








生物技术通报 ›› 2020, Vol. 36 ›› Issue (12): 42-53.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0241
收稿日期:2020-03-09
出版日期:2020-12-26
发布日期:2020-12-22
作者简介:鲁琳,女,副教授,研究方向:园林植物;E-mail:基金资助:
LU Lin1( ), YANG Shang-yu2, LIU Wei-dong1, LU Li-ming2(
), YANG Shang-yu2, LIU Wei-dong1, LU Li-ming2( )
)
Received:2020-03-09
Published:2020-12-26
Online:2020-12-22
摘要:
为了鉴定参与花烟草盐胁迫响应的活性氧清除相关基因,用200 mmol/L NaCl处理花烟草幼苗,并在处理后12 h采集其幼苗样本,提取总RNA,采用高通量测序技术,进行转录组测序。基于差异表达基因GO及KEGG分析的基础上,对参与其中的活性氧清除基因进行挖掘,利用qRT-PCR的方法验证转录组测序结果。结果表明,盐处理后,花烟草基因表达量变化在2倍以上的基因有7 239个(P<0.01),其中,上调表达基因4 037个,下调表达基因3 162个,将其功能归类于生物过程、细胞组分及分子功能三大类159个GO条目,并显著富集在12条KEGG代谢通路中。同时,在盐胁迫下,花烟草有45个活性氧清除相关基因的表达发生了显著改变,其中,上调表达基因28个,下调表达基因17个。此结果说明,活性氧的清除是花烟草抵御盐胁迫的重要机制之一。
鲁琳, 杨尚谕, 刘维东, 鲁黎明. 基于转录组测序花烟草响应盐胁迫活性氧清除相关基因的挖掘[J]. 生物技术通报, 2020, 36(12): 42-53.
LU Lin, YANG Shang-yu, LIU Wei-dong, LU Li-ming. Mining of Genes Related to Reactive Oxygen Species Scavenging in Response to Salt Stress in Nicotiana alata Based on Transcriptome Sequencing[J]. Biotechnology Bulletin, 2020, 36(12): 42-53.
| 序号 | 引物名称 | 引物序列(5'-3') |
|---|---|---|
| 1 | GST1-F | CCGGCAATAAGCATGAAGAT |
| GST1-R | TAGTATCCCCTTGGGTGTGG | |
| 2 | CAT1-F | CCAATAAGCTACCACTACGAAACG |
| CAT1-R | GGGAAAGAGGAGTGAACCCATT | |
| 3 | POD42-F | AAGAGCCATCACTATCTGGTACAAAG |
| POD42-R | CTGCCTCTCCGTCTGAACCT | |
| 4 | CIPK11-F | GCGTTGACGGAGCAAGTACA |
| CIPK11-R | ATGCTGGCGTTCCACAAAG | |
| 5 | ERF4-F | TCACTACGACTGGGCTTACCAA |
| ERF4-R | GGAATTGCTCAATGTTTGTCCTAA | |
| 6 | GPX4-F | CCCTGATGTTCCACATGAGATCT |
| GPX4-R | GGGTGGAAAGGAAACACAGTACA | |
| 7 | MYB5-F | GTGAAGGCCAATGGAGAAACTT |
| MYB5-R | TCTTAGCCTACAACTTTTTCCACATC | |
| 8 | CIPK6-F | TCCGCCTTGGTTTTCATCAG |
| CIPK6-R | TCGGATTCGGATCCAACATT | |
| 9 | Actin-F | CCAAGCAGCATGAAGATCAA |
| Actin-R | CGTACTCGGCCTTTGAAATC |
表1 qPCR反应所用引物
| 序号 | 引物名称 | 引物序列(5'-3') |
|---|---|---|
| 1 | GST1-F | CCGGCAATAAGCATGAAGAT |
| GST1-R | TAGTATCCCCTTGGGTGTGG | |
| 2 | CAT1-F | CCAATAAGCTACCACTACGAAACG |
| CAT1-R | GGGAAAGAGGAGTGAACCCATT | |
| 3 | POD42-F | AAGAGCCATCACTATCTGGTACAAAG |
| POD42-R | CTGCCTCTCCGTCTGAACCT | |
| 4 | CIPK11-F | GCGTTGACGGAGCAAGTACA |
| CIPK11-R | ATGCTGGCGTTCCACAAAG | |
| 5 | ERF4-F | TCACTACGACTGGGCTTACCAA |
| ERF4-R | GGAATTGCTCAATGTTTGTCCTAA | |
| 6 | GPX4-F | CCCTGATGTTCCACATGAGATCT |
| GPX4-R | GGGTGGAAAGGAAACACAGTACA | |
| 7 | MYB5-F | GTGAAGGCCAATGGAGAAACTT |
| MYB5-R | TCTTAGCCTACAACTTTTTCCACATC | |
| 8 | CIPK6-F | TCCGCCTTGGTTTTCATCAG |
| CIPK6-R | TCGGATTCGGATCCAACATT | |
| 9 | Actin-F | CCAAGCAGCATGAAGATCAA |
| Actin-R | CGTACTCGGCCTTTGAAATC |
| 样品名称 | 原始数据 | 筛选后数据 | 总映射 | 多重映射 | 唯一映射 | Q30/% | GC含量/% |
|---|---|---|---|---|---|---|---|
| CK_1 | 49583710 | 47599094 | 44085400(92.62%) | 969397(2.04%) | 43116003(90.58%) | 90.32 | 42.98 |
| CK_2 | 56621858 | 54483002 | 50100087(91.96%) | 1130193(2.07%) | 48969894(89.88%) | 90.99 | 43.03 |
| CK_3 | 50230428 | 47972778 | 44659130(93.09%) | 965148(2.01%) | 43693982(91.08%) | 90.53 | 43.03 |
| S12_1 | 64969188 | 62591696 | 57912308(92.52%) | 1294796(2.07%) | 56617512(90.46%) | 90.33 | 43.00 |
| S12_2 | 52595884 | 50762784 | 47212736(93.01%) | 1022342(2.01%) | 46190394(90.99%) | 90.38 | 42.97 |
| S12_3 | 53054528 | 51241836 | 47521067(92.74%) | 1099309(2.15%) | 46421758(90.59%) | 90.57 | 43.19 |
表2 各样品测序质量
| 样品名称 | 原始数据 | 筛选后数据 | 总映射 | 多重映射 | 唯一映射 | Q30/% | GC含量/% |
|---|---|---|---|---|---|---|---|
| CK_1 | 49583710 | 47599094 | 44085400(92.62%) | 969397(2.04%) | 43116003(90.58%) | 90.32 | 42.98 |
| CK_2 | 56621858 | 54483002 | 50100087(91.96%) | 1130193(2.07%) | 48969894(89.88%) | 90.99 | 43.03 |
| CK_3 | 50230428 | 47972778 | 44659130(93.09%) | 965148(2.01%) | 43693982(91.08%) | 90.53 | 43.03 |
| S12_1 | 64969188 | 62591696 | 57912308(92.52%) | 1294796(2.07%) | 56617512(90.46%) | 90.33 | 43.00 |
| S12_2 | 52595884 | 50762784 | 47212736(93.01%) | 1022342(2.01%) | 46190394(90.99%) | 90.38 | 42.97 |
| S12_3 | 53054528 | 51241836 | 47521067(92.74%) | 1099309(2.15%) | 46421758(90.59%) | 90.57 | 43.19 |
| GO分类号 | 功能描述 | 差异基因数量(DEGs) | 差异基因占比 /% |
|---|---|---|---|
| GO:0008150 | Biological_process | 4 595 | 76.97 |
| GO:0008152 | Metabolic process | 3 660 | 61.31 |
| GO:0044699 | Single-organism process | 2 808 | 47.04 |
| GO:0044763 | Single-organism cellular process | 2 047 | 34.29 |
| GO:0009058 | Biosynthetic process | 1 795 | 30.07 |
| GO:0044710 | Single-organism metabolic process | 1 730 | 28.98 |
| GO:0051234 | Establishment of localization | 1 121 | 18.78 |
| GO:0006810 | Transport | 1 119 | 18.74 |
| GO:1902578 | Single-organism localization | 845 | 14.15 |
| GO:0044765 | Single-organism transport | 836 | 14.00 |
| GO:0055114 | Oxidation-reduction process | 788 | 13.20 |
| GO:0006796 | Phosphate-containing compound metabolic process | 693 | 11.61 |
| GO:0006793 | Phosphorus metabolic process | 693 | 11.61 |
| GO:0044711 | Single-organism biosynthetic process | 646 | 10.82 |
| GO:0006464 | Cellular protein modification process | 563 | 9.43 |
| GO:0036211 | Protein modification process | 563 | 9.43 |
| GO:0005975 | Carbohydrate metabolic process | 549 | 9.20 |
| GO:0044281 | Small molecule metabolic process | 535 | 8.96 |
| GO:0006629 | Lipid metabolic process | 524 | 8.78 |
| GO:0055085 | Transmembrane transport | 464 | 7.77 |
| GO:0006811 | Ion transport | 460 | 7.71 |
| GO:0016310 | Phosphorylation | 449 | 7.52 |
| GO:1901135 | Carbohydrate derivative metabolic process | 428 | 7.17 |
| GO:0044255 | Cellular lipid metabolic process | 366 | 6.13 |
| GO:0006812 | Cation transport | 361 | 6.05 |
| GO:0044723 | Single-organism carbohydrate metabolic process | 359 | 6.01 |
| GO:0008610 | Lipid biosynthetic process | 320 | 5.36 |
| GO:0019637 | Organophosphate metabolic process | 309 | 5.18 |
| GO:1901137 | Carbohydrate derivative biosynthetic process | 267 | 4.47 |
| GO:0044262 | Cellular carbohydrate metabolic process | 240 | 4.02 |
表3 上调差异表达基因的GO功能注释分析(生物过程)
| GO分类号 | 功能描述 | 差异基因数量(DEGs) | 差异基因占比 /% |
|---|---|---|---|
| GO:0008150 | Biological_process | 4 595 | 76.97 |
| GO:0008152 | Metabolic process | 3 660 | 61.31 |
| GO:0044699 | Single-organism process | 2 808 | 47.04 |
| GO:0044763 | Single-organism cellular process | 2 047 | 34.29 |
| GO:0009058 | Biosynthetic process | 1 795 | 30.07 |
| GO:0044710 | Single-organism metabolic process | 1 730 | 28.98 |
| GO:0051234 | Establishment of localization | 1 121 | 18.78 |
| GO:0006810 | Transport | 1 119 | 18.74 |
| GO:1902578 | Single-organism localization | 845 | 14.15 |
| GO:0044765 | Single-organism transport | 836 | 14.00 |
| GO:0055114 | Oxidation-reduction process | 788 | 13.20 |
| GO:0006796 | Phosphate-containing compound metabolic process | 693 | 11.61 |
| GO:0006793 | Phosphorus metabolic process | 693 | 11.61 |
| GO:0044711 | Single-organism biosynthetic process | 646 | 10.82 |
| GO:0006464 | Cellular protein modification process | 563 | 9.43 |
| GO:0036211 | Protein modification process | 563 | 9.43 |
| GO:0005975 | Carbohydrate metabolic process | 549 | 9.20 |
| GO:0044281 | Small molecule metabolic process | 535 | 8.96 |
| GO:0006629 | Lipid metabolic process | 524 | 8.78 |
| GO:0055085 | Transmembrane transport | 464 | 7.77 |
| GO:0006811 | Ion transport | 460 | 7.71 |
| GO:0016310 | Phosphorylation | 449 | 7.52 |
| GO:1901135 | Carbohydrate derivative metabolic process | 428 | 7.17 |
| GO:0044255 | Cellular lipid metabolic process | 366 | 6.13 |
| GO:0006812 | Cation transport | 361 | 6.05 |
| GO:0044723 | Single-organism carbohydrate metabolic process | 359 | 6.01 |
| GO:0008610 | Lipid biosynthetic process | 320 | 5.36 |
| GO:0019637 | Organophosphate metabolic process | 309 | 5.18 |
| GO:1901137 | Carbohydrate derivative biosynthetic process | 267 | 4.47 |
| GO:0044262 | Cellular carbohydrate metabolic process | 240 | 4.02 |
| GO分类号 | 功能描述 | 差异基因数量(DEGs) | 差异基因占比/% |
|---|---|---|---|
| GO:0003824 | Catalytic activity | 3 314 | 55.51 |
| GO:0016491 | Oxidoreductase activity | 852 | 14.27 |
| GO:0005215 | Transporter activity | 658 | 11.02 |
| GO:0022857 | Transmembrane transporter activity | 555 | 9.30 |
| GO:0016301 | Kinase activity | 539 | 9.03 |
| GO:0016773 | Phosphotransferase activity,alcohol group as acceptor | 516 | 8.64 |
| GO:0022891 | Substrate-specific transmembrane transporter Activity | 437 | 7.32 |
| GO:0004672 | Protein kinase activity | 413 | 6.92 |
| GO:0048037 | Cofactor binding | 287 | 4.81 |
| GO:0016705 | Oxidoreductase activity,acting on paired donors,with incorporation or reduction of molecular oxygen | 238 | 3.99 |
| GO:0050662 | Coenzyme binding | 209 | 3.50 |
| GO:0016614 | Oxidoreductase activity,acting on CH-OH group of donors | 168 | 2.81 |
| GO:0020037 | Heme binding | 166 | 2.78 |
| GO:0016616 | Oxidoreductase activity,acting on the CH-OH group of donors,NAD or NADP as acceptor | 163 | 2.73 |
| GO:0005506 | Iron ion binding | 161 | 2.70 |
| GO:0022804 | Active transmembrane transporter activity | 152 | 2.55 |
| GO:0005509 | Calcium ion binding | 129 | 2.16 |
| GO:0015291 | Secondary active transmembrane transporter activity | 84 | 1.41 |
| GO:0016706 | Oxidoreductase activity,acting on paired donors,with incorporation or reduction of molecular oxygen,2-oxoglutarate as one donor,and incorporation of one atom each of oxygen into both donors | 70 | 1.17 |
| GO:0004497 | Monooxygenase activity | 48 | 0.80 |
| GO:0003857 | 3-hydroxyacyl-CoA dehydrogenase activity | 39 | 0.65 |
| GO:0004616 | Phosphogluconate dehydrogenase(decarboxylating)activity | 36 | 0.60 |
| GO:0016709 | Oxidoreductase activity,acting on paired donors,with incorporation or reduction of molecular oxygen,NAD(P)H as one donor,and incorporation of one atom of oxygen | 29 | 0.49 |
| GO:0008677 | 2-dehydropantoate 2-reductase activity | 27 | 0.45 |
| GO:0004312 | fatty acid synthase activity | 21 | 0.35 |
| GO:0004315 | 3-oxoacyl-[acyl-carrier-protein]synthase activity | 20 | 0.34 |
| GO:0016832 | Aldehyde-lyase activity | 19 | 0.32 |
| GO:0004332 | Fructose-bisphosphate aldolase activity | 16 | 0.27 |
| GO:0016872 | Intramolecular lyase activity | 13 | 0.22 |
| GO:0004506 | Squalene monooxygenase activity | 13 | 0.22 |
| GO:0003913 | DNA photolyase activity | 9 | 0.15 |
| GO:0004512 | Inositol-3-phosphate synthase activity | 6 | 0.10 |
表4 上调差异表达基因的GO功能注释分析(分子功能)
| GO分类号 | 功能描述 | 差异基因数量(DEGs) | 差异基因占比/% |
|---|---|---|---|
| GO:0003824 | Catalytic activity | 3 314 | 55.51 |
| GO:0016491 | Oxidoreductase activity | 852 | 14.27 |
| GO:0005215 | Transporter activity | 658 | 11.02 |
| GO:0022857 | Transmembrane transporter activity | 555 | 9.30 |
| GO:0016301 | Kinase activity | 539 | 9.03 |
| GO:0016773 | Phosphotransferase activity,alcohol group as acceptor | 516 | 8.64 |
| GO:0022891 | Substrate-specific transmembrane transporter Activity | 437 | 7.32 |
| GO:0004672 | Protein kinase activity | 413 | 6.92 |
| GO:0048037 | Cofactor binding | 287 | 4.81 |
| GO:0016705 | Oxidoreductase activity,acting on paired donors,with incorporation or reduction of molecular oxygen | 238 | 3.99 |
| GO:0050662 | Coenzyme binding | 209 | 3.50 |
| GO:0016614 | Oxidoreductase activity,acting on CH-OH group of donors | 168 | 2.81 |
| GO:0020037 | Heme binding | 166 | 2.78 |
| GO:0016616 | Oxidoreductase activity,acting on the CH-OH group of donors,NAD or NADP as acceptor | 163 | 2.73 |
| GO:0005506 | Iron ion binding | 161 | 2.70 |
| GO:0022804 | Active transmembrane transporter activity | 152 | 2.55 |
| GO:0005509 | Calcium ion binding | 129 | 2.16 |
| GO:0015291 | Secondary active transmembrane transporter activity | 84 | 1.41 |
| GO:0016706 | Oxidoreductase activity,acting on paired donors,with incorporation or reduction of molecular oxygen,2-oxoglutarate as one donor,and incorporation of one atom each of oxygen into both donors | 70 | 1.17 |
| GO:0004497 | Monooxygenase activity | 48 | 0.80 |
| GO:0003857 | 3-hydroxyacyl-CoA dehydrogenase activity | 39 | 0.65 |
| GO:0004616 | Phosphogluconate dehydrogenase(decarboxylating)activity | 36 | 0.60 |
| GO:0016709 | Oxidoreductase activity,acting on paired donors,with incorporation or reduction of molecular oxygen,NAD(P)H as one donor,and incorporation of one atom of oxygen | 29 | 0.49 |
| GO:0008677 | 2-dehydropantoate 2-reductase activity | 27 | 0.45 |
| GO:0004312 | fatty acid synthase activity | 21 | 0.35 |
| GO:0004315 | 3-oxoacyl-[acyl-carrier-protein]synthase activity | 20 | 0.34 |
| GO:0016832 | Aldehyde-lyase activity | 19 | 0.32 |
| GO:0004332 | Fructose-bisphosphate aldolase activity | 16 | 0.27 |
| GO:0016872 | Intramolecular lyase activity | 13 | 0.22 |
| GO:0004506 | Squalene monooxygenase activity | 13 | 0.22 |
| GO:0003913 | DNA photolyase activity | 9 | 0.15 |
| GO:0004512 | Inositol-3-phosphate synthase activity | 6 | 0.10 |
| GO分类号 | 功能描述 | 类型 | 差异基因数量(DEGs) | 差异基因占比/% |
|---|---|---|---|---|
| GO:0000041 | Transition metal ion transport | 生物过程 | 71 | 1.39 |
| GO:0072511 | Divalent inorganic cation transport | 75 | 1.46 | |
| GO:0070838 | Divalent metal ion transport | 59 | 1.15 | |
| GO:0015684 | Ferrous iron transport | 41 | 0.80 | |
| GO:0006826 | Iron ion transport | 44 | 0.86 | |
| GO:0046915 | Transition metal ion transmembrane transporter activity | 分子功能 | 68 | 1.33 |
| GO:0072509 | Divalent inorganic cation Transmembrane transporter activity | 75 | 1.46 | |
| GO:0015093 | Ferrous iron transmembrane transporter activity | 41 | 0.80 | |
| GO:0004748 | Ribonucleoside-diphosphate reductase activity,thioredoxin disulfide as acceptor | 14 | 0.27 | |
| GO:0016728 | Oxidoreductase activity,acting on CH or CH2 groups,disulfide as acceptor | 14 | 0.27 | |
| GO:0061731 | Ribonucleoside-diphosphate reductase activity | 14 | 0.27 | |
| GO:0005381 | Iron ion transmembrane transporter activity | 44 | 0.86 |
表5 下调差异表达基因的GO功能注释分析
| GO分类号 | 功能描述 | 类型 | 差异基因数量(DEGs) | 差异基因占比/% |
|---|---|---|---|---|
| GO:0000041 | Transition metal ion transport | 生物过程 | 71 | 1.39 |
| GO:0072511 | Divalent inorganic cation transport | 75 | 1.46 | |
| GO:0070838 | Divalent metal ion transport | 59 | 1.15 | |
| GO:0015684 | Ferrous iron transport | 41 | 0.80 | |
| GO:0006826 | Iron ion transport | 44 | 0.86 | |
| GO:0046915 | Transition metal ion transmembrane transporter activity | 分子功能 | 68 | 1.33 |
| GO:0072509 | Divalent inorganic cation Transmembrane transporter activity | 75 | 1.46 | |
| GO:0015093 | Ferrous iron transmembrane transporter activity | 41 | 0.80 | |
| GO:0004748 | Ribonucleoside-diphosphate reductase activity,thioredoxin disulfide as acceptor | 14 | 0.27 | |
| GO:0016728 | Oxidoreductase activity,acting on CH or CH2 groups,disulfide as acceptor | 14 | 0.27 | |
| GO:0061731 | Ribonucleoside-diphosphate reductase activity | 14 | 0.27 | |
| GO:0005381 | Iron ion transmembrane transporter activity | 44 | 0.86 |
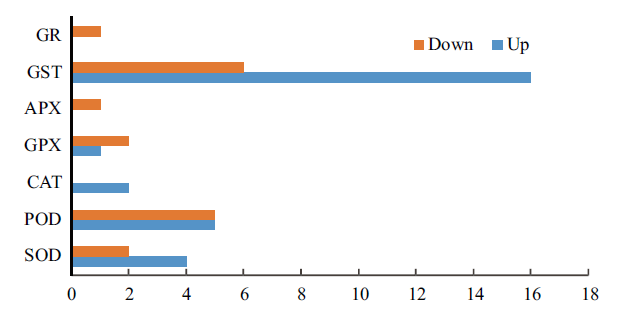
图3 花烟草响应高盐胁迫的抗氧化基因 GR:glutathione reductase;GST:glutathione S-transferases;APX:ascorbate peroxidase;GPX:glutathione peroxidase;CAT:Catalases;POD:peroxidase;SOD:Superoxide dismutase
| [1] | Soares ALC, Geilfus CM, Carpentier SC. Genotype-specific growth and proteomic responses of maize toward salt stress[J]. Frontier in Plant Science, 2018,9:661. |
| [2] |
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, et al. Plant responses to salt stress:adaptive mechanisms[J]. Agronomy, 2017,7:1-38.
doi: 10.3390/agronomy7010001 URL |
| [3] | Yang Y, Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses[J]. New Phytologist, 2018,217:523-539. |
| [4] | Noctor G, Reichheld JP, Foyer CH. ROS-related redox regulation and signaling in plants[J]. Seminars in Cell & Developmental Biology, 2018,80:3-12. |
| [5] | 马静, 王静, 姚鹏伟, 等. 盐分胁迫对烤烟叶片亚细胞结构及生理生化指标的影响[J]. 中国烟草学报, 2019,25(5):44-52. |
| Ma J, Wang J, Yao PW, et al. Effects of salt stress on subcellular structure and physiological and biochemical indexes of flue-cured tobacco leaves[J]. Acta Tabacaria Sinica, 2019,25(5):44-52. | |
| [6] | 李猛, 陈栋, 李秀妮, 等. 盐胁迫下外源褪黑素对烟草幼苗抗氧化特性和光合特性的影响[J]. 中国农业科技导报, 2019,21(2):141-147. |
| Li M, Chen D, Li XN, et al. Influenes of exogenous melatonin on antioxidant and photosynthetic characteristics of tobacco seedlings under salt stress[J]. Journal of Agricultural Science and Technology, 2019,21(2):141-147. | |
| [7] |
Singh N, Mishra A, Jha B. Over-expression of the peroxisomal ascorbate peroxidase(SbpAPX)gene cloned from halophyte Salicornia brachiate confers salt and drought stress tolerance in transgenic tobacco[J]. Marine Biotechnology, 2014,16(3):321-332.
URL pmid: 24197564 |
| [8] | Badawi GH, Yamauchi Y, Shimada E, et al. Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco(Nicotiana tabacum)chloroplasts[J]. Plant Science, 2004,166:919-928. |
| [9] |
Prashanth SR, Sadhasivam V, Parida A. Overexpression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia marina in indica rice var Pusa Basmati-1 confers abiotic stress tolerance[J]. Transgenic Research, 2008,17:281-291.
URL pmid: 17541718 |
| [10] | Wang Y, Ying Y, Chen J, et al. Transgenic Arabidopsis overexpressing Mn-SOD enhanced salt-tolerance[J]. Plant Science, 2004,167:671-677. |
| [11] | Wang YC, Qu GZ, Li HY, et al. Enhanced salt tolerance of transgenic poplar plants expressing a manganese superoxide dismutase from Tamarix androssowii[J]. Molecular Biology Report, 2010,37:1119-1124. |
| [12] |
Kim YH, Kim CY, Song WK, et al. Overexpression of sweet potato swpa4 peroxidase results in increased hydrogen peroxide production and enhances stress tolerance in tobacco[J]. Planta, 2008,227(4):867-881.
doi: 10.1007/s00425-007-0663-3 URL pmid: 18224366 |
| [13] |
Al-Taweel K, Iwaki T, Yabuta Y, et al. A bacterial transgene for catalase protects translation of d1 protein during exposure of salt-stressed tobacco leaves to strong light[J]. Plant Physiology, 2007,145(1):258-265.
doi: 10.1104/pp.107.101733 URL pmid: 17660354 |
| [14] | Xu WF, Shi WM, Ueda A, et al. Mechanisms of salt tolerance in transgenic Arabidopsis thaliana carrying a peroxisomal ascorbate peroxidase gene from barley[J]. Pedosphere, 2008,18:486-495. |
| [15] | Diaz-Vivancos P, Faize M, Barba-Espin G, et al. Ectopic expression of cytosolic superoxide dismutase and ascorbate per-oxidase leads to salt stress tolerance in transgenic plums[J]. Plant Biotechnology, 2013,11:976-985. |
| [16] | Gaber A, Yoshimura K, Yamamoto T, et al. Glutathione peroxidase-like protein of Synechocystis PCC 6803 confers tolerance to oxidative and environmental stresses in transgenic Arabidopsis[J]. Physiology Plantarum, 2006,128:251-262. |
| [17] | Yoshimura K, Miyao K, Gaber A, et al. Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol[J]. Plant Journal, 2004,37:21-33. |
| [18] | Luo Q, Teng W, Fang S, et al. Transcriptome analysis of salt-stress response in three seedling tissues of common wheat[J]. The Crop Journal, 2019,3:378-392. |
| [19] | Zenda T, Liu S, Wang X, et al. Key maize drought-responsive genes and pathways revealed by comparative transcriptome and physiological analyses of contrasting inbred lines[J]. International Journal of Molecular Sciences, 2019,20, 1268. |
| [20] | 朱琳, 袁梦, 高红秀, 等. 水稻苗期低温应答转录组分析[J]. 华北农学报, 2018,33(5):40-51. |
| Zhu L, Yuan M, Gao H, et al. Transcriptomic analysis of rice seedling responsive to low temperature[J]. Acta Agriculturae Boreali-Sinica, 2018,33(5):40-51. | |
| [21] | 鲁黎明, 陈勇, 鲁逸飞, 等. 低钾胁迫对烟草幼苗叶中基因表达的影响[J]. 核农学报, 2016,30(7):1273-1280. |
| Lu LM, Chen Y, Lu YF, et al. Effect of low potassium stress on gene expression profile in leaves of tobacco seedlings[J]. Journal of Nuclear Agricultural Sciences, 2016,30(7):1273-1280. | |
| [22] | Shigeoka S, Ishikawa T, Tamoi M, et al. Regulation and function of ascorbate peroxidase isoenzymes[J]. Jounal of Experimental Botany, 2002,53(372):1305-1319. |
| [23] | 马长乐, 王萍萍, 曹子谊, 等. 盐地碱蓬(Suaede sdlsa)APX 基因的克隆及盐胁迫下的表达[J]. 植物生理与分子生物学学报, 2002,28(4):261-266. |
| Ma CL, Wang PP, Cao ZY, et al. cDNA cloning and gene expression of APX in Suaeda salsa in response to salt stress[J]. Journal of Plant Physiology and Molecular Biology, 2002,28(4):261-266. | |
| [24] | 王超, 杨传平, 王玉成. 白桦抗坏血酸过氧化物酶(APX)基因克隆及表达分析[J]. 东北林业大学学报, 2009,37(3):70-81. |
| Wang C, Yang CP, Wang YC, et al. Cloning and expression analysis of an APX gene from Betula platyphylla[J]. Journal of Northeast Forestry University, 2009,37(3):70-81. | |
| [25] | Badawi GH, Kawano N, Yamauchi Y, et al. Overexpression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit[J]. Physiology Plantarum, 2004,121(2):231-238. |
| [26] | 郭慧娜, 孟玉平, 郝子琪, 等. 转ZjAPX 基因拟南芥对NaCl、干旱胁迫的耐性研究[J]. 生物技术通报, 2013,29(1):78-82. |
| Guo HN, Meng YP, Hao ZQ, et al. ZjAPX gene improving resistance to NaCl and drought stress in Arabidopsis[J]. Biotechnology Bulletin, 2013,29(1):78-82. | |
| [27] | 乔新荣, 张继英. 植物谷胱甘肽过氧化物酶(GPX)研究进展 [J]. 生物技术通报, 2016,32(9):7-13. |
| Qiao XR, Zhang JY. Research progress on GPX in plants[J]. Biotechnology Bulletin, 2016,32(9):7-13. | |
| [28] | Yoshimura K, Miyao K, Gaber A, et al. Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydom-onas glutathione peroxidase in cytosol or chloroplast[J]. Plant Journal, 2004,37(1):21-33. |
| [29] |
Zhai CZ, Zhao L, Yin LJ, et al. Two wheat glutathione peroxidase genes whose products are located in chloroplasts improve salt and H2O2 tolerances in Arabidopsis[J]. PLoS One, 2013,8(10):e73989.
URL pmid: 24098330 |
| [30] | Akbudak MA, Filiz E, Vatansever R, et al. Genome wide identification and expression profile of ascorbate peroxidase(APX)and glutathione peroxidase(GPX)genes under drought stress in Sorghum(Sorghum bicolor L.)[J]. Journal of Plant Growth Regulation, 2018,37(3):925-936. |
| [31] |
Labrou NE, Papageorgiou AC, Pavli O, et al. Plant GSTome:Structure and functional role in xenome network and plant stress response[J]. Current Opinion of Biotechnology, 2015,32:186-194.
doi: 10.1016/j.copbio.2014.12.024 URL |
| [32] | 陈秀华, 王臻昱, 李先平, 等. 谷胱甘肽S-转移酶的研究进展[J]. 东北农业大学学报, 2013,44(1):149-153. |
| Chen XH, Wang ZY, Li XP, et al. Research progress on glutathione S-transferases[J]. Journal of Northeast Agricultural University, 2013,44(1):149-153. | |
| [33] |
Xu J, Tian YS, Xing XJ, et al. Over-expression of AtGSTU19 provides tolerance to salt, drought and methy viologen stresses in Arabidopsis[J]. Physiology Plantarum, 2015,156(2):164-175.
doi: 10.1111/ppl.2016.156.issue-2 URL |
| [34] |
Roxas VP, Smith RK Jr, Allen ER, et al. Overexpression of glutathione S-transferase glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress[J]. Nature Biotechnology, 1997,15:988-991.
URL pmid: 9335051 |
| [35] |
戚元成, 张世敏, 王丽萍, 等. 谷胱甘肽转移酶基因过量表达能加速盐胁迫下转基因拟南芥的生长[J]. 植物生理与分子生物学学报, 2004,30(5):517-522.
pmid: 15627705 |
|
Qi YC, Zhang SM, Wang LP, et al. Overexpression of GST gene accelerates the growth of transgenic Arabidopsis under salt stress[J]. Journal of Plant Physiology and Molecular Biology, 2004,30(5):517-522.
URL pmid: 15627705 |
|
| [36] |
Chen JH, Jiang HW, Hsieh EJ, et al. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid[J]. Plant Physiology, 2012,158(1):340-351.
doi: 10.1104/pp.111.181875 URL pmid: 22095046 |
| [37] |
Gill SS, Anjum NA, Hasanuzzaman M, et al. Glutathione and glutathione reductase:A boon in disguise for plant abiotic stress defense operations[J]. Plant Physiology and Biochemistry, 2013,70:204-212.
doi: 10.1016/j.plaphy.2013.05.032 URL pmid: 23792825 |
| [38] |
Wu T, Lin W, Kao Y, et al. Identification and characterization of a novel chloroplast /mitochondria co-localized glutathione reductase 3 involved in salt stress response in rice[J]. Plant Molecular Biology, 2013,83:379-390.
doi: 10.1007/s11103-013-0095-3 URL pmid: 23783412 |
| [39] |
岳川, 曹红利, 周艳华, 等. 茶树谷胱甘肽还原酶基因 CsGRs 的克隆与表达分析[J]. 中国农业科学, 2014,47(16):3277-3289.
doi: 10.3864/j.issn.0578-1752.2014.16.013 URL |
|
Yue C, Cao HL, Zhou YH, et al. Cloning and expression analysis of glutathione reductase genes(CsGRs) in tea plant(Camellia sinensis)[J]. Scientia Agricultura Sinica, 2014,47(16):3277-3289.
doi: 10.3864/j.issn.0578-1752.2014.16.013 URL |
|
| [40] |
张腾国, 聂亭亭, 孙万仓, 等. 逆境胁迫对油菜谷胱甘肽还原酶基因表达及其酶活性的影响[J]. 应用生态学报, 2018,29(1):213-222.
pmid: 29692030 |
|
Zhang TG, Nie TT, Sun WC, et al. Effects of diverse stresses on gene expression and enzyme activity of glutathione reductase in Brassica campestris[J]. Chinese Journal of Applied Ecology, 2018,29(1):213-222.
doi: 10.13287/j.1001-9332.201801.010 URL pmid: 29692030 |
|
| [41] |
Deng Z, Zhao M, Liu H, et al. Molecular cloning, expression profiles and characterization of a glutathione reductase in Hevea brasiliensis[J]. Plant Physiology and Biochemistry, 2015,96:53-63.
doi: 10.1016/j.plaphy.2015.07.022 URL pmid: 26232647 |
| [42] |
Wu TM, Lin WR, Kao CH, et al. Gene knockout of glutathione reductase 3 results in increased sensitivity to salt stress in rice[J]. Plant Molecular Biology, 2015,87:555-564.
doi: 10.1007/s11103-015-0290-5 URL pmid: 25636203 |
| [1] | 林红妍, 郭晓蕊, 刘迪, 李慧, 陆海. 转录组分析转录因子AtbHLH68调控细胞壁发育的分子机制[J]. 生物技术通报, 2023, 39(9): 105-116. |
| [2] | 苗永美, 苗翠苹, 于庆才. 枯草芽孢杆菌BBs-27发酵液性质及脂肽对黄色镰刀菌的抑菌作用[J]. 生物技术通报, 2023, 39(9): 255-267. |
| [3] | 付钰, 贾瑞瑞, 何荷, 王良桂, 杨秀莲. 两种砧木楸树嫁接苗生长差异及转录组比较分析[J]. 生物技术通报, 2023, 39(8): 251-261. |
| [4] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [5] | 魏茜雅, 秦中维, 梁腊梅, 林欣琪, 李映志. 褪黑素种子引发处理提高朝天椒耐盐性的作用机制[J]. 生物技术通报, 2023, 39(7): 160-172. |
| [6] | 赵金玲, 安磊, 任晓亮. 单细胞转录组测序技术及其在秀丽隐杆线虫中的应用[J]. 生物技术通报, 2023, 39(6): 158-170. |
| [7] | 孔德真, 段震宇, 王刚, 张鑫, 席琳乔. 盐、碱胁迫下高丹草苗期生理特征及转录组学分析[J]. 生物技术通报, 2023, 39(6): 199-207. |
| [8] | 刘辉, 卢扬, 叶夕苗, 周帅, 李俊, 唐健波, 陈恩发. 外源硫诱导苦荞镉胁迫响应的比较转录组学分析[J]. 生物技术通报, 2023, 39(5): 177-191. |
| [9] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [10] | 谢洋, 邢雨蒙, 周国彦, 刘美妍, 银珊珊, 闫立英. 黄瓜二倍体及其同源四倍体果实转录组分析[J]. 生物技术通报, 2023, 39(3): 152-162. |
| [11] | 扈丽丽, 林柏荣, 王宏洪, 陈建松, 廖金铃, 卓侃. 最短尾短体线虫转录组及潜在效应蛋白分析[J]. 生物技术通报, 2023, 39(3): 254-266. |
| [12] | 杜清洁, 周璐瑶, 杨思震, 张嘉欣, 陈春林, 李娟起, 李猛, 赵士文, 肖怀娟, 王吉庆. 过表达CaCP1提高转基因烟草对盐胁迫的敏感性[J]. 生物技术通报, 2023, 39(2): 172-182. |
| [13] | 汪明滔, 刘建伟, 赵春钊. 植物调控盐胁迫下细胞壁完整性的分子机制[J]. 生物技术通报, 2023, 39(11): 18-27. |
| [14] | 周恒, 谢彦杰. 植物氧化胁迫信号应答的研究进展[J]. 生物技术通报, 2023, 39(11): 36-43. |
| [15] | 赵佳, 赵飞燕, 沈馨, 高广琦, 孙志宏. 乳酸菌抗氧化活性及其应用研究进展[J]. 生物技术通报, 2023, 39(11): 182-190. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||