








生物技术通报 ›› 2022, Vol. 38 ›› Issue (1): 194-204.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0177
杨威1( ), 伍茜1, 程建国2, 罗燕1(
), 伍茜1, 程建国2, 罗燕1( ), 王印1, 杨泽晓1, 姚学萍1
), 王印1, 杨泽晓1, 姚学萍1
收稿日期:2021-02-23
出版日期:2022-01-26
发布日期:2022-02-22
作者简介:杨威,男,硕士,研究方向:预防兽医学;E-mail: 基金资助:
YANG Wei1( ), WU Xi1, CHENG Jian-guo2, LUO Yan1(
), WU Xi1, CHENG Jian-guo2, LUO Yan1( ), WANG Yin1, YANG Ze-xiao1, YAO Xue-ping1
), WANG Yin1, YANG Ze-xiao1, YAO Xue-ping1
Received:2021-02-23
Published:2022-01-26
Online:2022-02-22
摘要:
干扰素在病毒性疾病的防治上,具有很高的临床应用前景。为了林麝干扰素的研究与应用,使用同源克隆方法首次克隆得到9条林麝干扰素α基因序列,序列全长均为570 bp,编码189个氨基酸,前23个氨基酸为信号肽,具有4个保守的半胱氨酸残基和5个保守的脯氨酸残基。9种亚型之间,核酸序列同源性为97.0%-99.6%,氨基酸序列同源性为93.7%-99.5%。蛋白结构主要由5个α-螺旋和无规则卷曲构成,三级结构与牛和人干扰素α高度相似。结构域预测包含干扰素受体IFNAR结合位点。首次构建了林麝干扰素α表达质粒并成功使用毕赤酵母表达出林麝干扰素α,确定了1%的最佳甲醇诱导浓度与96 h的最佳诱导时间。使用qPCR首次确定了林麝干扰素α对林麝肺成纤维细胞FMD-C1干扰素刺激基因具有转录调控作用,并具有剂量依赖性,调控作用在6 h达到最强。CCK-8法确定了林麝干扰素α可以抑制FMD-C1细胞的增殖,抗增殖作用也具有剂量依赖性。以上结果为林麝干扰素α的抗病毒研究与临床应用提供了理论基础。
杨威, 伍茜, 程建国, 罗燕, 王印, 杨泽晓, 姚学萍. 林麝干扰素α基因克隆、表达及转录调控分析[J]. 生物技术通报, 2022, 38(1): 194-204.
YANG Wei, WU Xi, CHENG Jian-guo, LUO Yan, WANG Yin, YANG Ze-xiao, YAO Xue-ping. Cloning,Expression and Transcriptional Regulation of Interferon-α in Forest Musk Deer[J]. Biotechnology Bulletin, 2022, 38(1): 194-204.
| 引物名Primer name | 引物序列Primer sequence(5'-3') | Tm/℃ | 产物长度Product length/bp |
|---|---|---|---|
| IFN-α | F:ATGGCCCCAGCCTGGTC R:TCAGTCCTTTCTCCTGAATCTCTCC | 58 | 570 |
| ExIFNα | F:CGGAATTCTGCCACCTGCCTCACAC R:ATAAGAATGCGGCCGCGTCCTTTCTCCTGAATCTCTCC | 57 | 522 |
| OAS | F:TGCTGACCTCGTCGTCTTCC R:TGGGGGACCTCAGCACAAAG | 58 | 194 |
| Viperin | F:TGGTGCCCGAGTCTAACCAG R:TCCATACATATTTCCCTCCTCGC | 57 | 195 |
| Mx1 | F:TCAACCTCCACCGAACTG R:TCTTCTTCTGCCTCCTTCTC | 56 | 172 |
| ISG15 | F:CAGCCAACCAGTGTCTG R:CCTAGCATCTTCACCGTCAG | 56 | 79 |
| ISG56 | F:TGGACTGTGAGGAAGGATGG R:GGCGATAGACAACGATTGC | 56 | 142 |
| β-actin | F:GAATCCTGCGGCATTCACG R:TCTTCATCGTGCTGGGTGC | 58 | 172 |
表1 引物序列
Table 1 Primer sequence
| 引物名Primer name | 引物序列Primer sequence(5'-3') | Tm/℃ | 产物长度Product length/bp |
|---|---|---|---|
| IFN-α | F:ATGGCCCCAGCCTGGTC R:TCAGTCCTTTCTCCTGAATCTCTCC | 58 | 570 |
| ExIFNα | F:CGGAATTCTGCCACCTGCCTCACAC R:ATAAGAATGCGGCCGCGTCCTTTCTCCTGAATCTCTCC | 57 | 522 |
| OAS | F:TGCTGACCTCGTCGTCTTCC R:TGGGGGACCTCAGCACAAAG | 58 | 194 |
| Viperin | F:TGGTGCCCGAGTCTAACCAG R:TCCATACATATTTCCCTCCTCGC | 57 | 195 |
| Mx1 | F:TCAACCTCCACCGAACTG R:TCTTCTTCTGCCTCCTTCTC | 56 | 172 |
| ISG15 | F:CAGCCAACCAGTGTCTG R:CCTAGCATCTTCACCGTCAG | 56 | 79 |
| ISG56 | F:TGGACTGTGAGGAAGGATGG R:GGCGATAGACAACGATTGC | 56 | 142 |
| β-actin | F:GAATCCTGCGGCATTCACG R:TCTTCATCGTGCTGGGTGC | 58 | 172 |

图1 FMD-IFNα的克隆及鉴定 A:FMD-IFNα的RT-PCR扩增(M:DL2 000 maker;1:阴性对照;2:目的片段);B:重组质粒pMD19-T-FMD-IFNα鉴定(M:DL2 000 Maker:1:阴性对照;2:目的片段)
Fig. 1 Cloning and identification of FMD-IFNα A:RT-PCR of FMD-IFNα. M:DL2 000 maker(1:Negative control. 2:Target fragment). B:Identification of recombinant plasmid pMD19-T-FMD-IFNα. (M:DL2 000 maker. 1:Negative control. 2:Target fragment)

图2 各亚型FMD-IFNα氨基酸序列同源性 灰色背景氨基酸为保守半胱氨酸残基位点;黑色方框氨基酸为保守脯氨酸位点;BosIFN-αA:NP_001017411.1;SusIFN-α4:NM_001166319.1;HomoIFN-α2:NM_000605.4
Fig. 2 Homology of amino acid sequence of each subtype of FMD-IFNα The gray background amino acids are conserved cysteine residue positions. The black boxed amino acids are conserved proline positions. BosIFN-αA:NP_001017411.1, SusIFN-α4:NM_001166319.1, HomoIFN-α2:NM_000605.4
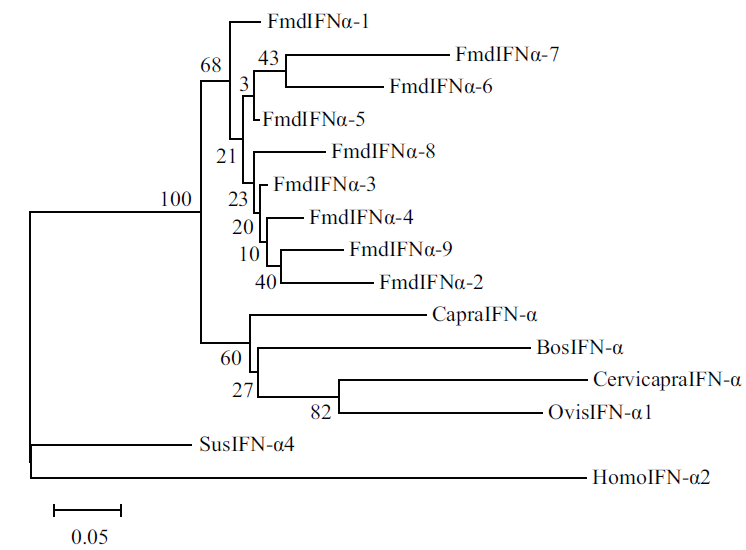
图3 IFNα氨基酸序列系统发生树
Fig. 3 Phylogenetic tree of IFNα amino acid sequence BosIFN-αA:NP_001017411.1, CapralIFN-α:NP_001272633.1, CervicapraIFN-α:ACR61636.1,OvisIFN-α:XP_004005368.4, SusIFN-α4:NP_001159791.1, HomoIFN-α2:NP_000596.2

图6 PCR产物的琼脂糖凝胶电泳图 A:FMD-IFNα表达片段PCR产物(M:DL2000 maker;1-4:阳性转化子;5:阴性对照);B:pPICZα-IFNα的鉴定PCR产物(M:DL2 000 maker;1-5:阳性转化子;6:阴性对照)
Fig. 6 Agarose gel electrophoresis of PCR products A:FMD-IFNα expression fragment(M:DL2000 maker. 1-4:Positive transformants. 5:Negative control). B:Identification PCR product of pPICZα-IFNα(M:DL2000 maker. 1-5:Positive transformant. 6:Negative control)
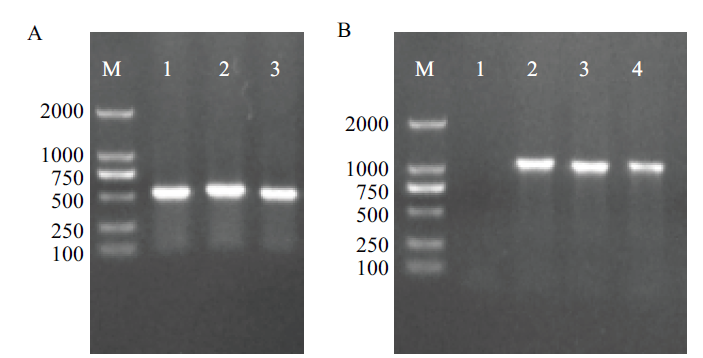
图7 阳性毕赤酵母转化子PCR鉴定结果 A:ExIFNα-F/R鉴定结果(M:DL2000 maker;1-3:阳性转化子);B:AOX1-F/R鉴定结果(M:DL2000 maker;1:阴性对照;2-4:阳性转化子)
Fig. 7 PCR identification results of positive Pichia pastoris transformants A:Identification result of ExIFNα-F/R(M:DL2 000 maker. 1-3:Positive transformants). B:Identification result of AOX1-F/R(M:DL2 000 maker. 1:Negative control. 2-4:Positive transformants)
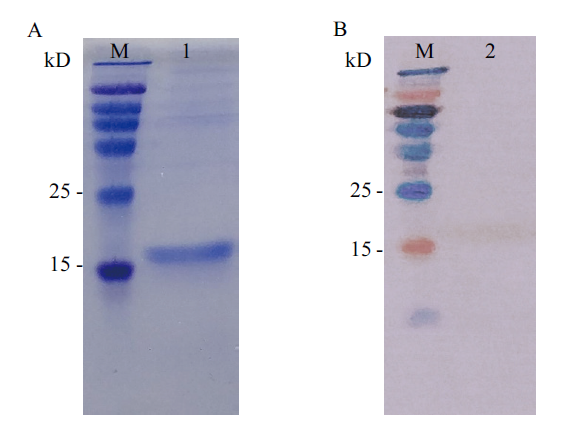
图8 FMD-IFNα初步诱导结果 A:SDS-PAGE结果图(M:彩色预染蛋白分子量标准(15-120 kD);1:重组酵母表达上清);B:Western blot结果图(M:彩色预染蛋白分子量标准(15-120 kD);2:重组酵母表达上清)
Fig. 8 Preliminary expression results of FMD-IFNα A:SDS-PAGE results(M:Color pre-stained protein molecular weight standard(15-120 kD). 1:Recombinant yeast expression supernatant). B:Western blot results(M:Color pre-stained protein molecular weight standard(15-120 kD). 2:Recombinant yeast expression supernatant)
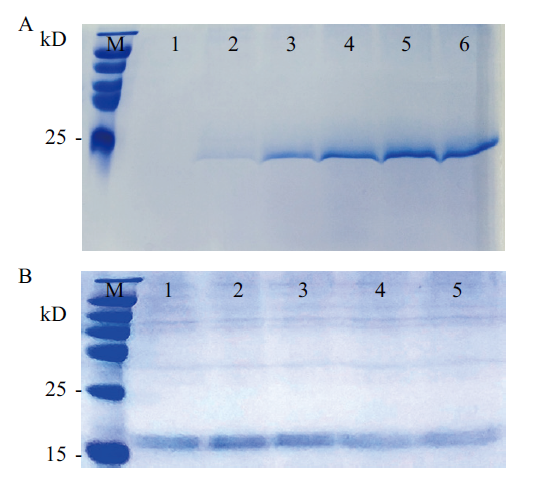
图9 重组毕赤酵母诱导表达优化结果 A:时间优化结果(M:彩色预染蛋白分子量标准(15-120 kD);1-6分别是诱导0 h,24 h,48 h,72 h,96 h,120 h的表达上清液); B:浓度优化结果(M:彩色预染蛋白分子量标准(15-120 kD);1-5泳道分别是甲醇浓度0.5%,1%,1.5%,2%,2.5%诱导表达上清液)
Fig. 9 Recombinant Pichia pastoris induced expression optimization results A:Time optimization results(M:Color pre-stained protein molecular weight standard(15-120 kD). 1-6 are expression supernatant after induced 0 h,24 h,48 h,72 h,96 h,and 120 h,respectively). B:Concentration optimization results(M:Molecular weight standard of color predyed protein(15-120 kD). 1-5 are expression supernatants induced by methanol concentration 0.5%,1%,1.5%,2%,and 2.5%)
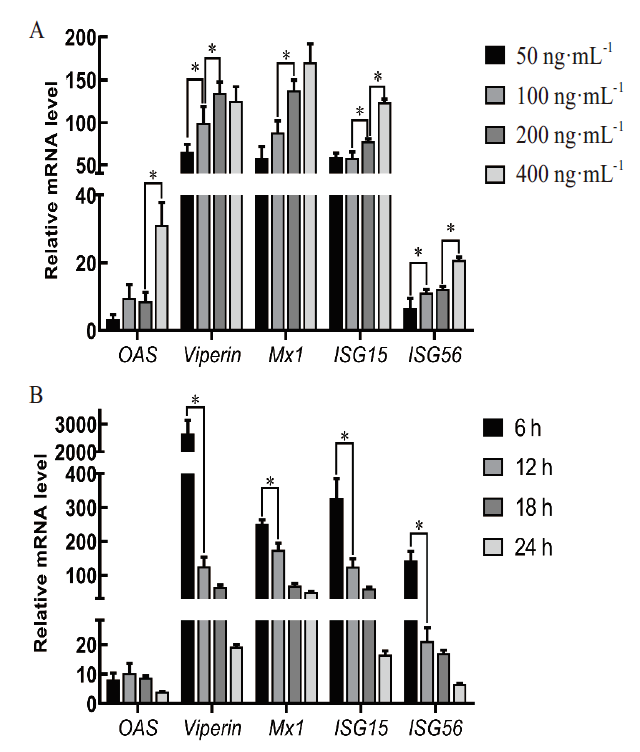
图10 FMD-IFNα调控ISG转录结果 A:剂量依赖性结果;B:时间依赖性结果;*:P<0.05
Fig. 10 Regulation results of FMD-IFNα on ISG transcription A:Dose-dependent results. B:Time-dependent results. *:P<0.05
| [1] | 王淯, 姜海瑞, 薛文杰, 等. 林麝(Moschus berezovskii)研究概况和进展[J]. 四川动物, 2006, 25(1):195-200. |
| Wang Y, Jiang HR, Xue WJ, et al. Advances in research of forest musk deer(Moschus berezovskii)[J]. Sichuan J Zool, 2006, 25(1):195-200. | |
| [2] | 巩海涛, 王雁群, 贺广彬, 等. 麝香药理及代用品的研究近况[J]. 山东医药工业, 2002, 21(1):26-27. |
| Gong HT, Wang YQ, He GB, et al. Research status of musk pharmacology and substitute[J]. Shangdong Pharm Ind, 2002, 21(1):26-27. | |
| [3] | 宋兴超, 杨福合, 邢秀梅. 中国麝科动物的种类、分布、价值及其保护对策[J]. 特种经济动植物, 2008, 11(9):5-7. |
| Song XC, Yang FH, Xing XM. Species, distribution, value and conservation strategies of musk deer in China[J]. Special Econ Animal Plant, 2008, 11(9):5-7. | |
| [4] | 戴晓阳, 王承旭, 王朋. 一种未知病毒导致家养林麝死亡的报道[J]. 湖北畜牧兽医, 2011, 32(10):25-26. |
| Dai XY, Wang CX, Wang P. A report of an unknown virus causing the death of forest musk deer[J]. Hubei J Animal Vet Sci, 2011, 32(10):25-26. | |
| [5] | 赵婷婷. 鹅干扰素-α在毕赤酵母中的分泌表达及抗病毒活性研究[D]. 雅安:四川农业大学, 2011. |
| Zhao TT. Secretory expression of Tianfu goose interferon-alpha in Pichia pastoris and its bioactive research[D]. Yaan:Sichuan Agricultural University, 2011. | |
| [6] |
Sen GC. Viruses and interferons[J]. Annu Rev Microbiol, 2001, 55(1):255-281.
doi: 10.1146/micro.2001.55.issue-1 URL |
| [7] |
Tan XM, Tang Y, Yang YF, et al. Gene cloning, sequencing, expression and biological activity of giant Panda(Ailuropoda melanoleuca)interferon-alpha[J]. Mol Immunol, 2007, 44(11):3061-3069.
doi: 10.1016/j.molimm.2006.12.017 URL |
| [8] | 李紫仟, 廉士珍, 胡博, 等. 北极狐、貉、犬IFN-基因克隆及表达[J]. 特产研究, 2020, 42(1):1-5. |
| Li ZQ, Lian SZ, Hu B, et al. Cloning and expression of IFN-gene from arctic fox, raccoon dog and canine[J]. Special Wild Econ Animal Plant Res, 2020, 42(1):1-5. | |
| [9] | 孙颖杰, 王哲. 水貂α-干扰素基因的克隆、测序和生物信息学分析[J]. 畜牧与兽医, 2009, 41(6):62-64. |
| Sun YJ, Wang Z. Cloning, sequencing and bioinformatics analysis of α-interferon gene in mink[J]. Animal Husb Vet Med, 2009, 41(6):62-64. | |
| [10] | 罗长财. 一种耐高温β-甘露聚糖酶在毕赤酵母中高效表达及其耐高温机理分析[D]. 无锡:江南大学, 2018. |
| Luo CC. High-level expression of A highly thermostable β-mannanase by Pichia pastoris and its mechanism analysis of thermostability[D]. Wuxi:Jiangnan University, 2018. | |
| [11] | 宋茧. 重组人PDGF-Fc融合蛋白的表达纯化及其活性测定[D]. 长春:吉林大学, 2019. |
| Song J. Expression, purification and activity determination of recombinant human PDGF-fc fusion protein[D]. Changchun:Jilin University, 2019. | |
| [12] |
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR[J]. Nucleic Acids Res, 2001, 29(9):e45.
doi: 10.1093/nar/29.9.e45 URL |
| [13] | 刘齐. 林麝和麋鹿肠道内病毒群落解析及新发病毒遗传特征研究[D]. 镇江:江苏大学, 2020. |
| LIU Q. Analysis of gut virus community and genetic characteristics of novel viruses between forest musk deer and elk[D]. Zhenjiang:Jiangsu University, 2020. | |
| [14] | 罗燕, 康纪平, 程建国, 等. 麝肺炎和化脓性疾病病毒的理化特性研究[J]. 安徽农业科学, 2010, 38(28):15649-15652. |
| Luo Y, Kang JP, Cheng JG, et al. Physical and chemical properties of pneumonia and suppurative disease virus in musk deer[J]. J Anhui Agric Sci, 2010, 38(28):15649-15652. | |
| [15] | 王照. 毕赤酵母表达犬长效干扰素融合蛋白的研究[D]. 长春:吉林农业大学, 2013. |
| Wang Z. The research of long-activity interferon fusion protein expressed by Pichia pastoris[D]. Changchun:Jilin Agricultural University, 2013. | |
| [16] | 林金祥, 杨可立. PEG-IFNα-2a与PEG-IFNα-2b治疗慢性乙型肝炎患者疗效研究[J]. 实用肝脏病杂志, 2021, 24(1):27-30. |
| Lin JX, Yang KL. Comparison of response to PEG-IFNα-2a or PEG-IFNα-2b in patients with chronic hepatitis B[J]. J Prac Hepatol, 2021, 24(1):27-30. | |
| [17] | 王华, 李娟红, 田雪琴, 等. 重组人干扰素α-2b凝胶联合聚甲酚磺醛溶液治疗宫颈病变人乳头瘤病毒感染患者的疗效[J]. 中华医院感染学杂志, 2021, 31(1):143-147. |
| Wang H, Li JH, Tian XQ, et al. Effect of recombinant human interferon α-2b combined with polycresol sulfonaldehyde solution on treatment of patients with cervical lesions and human papillomavirus infection[J]. Chin J Nosocomiology, 2021, 31(1):143-147. | |
| [18] |
Capon DJ, Shepard HM, Goeddel DV. Two distinct families of human and bovine interferon-alpha genes are coordinately expressed and encode functional polypeptides[J]. Mol Cell Biol, 1985, 5(4):768-779.
doi: 10.1128/mcb.5.4.768-779.1985 pmid: 2985969 |
| [19] |
Nisbet IT, Beilharz MW, Hertzog PJ, et al. Single amino acid substitutions at conserved residues of human interferon-alpha can effect antiviral specific activity[J]. Biochem Int, 1985, 11(3):301-309.
pmid: 3904757 |
| [20] | Roberts RM, Liu L, Alexenko A. New and atypical families of type I interferons in mammals:comparative functions, structures, and evolutionary relationships[J]. Prog Nucleic Acid Res Mol Biol, 1997, 56:287-325. |
| [21] | 亓立峰, 许梓荣. 干扰素的分子生物学研究进展[J]. 中国兽药杂志, 2003, 37(6):22-25. |
| Qi LF, Xu ZR. The molecular biological research of interferons[J]. Chin J Vet Drug, 2003, 37(6):22-25. | |
| [22] | de Veer MJ, Holko M, Frevel M, et al. Functional classification of interferon-stimulated genes identified using microarrays[J]. J Leukoc Biol, 2001, 69(6):912-920. |
| [23] | 白思宇, 杨倩, 仇华吉. 干扰素刺激基因的抗病毒机制[J]. 微生物学报, 2018, 58(3):361-371. |
| Bai SY, Yang Q, Qiu HJ. Antiviral mechanisms of interferon-stimulated genes[J]. Acta Microbiol Sin, 2018, 58(3):361-371. | |
| [24] |
Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions[J]. Curr Opin Virol, 2011, 1(6):519-525.
doi: 10.1016/j.coviro.2011.10.008 pmid: 22328912 |
| [25] |
Lanford RE, Guerra B, Lee H, et al. Genomic response to interferon-alpha in chimpanzees:implications of rapid downregulation for hepatitis C kinetics[J]. Hepatology, 2006, 43(5):961-972.
pmid: 16628626 |
| [26] |
Sarasin-Filipowicz M, Oakeley EJ, Duong FH, et al. Interferon signaling and treatment outcome in chronic hepatitis C[J]. PNAS, 2008, 105(19):7034-7039.
doi: 10.1073/pnas.0707882105 pmid: 18467494 |
| [27] |
Harada H, Fujita T, Miyamoto M, et al. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes[J]. Cell, 1989, 58(4):729-739.
doi: 10.1016/0092-8674(89)90107-4 pmid: 2475256 |
| [28] |
Nelson N, Marks MS, Driggers PH, et al. Interferon consensus sequence-binding protein, a member of the interferon regulatory factor family, suppresses interferon-induced gene transcription[J]. Mol Cell Biol, 1993, 13(1):588-599.
doi: 10.1128/mcb.13.1.588-599.1993 pmid: 7678054 |
| [29] | 黄金文, 许靖. 干扰素α对人骨髓成纤维细胞体外生长的影响[J]. 中华血液学杂志, 2004, 25(8):478-481 |
| Huang JW, Xu J. In vitro effect of interferon α on proliferation of human marrow fibroblast[J]. Chin J Hematol, 2004, 25(8):478-481 | |
| [30] |
Šantak G, Šantak M, Forčić D. Low concentration of PDGF-AB shows synergism with IFN-α in induction of IFN-β and -γ in MRC5 fibroblasts[J]. Cytokine, 2013, 64(2):494-496.
doi: 10.1016/j.cyto.2013.09.001 pmid: 24063997 |
| [31] | 陈君毅, 孙兴怀, 笪翠弟, 等. 干扰素α-2b对人眼tenon囊成纤维细胞纤维化作用的影响[J]. 中国眼耳鼻喉科杂志, 2011, 11(5):277-280. |
| Chen JY, Sun XH, Da CD, et al. Investigation of interferon α-2b in the fibrosis of human Tenon's capsule fibroblasts[J]. Chin J Ophthalmol Otorhinolaryngol, 2011, 11(5):277-280. | |
| [32] | 于宏, 孙立荣, 庞秀英, 等. 干扰素α-2b对HL-60细胞抑增殖与促凋亡的影响[J]. 中国实验血液学杂志, 2007, 15(1):56-58. |
| Yu H, Sun LR, Pang XY, et al. Influence of interferonα-2b on proliferation inhibition and apoptosis induction in HL-60 cells[J]. J Exp Hematol, 2007, 15(1):56-58. | |
| [33] |
Santak G, Santak M, Forcić D. Native human IFN-alpha is a more potent suppressor of HDF response to profibrotic stimuli than recombinant human IFN-alpha[J]. J Interferon Cytokine Res, 2007, 27(6):481-490.
doi: 10.1089/jir.2007.0174 URL |
| [34] |
Rosewicz S, Detjen K, Scholz A, et al. Interferon-alpha:regulatory effects on cell cycle and angiogenesis[J]. Neuroendocrinology, 2004, 80(Suppl 1):85-93.
doi: 10.1159/000080748 URL |
| [35] | Maeda S, Wada H, Naito Y, et al. Interferon-α Acts on the S/G2/M phases to induce apoptosis in the G1 phase of an IFNAR2-expressing hepatocellular carcinoma cell line[J]. J Biol Chem, 2014, 289(34):23786-23795. |
| [36] |
Yano H, Iemura A, Haramaki M, et al. Interferon Alfa receptor expression and growth inhibition by interferon Alfa in human liver cancer cell lines[J]. Hepatology, 1999, 29(6):1708-1717.
pmid: 10347112 |
| [37] |
Murphy D, Detjen KM, Welzel M, et al. Interferon-alpha delays S-phase progression in human hepatocellular carcinoma cells via inhibition of specific cyclin-dependent kinases[J]. Hepatol Baltim Md, 2001, 33(2):346-356.
doi: 10.1053/jhep.2001.21749 URL |
| [38] |
Detjen KM, Welzel M, Farwig K, et al. Molecular mechanism of interferon Alfa-Mediated growth inhibition in human neuroendocrine tumor cells[J]. Gastroenterology, 2000, 118(4):735-748.
pmid: 10734025 |
| [1] | 赵思佳, 王晓璐, 孙纪录, 田健, 张杰. 代谢工程改造毕赤酵母生产赤藓糖醇[J]. 生物技术通报, 2023, 39(8): 137-147. |
| [2] | 李英, 岳祥华. DNA甲基化在解析毛竹自然变异中的应用[J]. 生物技术通报, 2023, 39(7): 48-55. |
| [3] | 董聪, 高庆华, 王玥, 罗同阳, 王庆庆. 基于联合策略提高FAD依赖的葡萄糖脱氢酶的酵母表达[J]. 生物技术通报, 2023, 39(6): 316-324. |
| [4] | 陈强, 邹明康, 宋家敏, 张冲, 吴隆坤. 甜瓜LBD基因家族的鉴定和果实发育进程中的表达分析[J]. 生物技术通报, 2023, 39(3): 176-183. |
| [5] | 许睿, 祝英方. 中介体复合物在植物非生物胁迫应答中的功能[J]. 生物技术通报, 2023, 39(11): 54-60. |
| [6] | 赵昕, 杜玉瑶, 殷子扬, 毛淑红. 胆固醇7α-羟化酶在毕赤酵母中的异源表达[J]. 生物技术通报, 2023, 39(10): 304-310. |
| [7] | 张婵, 吴友根, 于靖, 杨东梅, 姚广龙, 杨华庚, 张军锋, 陈萍. 光与茉莉酸信号介导的萜类化合物合成分子机制[J]. 生物技术通报, 2022, 38(8): 32-40. |
| [8] | 王玥, 高庆华, 董聪, 罗同阳, 王庆庆. 密码子优化的吡喃糖氧化酶基因在毕赤酵母中的表达[J]. 生物技术通报, 2022, 38(4): 269-277. |
| [9] | 陈臣, 黄芝阳, 于海燕, 袁海彬, 田怀香. 原核生物转录调控研究技术及进展[J]. 生物技术通报, 2022, 38(10): 54-65. |
| [10] | 李丹, 杜梦潭, 修明霞, 刘兴健, 张志芳, 李轶女. 羊α干扰素在家蚕中的表达及抗小反刍兽疫病毒活性测定[J]. 生物技术通报, 2022, 38(1): 187-193. |
| [11] | 廖兆民, 蔡俊, 林建国, 杜馨, 王常高. 黑曲霉葡萄糖氧化酶基因在毕赤酵母中的表达及产酶条件的优化[J]. 生物技术通报, 2021, 37(6): 97-107. |
| [12] | 赵鸿远, 王朝, 成温玉, 马宁宁, 李曼, 魏小丽. 抗非洲猪瘟病毒制剂的研究进展[J]. 生物技术通报, 2021, 37(5): 174-181. |
| [13] | 杨悦, 陶妍, 谢晶, 钱韻芳. 基于重组毕赤酵母的草鱼C型溶菌酶生物合成及其抑菌活性[J]. 生物技术通报, 2021, 37(12): 169-179. |
| [14] | 徐楠, 徐宇娟, 孙盼, 宗仁杰, 郭敏亮. 根癌农杆菌vbp2基因启动子转录调控的探析[J]. 生物技术通报, 2021, 37(12): 41-49. |
| [15] | 马军, 徐通达. 植物非经典生长素信号转导通路解析[J]. 生物技术通报, 2020, 36(7): 15-22. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||