2转录因子验证 A:亚细胞定位结果;B:酵母自激活实验
Fig. 2 Validation experiments of transcription factor MeMYC2.2 A:Subcellular localization results. B:Yeast autoactivation assay
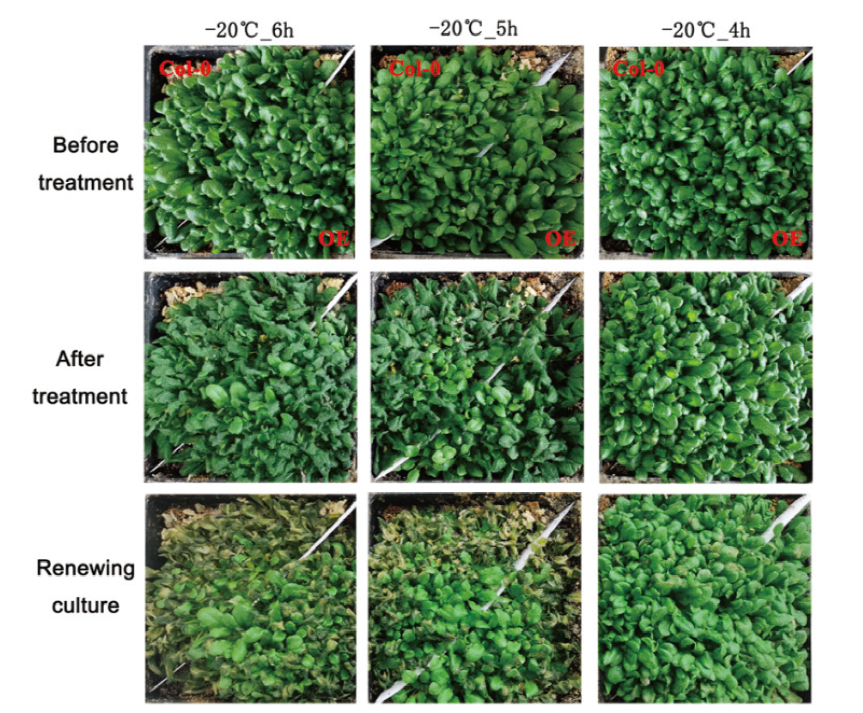
图4 转基因和野生型植株表现出不同的耐冻能力 两周龄的幼苗置于-20℃低温处理4-6 h,然后在正常培养条件下恢复培养5 d
Fig. 4 Wild-type and transgenic plants demonstrating different freezing tolerance abilities Two-week-old seedlings were treated by cold at -20℃ for 4-6 h, and then these treated seedlings were recovered for 5 d under normal conditions
| 基因型 Genotype | 冷冻处理 Freezing treatment/h | 存活率 Survival rate/% |
|---|---|---|
| Col-0 | 4 | 100 |
| 5 | 74.2±1.5 | |
| 6 | 29.4±7.1 | |
| MYC2.2 OE | 4 | 100 |
| 5 | 92.2±2.3 | |
| 6 | 83.6±3.6 |
表3 野生型和转基因植株冷冻处理后的存活率统计
Table 3 Survival rates of wild-type and transgenic plants with cold treatment (n>30)
| 基因型 Genotype | 冷冻处理 Freezing treatment/h | 存活率 Survival rate/% |
|---|---|---|
| Col-0 | 4 | 100 |
| 5 | 74.2±1.5 | |
| 6 | 29.4±7.1 | |
| MYC2.2 OE | 4 | 100 |
| 5 | 92.2±2.3 | |
| 6 | 83.6±3.6 |
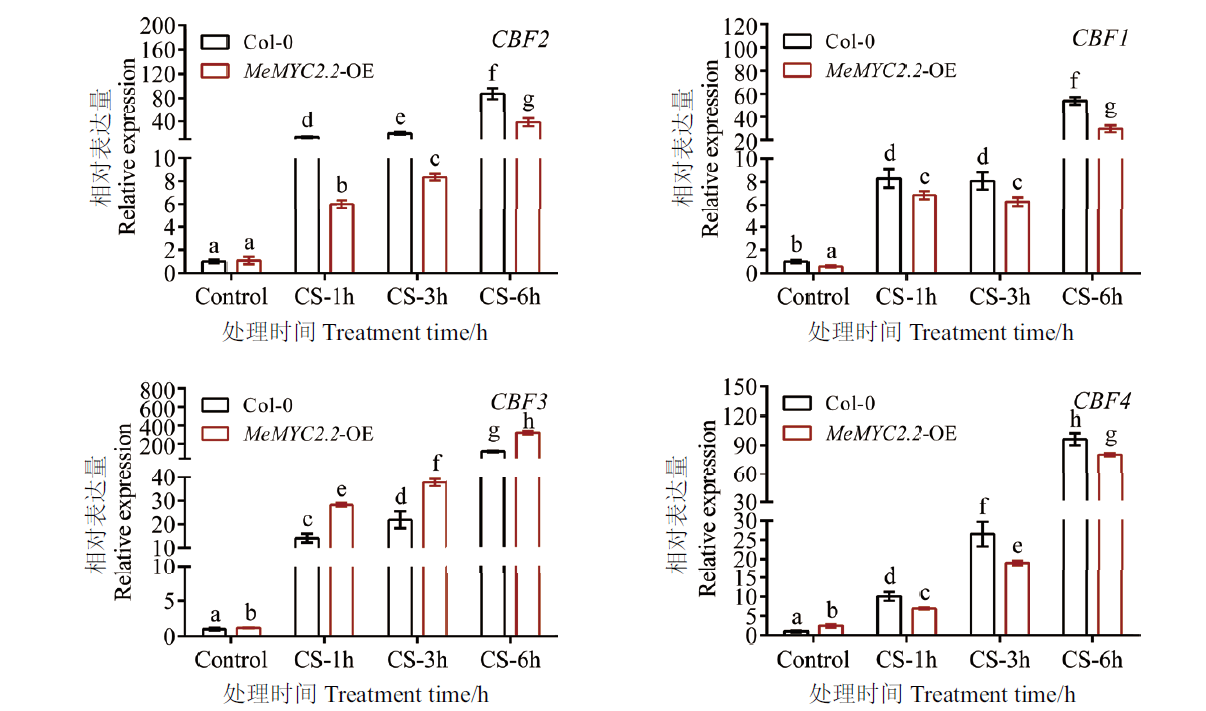
图5 冷冻胁迫下转基因拟南芥中CBF基因的表达分析 各CBF基因在未经处理的野生型拟南芥中的相对表达量设为1;显著差异用不同字母表示(P≤0.05);误差线为每组处理的标准误差(n=3)
Fig. 5 Expression analysis of CBF genes in transgenic Arabidopsis under cold stress The relative expression of indicated CBF gene in Col-0 Arabidopsis under normal conditions was set to 1. Error bars are ± SD(n=3), and different letters indicate differences with P≤0.05(ANOVA test)
| [1] |
Shan ZY, Luo XL, Wu MY, et al. Genome-wide identification and expression of GRAS gene family members in cassava[J]. BMC Plant Biol, 2020, 20(1): 46.
doi: 10.1186/s12870-020-2242-8 pmid: 31996133 |
| [2] | 但忠. 广西木薯的抗寒性与抗寒育种的初步研究[D]. 海口: 海南大学, 2010. |
| Dan Z. The cold-resisdance of cassva and preliminary investigation for breeding of cassava[D]. Haikou: Hainan University, 2010. | |
| [3] | 黄洁, 李开绵, 叶剑秋, 等. 我国的木薯优势区域概述[J]. 广西农业科学, 2008, 39(1): 104-108. |
| Huang J, Li KM, Ye JQ, et al. A summary review of dominant regions of cassava growing in China[J]. Guangxi Agric Sci, 2008, 39(1): 104-108. | |
| [4] | 吴远航, 刘秦, 刘攀道, 等. 木薯苯丙氨酸解氨酶基因的克隆及其对低温胁迫的响应[J]. 热带作物学报, 2019, 40(3): 483-489. |
| Wu YH, Liu Q, Liu PD, et al. Cloning of cassava phenylalanine ammonia lyase genes and their responses to low temperature stress[J]. Chin J Trop Crops, 2019, 40(3): 483-489. | |
| [5] |
Xu J, Duan XG, Yang J, et al. Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots[J]. Plant Physiol, 2013, 161(3): 1517-1528.
doi: 10.1104/pp.112.212803 pmid: 23344905 |
| [6] |
Xu J, Yang J, Duan XG, et al. Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava(Manihot esculenta Crantz)[J]. BMC Plant Biol, 2014, 14: 208.
doi: 10.1186/s12870-014-0208-4 URL |
| [7] |
Cheng ZH, Lei N, Li SX, et al. The regulatory effects of MeTCP4 on cold stress tolerance in Arabidopsis thaliana: a transcriptome analysis[J]. Plant Physiol Biochem, 2019, 138: 9-16.
doi: 10.1016/j.plaphy.2019.02.015 URL |
| [8] | An D, Ma QX, Yan W, et al. Divergent regulation of CBF regulon on cold tolerance and plant phenotype in cassava overexpressing Arabidopsis CBF3 gene[J]. Front Plant Sci, 2016, 7: 1866. |
| [9] |
Ruan MB, Guo X, Wang B, et al. Genome-wide characterization and expression analysis enables identification of abiotic stress-responsive MYB transcription factors in cassava(Manihot esculenta)[J]. J Exp Bot, 2017, 68(13): 3657-3672.
doi: 10.1093/jxb/erx202 URL |
| [10] |
Omondi JO, Yermiyahu U, Rachmilevitch S, et al. Optimizing root yield of cassava under fertigation and the masked effect of atmospheric temperature[J]. J Sci Food Agric, 2020, 100(12): 4592-4600.
doi: 10.1002/jsfa.10519 URL |
| [11] | 李罡, 李文龙, 许雪梅, 等. MYC2转录因子参与植物发育调控的研究进展[J]. 植物生理学报, 2019, 55(2): 125-132. |
| Li G, Li WL, Xu XM, et al. Research progress of MYC2 transcription factors participating in plant development and regulation[J]. Plant Physiol J, 2019, 55(2): 125-132. | |
| [12] | 周文平, 王怀琴, 郭晓荣, 等. 丹参bHLH转录因子基因SmMYC2的克隆和表达分析[J]. 植物科学学报, 2016, 34(2): 246-254. |
| Zhou WP, Wang HQ, Guo XR, et al. Cloning and expression analysis of SmMYC2, a bHLH transcription factor gene from Salvia miltiorrhiza bunge[J]. Plant Sci J, 2016, 34(2): 246-254. | |
| [13] |
Hu YR, Jiang YJ, Han X, et al. Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones[J]. J Exp Bot, 2017, 68(6): 1361-1369.
doi: 10.1093/jxb/erx004 pmid: 28201612 |
| [14] |
Hong GJ, Xue XY, Mao YB, et al. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression[J]. Plant Cell, 2012, 24(6): 2635-2648.
doi: 10.1105/tpc.112.098749 URL |
| [15] |
Aleman F, Yazaki J, Lee M, et al. An ABA-increased interaction of the PYL6 ABA receptor with MYC2 Transcription Factor: a putative link of ABA and JA signaling[J]. Sci Rep, 2016, 6: 28941.
doi: 10.1038/srep28941 pmid: 27357749 |
| [16] |
Hiruma K, Nishiuchi T, Kato T, et al. Arabidopsis enhanced disease resistance 1 is required for pathogen-induced expression of plant defensins in nonhost resistance, and acts through interference of MYC2-mediated repressor function[J]. Plant J, 2011, 67(6): 980-992.
doi: 10.1111/j.1365-313X.2011.04651.x URL |
| [17] |
An JP, Wang XN, Yao JF, et al. Apple MdMYC2 reduces aluminum stress tolerance by directly regulating MdERF3 gene[J]. Plant Soil, 2017, 418(1/2): 255-266.
doi: 10.1007/s11104-017-3297-7 URL |
| [18] |
Zhao ML, Wang JN, Shan W, et al. Induction of jasmonate signalling regulators MaMYC2s and their physical interactions with MaICE1 in methyl jasmonate-induced chilling tolerance in banana fruit[J]. Plant Cell Environ, 2013, 36(1): 30-51.
doi: 10.1111/j.1365-3040.2012.02551.x URL |
| [19] | 于晓玲, 郭鑫, 李淑霞, 等. 逆境信号下木薯MeMYC2转录因子表达及功能分析[J]. 热带作物学报, 2021, 42(4): 927-935. |
| Yu XL, Guo X, Li SX, et al. Expression analysis of MeMYC2 transcription factor in cassava under stress signal[J]. Chin J Trop Crops, 2021, 42(4): 927-935. | |
| [20] |
An D, Yang J, Zhang P. Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plant to cold stress[J]. BMC Genomics, 2012, 13: 64.
doi: 10.1186/1471-2164-13-64 pmid: 22321773 |
| [21] |
Chen QL, Li Z, Fan GZ, et al. Indications of stratospheric anomalies in the freezing rain and snow disaster in South China, 2008[J]. Sci China Earth Sci, 2011, 54(8): 1248-1256.
doi: 10.1007/s11430-011-4192-3 URL |
| [22] | Alves AAC. Cassava botany and physiology[M]// Hillocks RJ, Thresh JM, Bellotti AC. In Cassava: biology, production and utilization CAB International, 2002: 67-89. |
| [23] |
Meshi T, Iwabuchi M. Plant transcription factors[J]. Plant Cell Physiol, 1995, 36(8): 1405-1420.
pmid: 8589926 |
| [24] |
Jia YX, Ding YL, Shi YT, et al. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis[J]. New Phytol, 2016, 212(2): 345-353.
doi: 10.1111/nph.14088 URL |
| [25] | Ritonga FN, Chen S. Physiological and molecular mechanism involved in cold stress tolerance in plants[J]. Plants(Basel), 2020, 9(5): 560. |
| [26] |
Zhao CZ, Zhang ZJ, Xie SJ, et al. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis[J]. Plant Physiol, 2016, 171(4): 2744-2759.
doi: 10.1104/pp.16.00533 URL |
| [27] |
Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis -acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit[J]. Proc Natl Acad Sci USA, 1997, 94(3): 1035-1040.
doi: 10.1073/pnas.94.3.1035 pmid: 9023378 |
| [28] |
Gilmour SJ, Fowler SG, Thomashow MF. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities[J]. Plant Mol Biol, 2004, 54(5): 767-781.
doi: 10.1023/B:PLAN.0000040902.06881.d4 pmid: 15356394 |
| [29] |
Hao JJ, Yang JL, Dong JL, et al. Characterization of BdCBF genes and genome-wide transcriptome profiling of BdCBF3-dependent and-independent cold stress responses in Brachypodium distachyon[J]. Plant Sci, 2017, 262: 52-61.
doi: 10.1016/j.plantsci.2017.06.001 URL |
| [30] |
Miura K, Jin JB, Lee J, et al. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis[J]. Plant Cell, 2007, 19(4): 1403-1414.
doi: 10.1105/tpc.106.048397 URL |
| [31] |
Fursova OV, Pogorelko GV, Tarasov VA. Identification of ICE2, a gene involved in cold acclimation which determines freezing tolerance in Arabidopsis thaliana[J]. Gene, 2009, 429(1/2): 98-103.
doi: 10.1016/j.gene.2008.10.016 URL |
| [32] |
Gilmour SJ, Zarka DG, Stockinger EJ, et al. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression[J]. Plant J, 1998, 16(4): 433-442.
doi: 10.1046/j.1365-313x.1998.00310.x pmid: 9881163 |
| [33] |
An D, Ma QX, Wang HX, et al. Cassava C-repeat binding factor 1 gene responds to low temperature and enhances cold tolerance when overexpressed in Arabidopsis and cassava[J]. Plant Mol Biol, 2017, 94(1/2): 109-124.
doi: 10.1007/s11103-017-0596-6 URL |
| [34] |
Yang YL, Liao WB, Yu XL, et al. Overexpression of MeDREB1D confers tolerance to both drought and cold stresses in transgenic Arabidopsis[J]. Acta Physiol Plant, 2016, 38(10): 1-11.
doi: 10.1007/s11738-015-2023-4 URL |
| [1] | 陈晓, 于茗兰, 吴隆坤, 郑晓明, 逄洪波. 植物lncRNA及其对低温胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(7): 1-12. |
| [2] | 肖亮, 吴正丹, 陆柳英, 施平丽, 尚小红, 曹升, 曾文丹, 严华兵. 木薯重要性状基因的研究进展[J]. 生物技术通报, 2023, 39(6): 31-48. |
| [3] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [4] | 蒋路园, 丰美静, 杜雨晴, 邸葆, 陈段芬, 邱德有, 杨艳芳. 红豆杉低温半致死温度和低温胁迫下紫杉烷含量[J]. 生物技术通报, 2023, 39(3): 232-242. |
| [5] | 姚晓文, 梁晓, 陈青, 伍春玲, 刘迎, 刘小强, 税军, 乔阳, 毛奕茗, 陈银华, 张银东. 二斑叶螨抗性木薯木质素合成途径基因表达特性研究[J]. 生物技术通报, 2023, 39(2): 161-171. |
| [6] | 张晓燕, 杨淑华, 丁杨林. 植物感知和传递低温信号的分子机制[J]. 生物技术通报, 2023, 39(11): 28-35. |
| [7] | 邢媛, 宋健, 李俊怡, 郑婷婷, 刘思辰, 乔治军. 谷子AP基因家族鉴定及其对非生物胁迫的响应分析[J]. 生物技术通报, 2023, 39(11): 238-251. |
| [8] | 毛可欣, 王海荣, 安淼, 刘腾飞, 王世金, 李健, 李国田. 中华猕猴桃GRAS基因家族鉴定及低温胁迫表达分析[J]. 生物技术通报, 2023, 39(11): 297-307. |
| [9] | 尤垂淮, 谢津津, 张婷, 崔天真, 孙欣路, 臧守建, 武奕凝, 孙梦瑶, 阙友雄, 苏亚春. 钩吻脂氧合酶基因 GeLOX1 的鉴定及低温胁迫表达分析[J]. 生物技术通报, 2023, 39(11): 318-327. |
| [10] | 韩志玲, 陈青, 梁晓, 伍春玲, 刘迎, 伍牧锋, 徐雪莲. 二斑叶螨取食抗、感螨木薯品种对茉莉酸信号途径基因表达的影响[J]. 生物技术通报, 2022, 38(6): 211-220. |
| [11] | 金姣姣, 刘自刚, 米文博, 徐明霞, 邹娅, 徐春梅, 赵彩霞. 利用RNA-Seq鉴定调控甘蓝型油菜叶片光合特性的低温胁迫应答基因[J]. 生物技术通报, 2022, 38(4): 126-142. |
| [12] | 崔洁冰, 张萌, 张莹婷, 徐进. 低温胁迫对柳杉不同无性系的影响及抗寒性评价[J]. 生物技术通报, 2022, 38(3): 31-40. |
| [13] | 邹良平, 郭鑫, 起登凤, 翟敏, 李壮, 赵平娟, 彭明, 牛兴奎. 低氮胁迫诱导木薯幼苗花青素积累及其基因表达[J]. 生物技术通报, 2022, 38(2): 75-82. |
| [14] | 乌凤章, 王贺新. 蛋白质泛素化介导的植物低温胁迫反应[J]. 生物技术通报, 2021, 37(6): 225-235. |
| [15] | 孔春艳, 陈永坤, 王莎莎, 郝大海, 杨宇, 龚明. 小桐子低温胁迫下microRNA实时荧光定量PCR内参的筛选与比较[J]. 生物技术通报, 2019, 35(7): 25-32. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||