








生物技术通报 ›› 2022, Vol. 38 ›› Issue (9): 215-225.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1507
张开平1( ), 刘燕丽1, 涂绵亮1, 李继伟1, 吴文标2(
), 刘燕丽1, 涂绵亮1, 李继伟1, 吴文标2( )
)
收稿日期:2021-11-28
出版日期:2022-09-26
发布日期:2022-10-11
作者简介:张开平,男,硕士,高级实验师,研究方向:发酵工程;E-mail: 基金资助:
ZHANG Kai-ping1( ), LIU Yan-li1, TU Mian-liang1, LI Ji-wei1, WU Wen-biao2(
), LIU Yan-li1, TU Mian-liang1, LI Ji-wei1, WU Wen-biao2( )
)
Received:2021-11-28
Published:2022-09-26
Online:2022-10-11
摘要:
分离筛选高效降解稻草的菌株,研究菌株产纤维素酶工艺条件及酶学性质。采用刚果红染色法从腐败木质下的土壤中分离筛选到一株产纤维素酶菌株,结合菌株的形态特征和18S rDNA序列同源性比较进行鉴定;通过单因素试验和响应面分析法确定菌株最适产酶条件,并对纤维素酶的稳定性进行研究。分离纯化得到的菌株命名为烟曲霉(Aspergillus fumigatus A-16);响应面实验结果表明,最优产纤维素酶工艺参数为:稻草粉添加量7 g/100 mL,pH 6.0,温度65℃,发酵时间5 d;在此最优条件下,该菌产生的羧甲基纤维素酶(CMCase)和滤纸酶(FPA)活力分别为2 954.76 U/mL和1 086.37 U/mL;其总活力较优化前提高了26.4%。该纤维素酶的适宜反应温度为70℃,适宜pH 6.0。在80℃热处理90 min条件下酶活力可保持在80%以上,说明该酶热稳定性较好。同时,在pH 5.0-7.0范围内比较稳定,放置1 d后可保持70%以上的酶活力。该研究可为利用富含纤维素的生物质原料开发洁净能源及食品级葡萄糖资源提供了基础支撑。
张开平, 刘燕丽, 涂绵亮, 李继伟, 吴文标. 烟曲霉A-16产纤维素酶工艺优化及酶学特性[J]. 生物技术通报, 2022, 38(9): 215-225.
ZHANG Kai-ping, LIU Yan-li, TU Mian-liang, LI Ji-wei, WU Wen-biao. Optimization of Producing Cellulase by Aspergillus fumigatus A-16 and Its Enzymatic Properties[J]. Biotechnology Bulletin, 2022, 38(9): 215-225.
| 菌株编号 Number of strain | D/d值 D/d value | 羧甲基纤维素酶活力 CMCase /(U·mL-1) | 滤纸酶活力 FPA /(U·mL-1) |
|---|---|---|---|
| A-02 | 5.83±0.66 | 926.09±65.43 | 289.41±23.28 |
| A-07 | 5.65±0.71 | 757.26±17.55 | 116.52±16.65 |
| A-10 | 7.12±0.48 | 1487.39±85.23 | 418.14±43.12 |
| A-11 | 6.64±0.50 | 1004.58±36.52 | 396.41±40.33 |
| A-16 | 9.87±0.42 | 2516.24±117.41 | 686.48±43.20 |
| A-19 | 8.15±0.17 | 1986.61±123.28 | 768.87±27.40 |
| A-22 | 8.41±0.24 | 2387.40±67.20 | 718.41±33.11 |
| A-26 | 7.57±0.30 | 2060.24±25.64 | 671.92±40.29 |
表1 产纤维素酶菌株酶活性
Table 1 Enzymatic activity of the cellulase-producing strains
| 菌株编号 Number of strain | D/d值 D/d value | 羧甲基纤维素酶活力 CMCase /(U·mL-1) | 滤纸酶活力 FPA /(U·mL-1) |
|---|---|---|---|
| A-02 | 5.83±0.66 | 926.09±65.43 | 289.41±23.28 |
| A-07 | 5.65±0.71 | 757.26±17.55 | 116.52±16.65 |
| A-10 | 7.12±0.48 | 1487.39±85.23 | 418.14±43.12 |
| A-11 | 6.64±0.50 | 1004.58±36.52 | 396.41±40.33 |
| A-16 | 9.87±0.42 | 2516.24±117.41 | 686.48±43.20 |
| A-19 | 8.15±0.17 | 1986.61±123.28 | 768.87±27.40 |
| A-22 | 8.41±0.24 | 2387.40±67.20 | 718.41±33.11 |
| A-26 | 7.57±0.30 | 2060.24±25.64 | 671.92±40.29 |
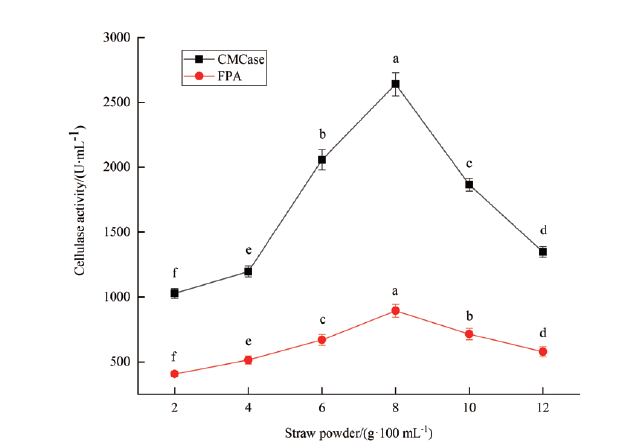
图3 稻草粉添加量对A. fumigatus A-16菌株纤维素酶活力的影响 不同小写字母表示差异显著(P<0.05);下同
Fig.3 Effects of straw powder on the cellulase activity of strain A. fumigatus A-16 Different lowercase letters indicate significant differences(P<0.05). The same below

图7 响应曲面图 A:稻草粉添加量和pH交互作用;B:稻草粉添加量和发酵温度交互作用;C:稻草粉添加量和发酵时间交互作用;D:pH和发酵温度交互作用;E:pH和发酵时间交互作用;F:发酵温度和发酵时间交互作用
Fig.7 Response surface diagram A:Interaction of straw powder and initial pH. B:Interaction of straw powder and fermentation temperature. C:Interaction of straw powder and fermentation time. D:Interaction of pH and fermentation temperature. E:Interaction of pH and fermentation time. F:Interaction of fermentation temperature and fermentation time
| 试验号 Test No. | 稻草粉添加量 Straw powder/ (g·100 mL-1) | pH | 发酵温度 Fermentation temperature/℃ | 发酵时间 Fermentation time/d | 羧甲基纤维素 酶活力 CMCase / (U·mL-1) |
|---|---|---|---|---|---|
| 1 | 10 | 6.0 | 75 | 5 | 2 063.71±3.15 |
| 2 | 6 | 5.0 | 65 | 5 | 2 531.72±4.86 |
| 3 | 6 | 6.0 | 65 | 6 | 2 657.27±5.22 |
| 4 | 6 | 7.0 | 65 | 5 | 2 513.37±1.82 |
| 5 | 8 | 7.0 | 65 | 6 | 2 086.14±5.44 |
| 6 | 8 | 5.0 | 65 | 6 | 2 447.22±2.18 |
| 7 | 10 | 5.0 | 65 | 5 | 2 073.50±2.97 |
| 8 | 8 | 7.0 | 65 | 4 | 2 413.23±4.92 |
| 9 | 8 | 6.0 | 65 | 5 | 2 888.50±2.83 |
| 10 | 6 | 6.0 | 65 | 4 | 2 481.15±2.24 |
| 11 | 8 | 6.0 | 55 | 6 | 2 370.83±3.01 |
| 12 | 8 | 6.0 | 65 | 5 | 2 954.07±2.16 |
| 13 | 10 | 7.0 | 65 | 5 | 2 018.95±3.68 |
| 14 | 8 | 7.0 | 55 | 5 | 2 004.27±6.20 |
| 15 | 8 | 5.0 | 55 | 5 | 2 274.48±4.97 |
| 16 | 10 | 6.0 | 65 | 6 | 2 168.28±2.94 |
| 17 | 10 | 6.0 | 55 | 5 | 2 075.56±4.99 |
| 18 | 8 | 7.0 | 75 | 5 | 2 635.90±3.13 |
| 19 | 8 | 6.0 | 65 | 5 | 2 951.56±3.51 |
| 20 | 10 | 6.0 | 65 | 4 | 2 217.78±4.23 |
| 21 | 6 | 6.0 | 75 | 5 | 2 406.44±7.46 |
| 22 | 8 | 6.0 | 65 | 5 | 2 898.67±4.35 |
| 23 | 8 | 5.0 | 65 | 4 | 2 536.43±3.79 |
| 24 | 6 | 6.0 | 55 | 5 | 2 618.73±4.34 |
| 25 | 8 | 6.0 | 75 | 6 | 2 267.33±5.75 |
| 26 | 8 | 5.0 | 75 | 5 | 2 288.70±5.14 |
| 27 | 8 | 6.0 | 75 | 4 | 2 298.64±2.95 |
| 28 | 8 | 6.0 | 65 | 5 | 2 820.48±3.77 |
| 29 | 8 | 6.0 | 55 | 4 | 2 258.87±3.49 |
表2 响应面设计及试验结果
Table 2 Response surface design and test results
| 试验号 Test No. | 稻草粉添加量 Straw powder/ (g·100 mL-1) | pH | 发酵温度 Fermentation temperature/℃ | 发酵时间 Fermentation time/d | 羧甲基纤维素 酶活力 CMCase / (U·mL-1) |
|---|---|---|---|---|---|
| 1 | 10 | 6.0 | 75 | 5 | 2 063.71±3.15 |
| 2 | 6 | 5.0 | 65 | 5 | 2 531.72±4.86 |
| 3 | 6 | 6.0 | 65 | 6 | 2 657.27±5.22 |
| 4 | 6 | 7.0 | 65 | 5 | 2 513.37±1.82 |
| 5 | 8 | 7.0 | 65 | 6 | 2 086.14±5.44 |
| 6 | 8 | 5.0 | 65 | 6 | 2 447.22±2.18 |
| 7 | 10 | 5.0 | 65 | 5 | 2 073.50±2.97 |
| 8 | 8 | 7.0 | 65 | 4 | 2 413.23±4.92 |
| 9 | 8 | 6.0 | 65 | 5 | 2 888.50±2.83 |
| 10 | 6 | 6.0 | 65 | 4 | 2 481.15±2.24 |
| 11 | 8 | 6.0 | 55 | 6 | 2 370.83±3.01 |
| 12 | 8 | 6.0 | 65 | 5 | 2 954.07±2.16 |
| 13 | 10 | 7.0 | 65 | 5 | 2 018.95±3.68 |
| 14 | 8 | 7.0 | 55 | 5 | 2 004.27±6.20 |
| 15 | 8 | 5.0 | 55 | 5 | 2 274.48±4.97 |
| 16 | 10 | 6.0 | 65 | 6 | 2 168.28±2.94 |
| 17 | 10 | 6.0 | 55 | 5 | 2 075.56±4.99 |
| 18 | 8 | 7.0 | 75 | 5 | 2 635.90±3.13 |
| 19 | 8 | 6.0 | 65 | 5 | 2 951.56±3.51 |
| 20 | 10 | 6.0 | 65 | 4 | 2 217.78±4.23 |
| 21 | 6 | 6.0 | 75 | 5 | 2 406.44±7.46 |
| 22 | 8 | 6.0 | 65 | 5 | 2 898.67±4.35 |
| 23 | 8 | 5.0 | 65 | 4 | 2 536.43±3.79 |
| 24 | 6 | 6.0 | 55 | 5 | 2 618.73±4.34 |
| 25 | 8 | 6.0 | 75 | 6 | 2 267.33±5.75 |
| 26 | 8 | 5.0 | 75 | 5 | 2 288.70±5.14 |
| 27 | 8 | 6.0 | 75 | 4 | 2 298.64±2.95 |
| 28 | 8 | 6.0 | 65 | 5 | 2 820.48±3.77 |
| 29 | 8 | 6.0 | 55 | 4 | 2 258.87±3.49 |
| 方差来源 Source | 总偏差平方和 Sum of squares | 自由度 Degree of freedom | 均方 Mean square | F | P | 显著性 Significance |
|---|---|---|---|---|---|---|
| Model | 2.224E+006 | 14 | 1.589E+005 | 9.61 | <0.0001 | ** |
| A | 5.593E+005 | 1 | 5.593E+005 | 33.84 | <0.0001 | ** |
| B | 19200.00 | 1 | 19200.00 | 1.16 | 0.2994 | |
| C | 10680.33 | 1 | 10680.33 | 0.65 | 0.4349 | |
| D | 3640.08 | 1 | 3640.08 | 0.22 | 0.6461 | |
| AB | 334.89 | 1 | 3640.08 | 0.02 | 0.8888 | |
| AC | 10040.04 | 1 | 10040.04 | 0.61 | 0.4487 | |
| AD | 12746.41 | 1 | 12746.41 | 0.77 | 0.3947 | |
| BC | 95326.56 | 1 | 95326.56 | 5.77 | 0.0308 | * |
| BD | 14149.10 | 1 | 14149.10 | 0.31 | 0.5861 | |
| CD | 5133.72 | 1 | 5133.72 | 0.86 | 0.3705 | |
| A2 | 5.886E+005 | 1 | 5.886E+005 | 35.61 | < 0.0001 | ** |
| B2 | 5.899E+005 | 1 | 5.899E+005 | 35.69 | < 0.0001 | ** |
| C2 | 7.240E+005 | 1 | 7.240E+005 | 43.80 | < 0.0001 | ** |
| D2 | 4.190E+005 | 1 | 4.190E+005 | 25.35 | 0.0002 | ** |
| 残差 | 2.314E+005 | 14 | 16529.14 | |||
| 失拟项 | 2.108E+005 | 10 | 21083.15 | 4.10 | 0.0932 | |
| 纯误差 | 20576.45 | 4 | 5144.11 | |||
| 所有项 | 2.455E+006 | 28 |
表3 响应面模型及回归方程的方差分析
Table 3 Analysis of variance of the response surface model and the regression equations
| 方差来源 Source | 总偏差平方和 Sum of squares | 自由度 Degree of freedom | 均方 Mean square | F | P | 显著性 Significance |
|---|---|---|---|---|---|---|
| Model | 2.224E+006 | 14 | 1.589E+005 | 9.61 | <0.0001 | ** |
| A | 5.593E+005 | 1 | 5.593E+005 | 33.84 | <0.0001 | ** |
| B | 19200.00 | 1 | 19200.00 | 1.16 | 0.2994 | |
| C | 10680.33 | 1 | 10680.33 | 0.65 | 0.4349 | |
| D | 3640.08 | 1 | 3640.08 | 0.22 | 0.6461 | |
| AB | 334.89 | 1 | 3640.08 | 0.02 | 0.8888 | |
| AC | 10040.04 | 1 | 10040.04 | 0.61 | 0.4487 | |
| AD | 12746.41 | 1 | 12746.41 | 0.77 | 0.3947 | |
| BC | 95326.56 | 1 | 95326.56 | 5.77 | 0.0308 | * |
| BD | 14149.10 | 1 | 14149.10 | 0.31 | 0.5861 | |
| CD | 5133.72 | 1 | 5133.72 | 0.86 | 0.3705 | |
| A2 | 5.886E+005 | 1 | 5.886E+005 | 35.61 | < 0.0001 | ** |
| B2 | 5.899E+005 | 1 | 5.899E+005 | 35.69 | < 0.0001 | ** |
| C2 | 7.240E+005 | 1 | 7.240E+005 | 43.80 | < 0.0001 | ** |
| D2 | 4.190E+005 | 1 | 4.190E+005 | 25.35 | 0.0002 | ** |
| 残差 | 2.314E+005 | 14 | 16529.14 | |||
| 失拟项 | 2.108E+005 | 10 | 21083.15 | 4.10 | 0.0932 | |
| 纯误差 | 20576.45 | 4 | 5144.11 | |||
| 所有项 | 2.455E+006 | 28 |

图8 菌株A-16产纤维素酶作用的最适温度及其热稳定性 A:CMCase和FPA酶作用最适反应温度;B:CMCase和FPA酶的热稳定性
Fig.8 Optimal temperature of cellulase activity of strain A-16 and and its thermo-stabilities A:Optimal temperature for CMCase and FPA enzymatic activity. B:Thermo-stabilities of the CMCase and FPA
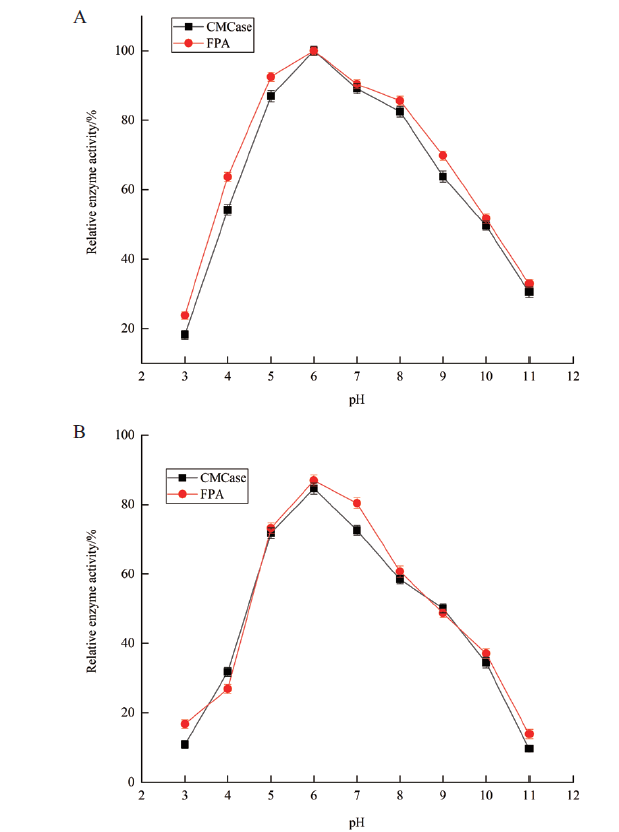
图9 菌株A-16产纤维素酶作用的最适pH及其pH稳定性 A:CMCase和FPA酶作用最适反应pH;B:CMCase和FPA酶的pH稳定性
Fig. 9 Optimal pH of cellulase activity of strain A-16 and its pH stabilities A:Optimal pH for CMCase and FPA enzymatic activity. B:pH stabilities of the CMCase and FPA
| [1] |
Souii A, Guesmi A, Ouertani R, et al. Carboxymethyl cellulase production by extremotolerant bacteria in low-cost media and application in enzymatic saccharification of Stevia biomass[J]. Waste Biomass Valorization, 2020, 11(5):2111-2122.
doi: 10.1007/s12649-018-0496-2 URL |
| [2] |
Mehboob N, Asad MJ, Asgher M, et al. Exploring thermophilic cellulolytic enzyme production potential of Aspergillus fumigatus by the solid-state fermentation of wheat straw[J]. Appl Biochem Biotechnol, 2014, 172(7):3646-3655.
doi: 10.1007/s12010-014-0796-3 pmid: 24562980 |
| [3] |
Sohail M, Ahmad A, Khan SA. Production of cellulase from Aspergillus terreus MS105 on crude and commercially purified substrates[J]. 3 Biotech, 2016, 6(1):103.
doi: 10.1007/s13205-016-0420-z URL |
| [4] |
Mahmood RT, Asad MJ, Mehboob N, et al. Production, purification, and characterization of exoglucanase by Aspergillus fumigatus[J]. Appl Biochem Biotechnol, 2013, 170(4):895-908.
doi: 10.1007/s12010-013-0227-x pmid: 23615734 |
| [5] |
Sharma HK, Xu CC, Qin WS. Co-culturing of novel Bacillus species isolated from municipal sludge and gut of red wiggler worm for improving CMCase activity[J]. Waste Biomass Valorization, 2020, 11(5):2047-2058.
doi: 10.1007/s12649-018-0448-x URL |
| [6] |
Zanirun Z, Bahrin EK, Lai-Yee P, et al. Effect of physical and chemical properties of oil palm empty fruit bunch, decanter cake and sago pith residue on cellulases production by Trichoderma asperellum UPM1 and Aspergillus fumigatus UPM2[J]. Appl Biochem Biotechnol, 2014, 172(1):423-435.
doi: 10.1007/s12010-013-0530-6 URL |
| [7] |
Horn SJ, Vaaje-Kolstad G, Westereng B, et al. Novel enzymes for the degradation of cellulose[J]. Biotechnol Biofuels, 2012, 5(1):45.
doi: 10.1186/1754-6834-5-45 pmid: 22747961 |
| [8] |
Zhang K, Pei ZJ, Wang DH. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals:a review[J]. Bioresour Technol, 2016, 199:21-33.
doi: 10.1016/j.biortech.2015.08.102 URL |
| [9] |
Wang D, Sun J, Yu HL, et al. Maximum saccharification of cellulose complex by an enzyme cocktail supplemented with cellulase from newly isolated Aspergillus fumigatus ECU0811[J]. Appl Biochem Biotechnol, 2012, 166(1):176-186.
doi: 10.1007/s12010-011-9414-9 pmid: 22086563 |
| [10] | 李乐, 李明星, 汤国雄, 等. 一株纤维素酶产生菌的筛选与产酶特性研究[J]. 环境科技, 2019, 32(1):24-29. |
| Li L, Li MX, Tang GX, et al. Optimization of enzyme production conditions for a cellulase-producing strain[J]. Environ Sci Technol, 2019, 32(1):24-29. | |
| [11] |
Miao JX, Wang MM, Ma L, et al. Effects of amino acids on the lignocellulose degradation by Aspergillus fumigatus Z5:insights into performance, transcriptional, and proteomic profiles[J]. Biotechnol Biofuels, 2019, 12:4.
doi: 10.1186/s13068-018-1350-2 URL |
| [12] |
Srivastava N, Rawat R, Sharma R, et al. Effect of nickel-cobaltite nanoparticles on production and thermostability of cellulases from newly isolated thermotolerant Aspergillus fumigatus NS(class:Eurotiomycetes)[J]. Appl Biochem Biotechnol, 2014, 174(3):1092-1103.
doi: 10.1007/s12010-014-0940-0 pmid: 24801407 |
| [13] |
Islam F, Roy N. Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses[J]. BMC Res Notes, 2018, 11(1):445.
doi: 10.1186/s13104-018-3558-4 pmid: 29973263 |
| [14] |
El-Ghonemy DH, Ali TH, El-Bondkly AM, et al. Improvement of Aspergillus oryzae NRRL 3484 by mutagenesis and optimization of culture conditions in solid-state fermentation for the hyper-production of extracellular cellulase[J]. Antonie Van Leeuwenhoek, 2014, 106(5):853-864.
doi: 10.1007/s10482-014-0255-8 pmid: 25119245 |
| [15] |
Grigorevski-Lima AL, da Vinha FNM, Souza DT, et al. Aspergillus fumigatus thermophilic and acidophilic endoglucanases[J]. Appl Biochem Biotechnol, 2009, 155(1-3):321-329.
doi: 10.1007/s12010-008-8482-y pmid: 19127443 |
| [16] |
Das A, Paul T, Ghosh P, et al. Kinetic study of a glucose tolerant β-glucosidase from Aspergillus fumigatus ABK9 entrapped into alginate beads[J]. Waste Biomass Valorization, 2015, 6(1):53-61.
doi: 10.1007/s12649-014-9329-0 URL |
| [17] |
Sharma M, Soni R, Nazir A, et al. Evaluation of glycosyl hydrolases in the secretome of Aspergillus fumigatus and saccharification of alkali-treated rice straw[J]. Appl Biochem Biotechnol, 2011, 163(5):577-591.
doi: 10.1007/s12010-010-9064-3 pmid: 20730507 |
| [18] | 于岚, 程芳, 邵文琦, 等. 一株嗜热纤维素酶生产菌的分离、鉴定及酶学研究[J]. 安徽农业科学, 2013, 41(17):7413-7417. |
| Yu L, Cheng F, Shao WQ, et al. Study on isolation, identification and characterization of a thermophilic cellulase-producing strain[J]. J Anhui Agric Sci, 2013, 41(17):7413-7417. | |
| [19] |
Lin CY, Shen ZC, Qin WS. Characterization of xylanase and cellulase produced by a newly isolated Aspergillus fumigatus N2 and its efficient saccharification of barley straw[J]. Appl Biochem Biotechnol, 2017, 182(2):559-569.
doi: 10.1007/s12010-016-2344-9 URL |
| [20] |
Saroj P, Manasa P, Narasimhulu K. Characterization of thermophilic fungi producing extracellular lignocellulolytic enzymes for lignocellulosic hydrolysis under solid-state fermentation[J]. Bioresour Bioprocess, 2018, 5:31.
doi: 10.1186/s40643-018-0216-6 URL |
| [21] | 谢洁, 商必志, 任慧爽, 等. 一株纤维素酶产生菌B. cereus JYMB2菌株的筛选鉴定[J]. 西南大学学报:自然科学版, 2016, 38(5):45-51. |
| Xie J, Shang BZ, Ren HS, et al. Screening and identification of a cellulase-producing strain of Bacillus cereus-JYMB2[J]. J Southwest Univ Nat Sci Ed, 2016, 38(5):45-51. | |
| [22] | 马振刚, 熊亮, 张真, 等. 高产碱性纤维素酶细菌的筛选鉴定及其酶学特性与发酵条件研究[J]. 南方农业学报, 2021, 52(3):722-731. |
| Ma ZG, Xiong L, Zhang Z, et al. Screening and identification of a strain with high yield of alkaline cellulase and its enzyme characterizations and fermentation conditions[J]. J South Agric, 2021, 52(3):722-731. | |
| [23] | 魏姣, 万学瑞, 吴润, 等. 产纤维素酶真菌菌株的分离筛选及产酶条件优化[J]. 甘肃农业大学学报, 2016, 51(2):8-15. |
| Wei J, Wan XR, Wu R, et al. Isolation and screening of fungi strains producing cellulase and optimization of conditions for enzyme production[J]. J Gansu Agric Univ, 2016, 51(2):8-15. | |
| [24] | 何楠, 令利军, 冯蕾, 等. 1株产纤维素酶细菌的筛选、鉴定及生长特性[J]. 微生物学杂志, 2017, 37(1):43-49. |
| He N, Ling LJ, Feng L, et al. Isolation, identification and growth characteristics of a cellulase-producing bacterium[J]. J Microbiol, 2017, 37(1):43-49. | |
| [25] | 李婷, 王玥, 刘中珊, 等. 一株降解纤维素的低温放线菌Streptomyces azureus及产酶条件优化[J]. 中国农学通报, 2021, 37(32):25-33. |
| Li T, Wang Y, Liu ZS, et al. A novel low temperature cellulose-degrading strain Streptomyces azureus and its enzymatic production condition optimization[J]. Chin Agric Sci Bull, 2021, 37(32):25-33. | |
| [26] | 赵龙妹, 陈林, 杜东晓, 等. 产纤维素酶细菌的筛选鉴定与特性分析[J]. 中国农学通报, 2021, 37(30):83-88. |
| Zhao LM, Chen L, Du DX, et al. Screening, identification and characteristic analysis of cellulase-producing bacteria[J]. Chin Agric Sci Bull, 2021, 37(30):83-88. | |
| [27] | 蒋芳, 刘松青, 甄阳光, 等. 一株产高温纤维素酶菌株的分离筛选[J]. 纤维素科学与技术, 2015, 23(2):50-54. |
| Jiang F, Liu SQ, Zhen YG, et al. Isolation and screening of a thermophilic cellulose bacterial strain[J]. J Cellul Sci Technol, 2015, 23(2):50-54. | |
| [28] | 金伟, 陈文静, 缪礼鸿, 等. 1株耐热纤维素酶产生菌的筛选及其酶学特性[J]. 江苏农业科学, 2017, 45(20):272-274. |
| Jin W, Chen WJ, Miao LH, et al. Screening of a heat-resistant cellulase-producing strain and enzymatic specificity[J]. Jiangsu Agric Sci, 2017, 45(20):272-274. |
| [1] | 饶紫环, 谢志雄. 一株Olivibacter jilunii 纤维素降解菌株的分离鉴定与降解能力分析[J]. 生物技术通报, 2023, 39(8): 283-290. |
| [2] | 张晶, 张浩睿, 曹云, 黄红英, 曲萍, 张志萍. 嗜热纤维素降解菌研究进展[J]. 生物技术通报, 2023, 39(6): 73-87. |
| [3] | 马玉倩, 孙东辉, 岳浩峰, 辛佳瑜, 刘宁, 曹志艳. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135. |
| [4] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [5] | 杨俊钊, 张新蕊, 孙清扬, 郑菲. Loop B3对GH7内切纤维素酶功能的影响机制[J]. 生物技术通报, 2023, 39(10): 281-291. |
| [6] | 付巧, 林啟兰, 薛强, 熊海容, 王亚伟. N端截短CBM41对枯草芽孢杆菌来源普鲁兰酶酶学性质的影响[J]. 生物技术通报, 2022, 38(6): 245-251. |
| [7] | 王新光, 田磊, 王恩泽, 钟成, 田春杰. 玉米秸秆高效降解微生物复合菌系的构建及降解效果评价[J]. 生物技术通报, 2022, 38(4): 217-229. |
| [8] | 张功友, 王一涵, 郭敏, 张婷婷, 王兵, 刘红美. 重楼中一株产纤维素酶内生真菌的分离及鉴定[J]. 生物技术通报, 2022, 38(2): 95-104. |
| [9] | 唐昊, 孙灿, 李沅秋, 罗朝兵. 纤维素降解菌Raoultella ornithinolytica LL1的筛选及基因组测序[J]. 生物技术通报, 2021, 37(6): 85-96. |
| [10] | 陶治东, 何艳慧, 邓子禾, 孙琳琳, 武占省. 香菇菌渣高效纤维素降解菌的筛选及产酶优化[J]. 生物技术通报, 2021, 37(11): 158-165. |
| [11] | 胡芳, 董旭, 史长伟, 吴学栋. 超声波强化木质纤维素酶解的研究进展[J]. 生物技术通报, 2021, 37(10): 234-244. |
| [12] | 刘登, 刘均洪. 嗜热性木质纤维素酶在纤维素乙醇生产中的研究进展[J]. 生物技术通报, 2020, 36(8): 185-193. |
| [13] | 冯光志, 石慧, 刘博, 吴玉婷, 王月琳, 石玉. 小龙虾肠道产纤维素酶细菌的分离与鉴定[J]. 生物技术通报, 2020, 36(2): 65-70. |
| [14] | 杨彬, 李小波, 周林, 区佩渝, 金小宝. 同步分泌高效纤维素酶和木聚糖酶菌株YB的鉴定及其酶学性质研究[J]. 生物技术通报, 2020, 36(2): 110-118. |
| [15] | 张家顺, 高丽莉, 马江山, 刘高强. 表面活性剂对纤维素酶解的影响及机理[J]. 生物技术通报, 2019, 35(9): 11-20. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||