








生物技术通报 ›› 2022, Vol. 38 ›› Issue (2): 1-9.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0498
• 研究报告 • 下一篇
杨佳慧( ), 孙玉萍, 陆雅宁, 刘欢, 卢存福(
), 孙玉萍, 陆雅宁, 刘欢, 卢存福( ), 陈玉珍(
), 陈玉珍( )
)
收稿日期:2021-04-15
出版日期:2022-02-26
发布日期:2022-03-09
作者简介:杨佳慧,女,硕士研究生,研究方向:植物分子生物学;E-mail: 基金资助:
YANG Jia-hui( ), SUN Yu-ping, LU Ya-ning, LIU huan, LU Cun-fu(
), SUN Yu-ping, LU Ya-ning, LIU huan, LU Cun-fu( ), CHEN Yu-zhen(
), CHEN Yu-zhen( )
)
Received:2021-04-15
Published:2022-02-26
Online:2022-03-09
摘要:
端粒酶是真核生物中维持染色体末端DNA完整性的一类特殊逆转录酶,研究拟南芥AtTERT对大肠杆菌生长及非生物胁迫的影响,为深入研究TERT蛋白非端粒功能奠定基础。将拟南芥AtTERT转入大肠杆菌,成功构建pET32a-AtTERT原核表达载体,优化诱导条件,纯化并鉴定GST-AtTERT融合蛋白,运用Western blotting验证,同时采用点板法检测转AtTERT大肠杆菌的非生物胁迫抗性。结果表明,优化诱导条件为感受态细胞Transetta(DE3)诱导温度20℃、诱导剂(IPTG)浓度为0.5 mmol/L; 纯化的GST-AtTERT融合蛋白相对分子量大小为156 kD;Western blotting验证结果显示在诱导全菌、上清及沉淀中均出现并与预期分子量大小结果一致的蛋白条带。转AtTERT重组菌在NaCl(400和500 mmol/L)、甘露醇(400和600 mmol/L)和H2O2(0.4 mmol/L)的LB固体培养基上的存活率显著高于空载对照菌,而利用液氮反复冻融6次时,转AtTERT重组菌存活率显著低于空载对照菌。转AtTERT大肠杆菌NaCl盐胁迫、甘露醇渗透胁迫、过氧化氢氧化胁迫抗性增强,但低温抗性减弱,表明拟南芥AtTERT具有抗非生物胁迫的非端粒功能。
杨佳慧, 孙玉萍, 陆雅宁, 刘欢, 卢存福, 陈玉珍. 拟南芥AtTERT对大肠杆菌非生物胁迫抗性的影响[J]. 生物技术通报, 2022, 38(2): 1-9.
YANG Jia-hui, SUN Yu-ping, LU Ya-ning, LIU huan, LU Cun-fu, CHEN Yu-zhen. Abiotic Stress Resistance of Escherichia coli Transformed with Arabidopsis thaliana AtTERT Gene[J]. Biotechnology Bulletin, 2022, 38(2): 1-9.
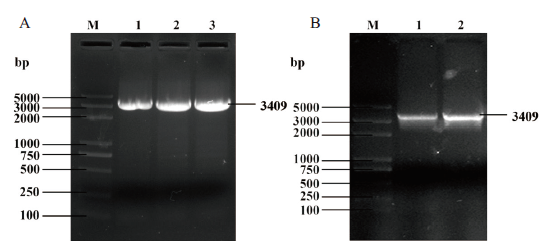
图1 AtTERT的PCR扩增 A:带有pET-32a载体序列的AtTERT;B:带有pGEX-4T-1载体序列的AtTERT。M:5 000 bp DNA marker
Fig. 1 PCR amplification of AtTERT gene A:AtTERT gene with pET32a vector sequence. B:AtTERT gene with pGEX-4T-1 vector sequence. M:5 000 bp DNA marker
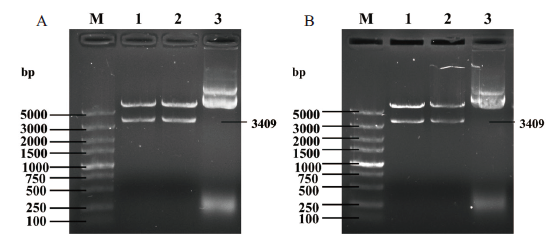
图2 pET32a/ pGEX-4T-1-AtTERT重组质粒的酶切鉴定 A:pET32a-AtTERT重组质粒酶切鉴定;B:pGEX-4T-1-AtTERT重组质粒酶切鉴定。M:5 000 bp marker;1、2:重组质粒酶切;3:对照质粒
Fig.2 Double digestion identification of pET32a/ pGEX-4T-1-AtTERT A:Identification of pET32a-AtTERT recombinant plasmid after enzyme digestion. B:Identification of pGEX-4T-1-AtTERT recombinant plasmid after enzyme digestion. M:5 000 bp marker. 1,2:Recombinant plasmid after enzyme digestion. 3:Control plasmid
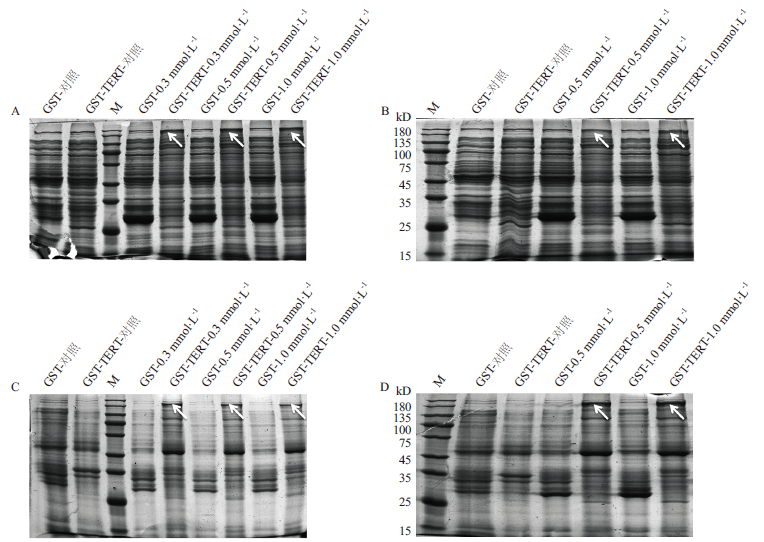
图3 GST-TERT蛋白的SDS-PAGE检测 A:20℃不同IPTG浓度下可溶性蛋白的表达;B:30℃不同IPTG浓度下可溶性蛋白的表达;C:20℃不同IPTG浓度下包涵体蛋白的表达;D:30℃不同IPTG浓度下包涵体蛋白的表达。M:180 kD marker
Fig. 3 Detection of GST-TERT protein by SDS-PAGE A:Expression of soluble protein under different IPTG concentrations at 20℃. B:Expression of soluble protein under different IPTG concentrations at 30℃. C:Expression of inclusion body protein under different IPTG concentrations at 20℃. D:Expression of inclusion body protein under different IPTG concentrations at 30℃. M:180 kD marker

图4 Western blotting鉴定GST-AtTERT融合蛋白 A:鉴定GST-AtTERT融合蛋白的表达形式(1,2:空载体对照菌及转基因重组菌诱导后全菌蛋白;3,4:空载体对照菌及转基因重组菌诱导后可溶性蛋白;5,6:空载体对照菌及转基因重组菌包涵体蛋白);B:鉴定纯化后GST-AtTERT融合蛋白(1,2:空载体对照菌及转基因重组菌诱导后可溶性蛋白;3孵育后流出液;4:杂蛋白洗脱后流出液;5-7:纯化后蛋白)
Fig. 4 Western blotting identification of GST-AtTERT fusion protein A:Identification of GST-AtTERT fusion protein pattern(1,2:bacterial protein induced by empty vector control bacteria and transgenic recombinant bacteria;3,4:soluble protein induced by empty vector control bacteria and transgenic recombinant bacteria;5,6:inclusion body protein induced by empty vector control bacteria and transgenic recombinant bacteria). B:Identification of purified GST-AtTERT fusion protein(1,2:soluble protein induced by empty vector control bacteria and transgenic recombinant bacteria;3:outflow after incubation;4:outflow after miscellaneous protein washing out;5-7:protein after elution)
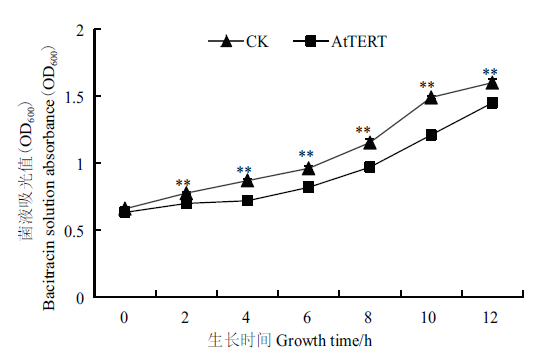
图5 AtTERT对大肠杆菌生长特性的影响 **:表示在P<0.01水平差异达到极显著水平;*:表示在P<0.05水平差异显著。下同
Fig.5 Effect of AtTERT on the growth characteristics of E.coli **indicates that the difference is extremely significant at P<0.01;*indicates that the difference is significant at P<0.05. The same below
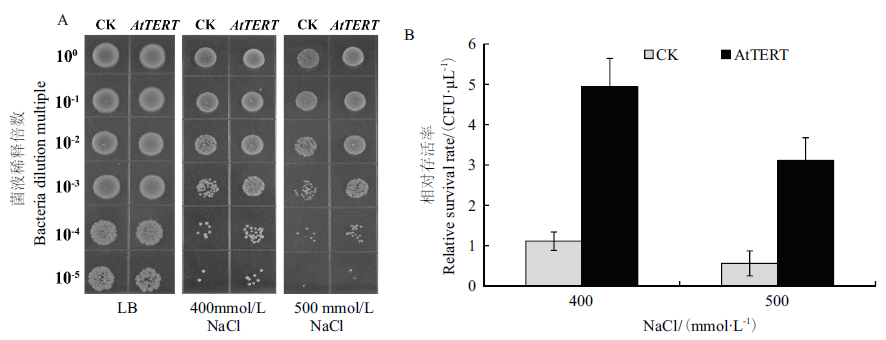
图6 盐胁迫下AtTERT对大肠杆菌存活率的影响 A:空载对照菌(CK)和转基因重组菌(pET32a-AtTERT)在LB(0 mmol/L NaCl)及含有NaCl(400和500 mmol/L)的LB固体培养基上生长状况;B:大肠杆菌菌液稀释104倍时存活率
Fig.6 Effect of AtTERT on the survival rate of E. coli under salt stress A:Growth status of empty control bacteria(pET32a)and transgenic recombinant bacteria(pET32a-AtTERT). B:Survival rate of E.coli when the bacterial solution diluted 104 times
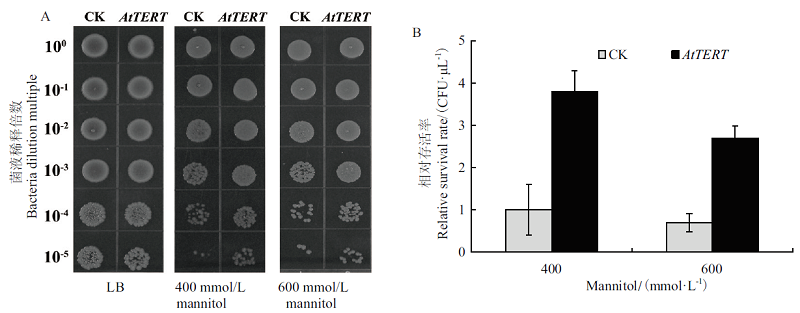
图7 甘露醇胁迫下AtTERT对大肠杆菌存活率的影响 A:空载对照菌(pET32a)和转基因重组菌(pET32a-AtTERT)在含有甘露醇的LB固体培养基上生长状况;B:大肠杆菌菌液稀释104倍时存活率
Fig.7 Effect of AtTERT on the survival rate of E. coli under mannitol stress A:Growth status of empty control bacteria(pET32a)and transgenic recombinant bacteria(pET32a-AtTERT)on the LB solid medium containing mannitol. B:Survival rate of E.coli when the bacterial solution diluted 104 times

图8 低温胁迫下AtTERT对大肠杆菌存活率的影响 A:空载对照菌(pET32a)和转基因重组菌(pET32a-AtTERT)经过液氮反复冻融后在LB固体培养基上生长状况;B:大肠杆菌菌液稀释104倍时存活率
Fig.8 Effect of AtTERT on the survival rate of E. coli under cold stress A:Growth status of empty control bacteria(pET32a)and transgenic recombinant bacteria(pET32a-AtTERT)on the LB solid medium after repeated freezing and thawing in liquid nitrogen. B:Survival rate of E.coli when the bacterial solution diluted 104 times
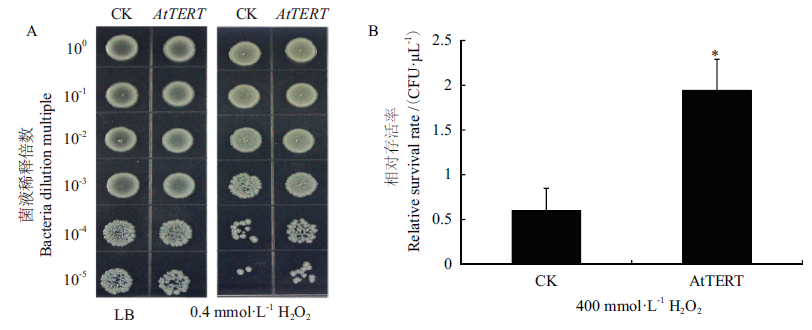
图9 H2O2胁迫下AtTERT对大肠杆菌存活率的影响 A:空载对照菌(pET32a)和转基因重组菌(pET32a-AtTERT)在含有0 mmol/L过氧化氢LB固体培养基上生长状况;B:大肠杆菌菌液稀释104倍时存活率
Fig. 9 Effect of AtTERT on the survival rate of E. coli under hydrogen peroxide stress
| [1] |
Chan H, Wang Y, Feigon J. Progress in human and Tetrahymena telomerase structure determination[J]. Annu Rev Biophys, 2017, 46:199-225.
doi: 10.1146/biophys.2017.46.issue-1 URL |
| [2] |
Jiang JS, Wang YQ, Sušac L, et al. Structure of telomerase with telomeric DNA[J]. Cell, 2018, 173(5):1179-1190. e13.
doi: 10.1016/j.cell.2018.04.038 URL |
| [3] |
Tomlinson RL, Abreu EB, Ziegler T, et al. Telomerase reverse transcriptase is required for the localization of telomerase RNA to Cajal bodies and telomeres in human cancer cells[J]. Mol Biol Cell, 2008, 19(9):3793-3800.
doi: 10.1091/mbc.E08-02-0184 pmid: 18562689 |
| [4] |
Majerská J, Schrumpfová PP, Dokládal L, et al. Tandem affinity purification of AtTERT reveals putative interaction partners of plant telomerase in vivo[J]. Protoplasma, 2017, 254(4):1547-1562.
doi: 10.1007/s00709-016-1042-3 pmid: 27853871 |
| [5] |
Dvořáčková M, Fojtová M, Fajkus J. Chromatin dynamics of plant telomeres and ribosomal genes[J]. Plant J, 2015, 83(1):18-37.
doi: 10.1111/tpj.2015.83.issue-1 URL |
| [6] |
Doksani Y, de Lange T. Telomere-internal double-strand breaks are repaired by homologous recombination and PARP1/Lig3-dependent end-joining[J]. Cell Rep, 2016, 17(6):1646-1656.
doi: S2211-1247(16)31394-8 pmid: 27806302 |
| [7] | 刘颖, 吴晓飞, 门璟煜, 等. 端粒酶的非端粒功能研究进展[J]. 中国细胞生物学学报, 2016, 38(5):640-646. |
| Liu Y, Wu XF, Men JY, et al. Research progress of non-telomeric functions of telomerase[J]. Chin J Cell Biol, 2016, 38(5):640-646. | |
| [8] |
Ding D, Zhou J, Wang M, et al. Implications of telomere-independent activities of telomerase reverse transcriptase in human cancer[J]. Febs J, 2013, 280(14):3205-3211.
doi: 10.1111/febs.12258 URL |
| [9] | Chiodi I, Mondello C. Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria[J]. Front Oncol, 2012, 2:133. |
| [10] |
Masutomi K, Possemato R, Wong JM, et al. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses[J]. PNAS, 2005, 102(23):8222-8227.
doi: 10.1073/pnas.0503095102 URL |
| [11] |
Park JI, Venteicher AS, Hong JY, et al. Telomerase modulates Wnt signalling by association with target gene chromatin[J]. Nature, 2009, 460(7251):66-72.
doi: 10.1038/nature08137 URL |
| [12] |
Dokládal L, Benková E, Honys D, et al. An Armadillo-domain protein participates in a telomerase interaction network[J]. Plant Mol Biol, 2018, 97(4/5):407-420.
doi: 10.1007/s11103-018-0747-4 URL |
| [13] |
Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity[J]. Cell, 1987, 51(6):887-898.
pmid: 3319189 |
| [14] |
Le Blancq SM, Kase RS, Van der Ploeg LH. Analysis of a Giardia lamblia rRNA encoding telomere with[TAGGG]n as the telomere repeat[J]. Nucleic Acids Res, 1991, 19(20):5790.
pmid: 1840670 |
| [15] |
Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice[J]. Nature, 1990, 347(6291):400-402.
doi: 10.1038/347400a0 URL |
| [16] |
Xiao Z, Zhang A, Lin J, et al. Telomerase:a target for therapeutic effects of curcumin and a curcumin derivative in Aβ1-42 insult in vitro[J]. PLoS One, 2014, 9(7):e101251.
doi: 10.1371/journal.pone.0101251 URL |
| [17] |
Fajkus J, Kovarík A, Královics R. Telomerase activity in plant cells[J]. FEBS Lett, 1996, 391(3):307-309.
pmid: 8764995 |
| [18] |
Oguchi K, Liu HT, Tamura K, et al. Molecular cloning and characterization of AtTERT, a telomerase reverse transcriptase homolog in Arabidopsis thaliana[J]. FEBS Lett, 1999, 457(3):465-469.
pmid: 10471830 |
| [19] |
Heller-Uszynska K, Schnippenkoetter W, Kilian A. Cloning and characterization of rice(Oryza sativa L)telomerase reverse transcriptase, which reveals complex splicing patterns[J]. Plant J, 2002, 31(1):75-86.
pmid: 12100484 |
| [20] | 王瑾瑜, 张徐俞, 王雅群, 等. 用改进的TRAP法测定树木端粒酶活性[J]. 应用与环境生物学报, 2012, 18(4):682-686. |
|
Wang JY, Zhang XY, Wang YQ, et al. Detection of tree telomerase activity by a modified TRAP assay method[J]. Chin J Appl Environ Biol, 2012, 18(4):682-686.
doi: 10.3724/SP.J.1145.2012.00682 URL |
|
| [21] | 慕莹, 赵晓燕, 景丹龙, 等. 银杏不同组织器官及愈伤组织培养中端粒酶活性测定[J]. 北京林业大学学报, 2014, 36(3):95-99. |
| Mu Y, Zhao XY, Jing DL, et al. Telomerase activity assay in different organs and callus culture of Ginkgo biloba L[J]. J Beijing For Univ, 2014, 36(3):95-99. | |
| [22] |
Tanese N, Roth M, Goff SP. Expression of enzymatically active reverse transcriptase in Escherichia coli[J]. PNAS, 1985, 82(15):4944-4948.
pmid: 2410910 |
| [23] |
Hansen DT, Thiyagarajan T, Larson AC, et al. Telomerase repeat amplification protocol(TRAP)activity upon recombinant expression and purification of human telomerase in a bacterial system[J]. Protein Expr Purif, 2016, 123:6-13.
doi: 10.1016/j.pep.2016.03.001 URL |
| [24] |
Geserick C, Tejera A, González-Suárez E, et al. Expression of mTert in primary murine cells links the growth-promoting effects of telomerase to transforming growth factor-beta signaling[J]. Oncogene, 2006, 25(31):4310-4319.
pmid: 16501597 |
| [25] |
Sharma GG, Gupta A, Wang HC, et al. hTERT associates with human telomeres and enhances genomic stability and DNA repair[J]. Oncogene, 2003, 22(1):131-146.
doi: 10.1038/sj.onc.1206063 URL |
| [26] |
Smith LL, Coller HA, Roberts JM. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation[J]. Nat Cell Biol, 2003, 5(5):474-479.
doi: 10.1038/ncb985 URL |
| [27] |
Zhang PS, Chan SL, Fu WM, et al. TERT suppresses apoptotis at a premitochondrial step by a mechanism requiring reverse transcriptase activity and 14-3-3 protein binding ability[J]. FASEB J, 2003, 17(6):767-769.
doi: 10.1096/fsb2.v17.6 URL |
| [28] |
Dudognon C, Pendino F, Hillion J, et al. Death receptor signaling regulatory function for telomerase:hTERT abolishes TRAIL-induced apoptosis, independently of telomere maintenance[J]. Oncogene, 2004, 23(45):7469-7474.
pmid: 15326479 |
| [29] |
Ahmed S, Passos JF, Birket MJ, et al. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress[J]. J Cell Sci, 2008, 121(Pt 7):1046-1053.
doi: 10.1242/jcs.019372 URL |
| [30] |
Okamoto N, Yasukawa M, Nguyen C, et al. Maintenance of tumor initiating cells of defined genetic composition by nucleostemin[J]. PNAS, 2011, 108(51):20388-20393.
doi: 10.1073/pnas.1015171108 pmid: 21730156 |
| [31] | Gordon DM, Santos JH. The emerging role of telomerase reverse transcriptase in mitochondrial DNA metabolism[J]. J Nucleic Acids, 2010, 2010:1-7. |
| [32] | 张徐俞, 王瑾瑜, 郑广顺, 等. 盐胁迫下沙冬青细胞端粒酶活性的变化与DNA稳定性的关系[J]. 生物技术通报, 2014(10):134-138. |
| Zhang XY, Wang JY, Zheng GS, et al. Effects of salt stress on telomerase activity in relation to DNA stability of Ammopiptanthus mongolicus cells[J]. Biotechnol Bull, 2014(10):134-138. | |
| [33] |
Fojtová M, Fulnecková J, Fajkus J, et al. Recovery of tobacco cells from cadmium stress is accompanied by DNA repair and increased telomerase activity[J]. J Exp Bot, 2002, 53(378):2151-2158.
doi: 10.1093/jxb/erf080 URL |
| [34] |
吴晓飞, 王瑾瑜, 张徐俞, 等. 盐胁迫下胡杨和合作杨悬浮细胞端粒酶与抵御氧化损伤的关系[J]. 生物技术通报, 2017, 33(8):111-119.
doi: 10.13560/j.cnki.biotech.bull.1985.2017-0136 |
| Wu XF, Wang JY, Zhang XY, et al. Telomerase activity in relation to oxidative damage resistance in cells of Populus euphratica and×P. simonii P. pyramibalis cv under salt stress[J]. Biotechnol Bull, 2017, 33(8):111-119. | |
| [35] | 孙丽春, 杨颖, 于婷乔, 等. 沙冬青端粒酶逆转录酶基因(AmTERT)克隆及表达分析[J]. 中国细胞生物学学报, 2018, 40(7):1088-1100. |
| Sun LC, Yang Y, Yu TQ, et al. Cloning and expression analysis of telomerase reverse transcriptase(AmTERT)gene from Ammopiptanthus mongolicus[J]. Chin J Cell Biol, 2018, 40(7):1088-1100. | |
| [36] | 王杨, 孙玉萍, 董琦, 等. 拟南芥AtTERT基因特性分析及其对盐与过氧化氢胁迫的响应[J]. 农业生物技术学报, 2019, 27(4):581-592. |
| Wang Y, Sun YP, Dong Q, et al. Characterization analysis and responses to NaCl and H2O2 stress of AtTERT in Arabidopsis thaliana[J]. J Agric Biotechnol, 2019, 27(4):581-592. |
| [1] | 李宇, 李素贞, 陈茹梅, 卢海强. 植物bHLH转录因子调控铁稳态的研究进展[J]. 生物技术通报, 2023, 39(7): 26-36. |
| [2] | 赵雪婷, 高利燕, 王俊刚, 沈庆庆, 张树珍, 李富生. 甘蔗AP2/ERF转录因子基因ShERF3的克隆、表达及其编码蛋白的定位[J]. 生物技术通报, 2023, 39(6): 208-216. |
| [3] | 李苑虹, 郭昱昊, 曹燕, 祝振洲, 王飞飞. 外源植物激素调控微藻生长及目标产物积累研究进展[J]. 生物技术通报, 2023, 39(6): 61-72. |
| [4] | 冯珊珊, 王璐, 周益, 王幼平, 方玉洁. WOX家族基因调控植物生长发育和非生物胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(5): 1-13. |
| [5] | 李帜奇, 袁月, 苗荣庆, 庞秋颖, 张爱琴. 盐胁迫盐芥和拟南芥褪黑素含量及合成相关基因表达模式分析[J]. 生物技术通报, 2023, 39(5): 142-151. |
| [6] | 翟莹, 李铭杨, 张军, 赵旭, 于海伟, 李珊珊, 赵艳, 张梅娟, 孙天国. 异源表达大豆转录因子GmNF-YA19提高转基因烟草抗旱性[J]. 生物技术通报, 2023, 39(5): 224-232. |
| [7] | 姚姿婷, 曹雪颖, 肖雪, 李瑞芳, 韦小妹, 邹承武, 朱桂宁. 火龙果溃疡病菌实时荧光定量PCR内参基因的筛选[J]. 生物技术通报, 2023, 39(5): 92-102. |
| [8] | 杨春洪, 董璐, 陈林, 宋丽. 大豆VAS1基因家族的鉴定及参与侧根发育的研究[J]. 生物技术通报, 2023, 39(3): 133-142. |
| [9] | 崔吉洁, 蔡文波, 庄庆辉, 高爱平, 黄建峰, 陈亚辉, 宋志忠. 杧果Fe-S簇装配基因MiISU1的生物学功能[J]. 生物技术通报, 2023, 39(2): 139-146. |
| [10] | 黄文莉, 李香香, 周炆婷, 罗莎, 姚维嘉, 马杰, 张芬, 沈钰森, 顾宏辉, 王建升, 孙勃. 利用CRISPR/Cas9技术靶向编辑青花菜BoZDS[J]. 生物技术通报, 2023, 39(2): 80-87. |
| [11] | 苗淑楠, 高宇, 李昕儒, 蔡桂萍, 张飞, 薛金爱, 季春丽, 李润植. 大豆GmPDAT1参与油脂合成和非生物胁迫应答的功能分析[J]. 生物技术通报, 2023, 39(2): 96-106. |
| [12] | 许睿, 祝英方. 中介体复合物在植物非生物胁迫应答中的功能[J]. 生物技术通报, 2023, 39(11): 54-60. |
| [13] | 孙雨桐, 刘德帅, 齐迅, 冯美, 黄栩筝, 姚文孔. 茉莉酸调控植物生长发育和胁迫的研究进展[J]. 生物技术通报, 2023, 39(11): 99-109. |
| [14] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [15] | 鄢梦雨, 韦晓薇, 曹婧, 兰海燕. 异子蓬SabHLH169基因的克隆及抗旱功能分析[J]. 生物技术通报, 2023, 39(11): 328-339. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||