








生物技术通报 ›› 2022, Vol. 38 ›› Issue (10): 273-280.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1564
王海杰( ), 王成稷, 郭洋, 王云, 陈艳娟, 梁敏, 王珏, 龚慧, 沈如凌(
), 王成稷, 郭洋, 王云, 陈艳娟, 梁敏, 王珏, 龚慧, 沈如凌( )
)
收稿日期:2022-02-21
出版日期:2022-10-26
发布日期:2022-11-11
作者简介:王海杰,男,实验师,研究方向:模式生物表型;E-mail:
WANG Hai-jie( ), WANG Cheng-ji, GUO Yang, WANG Yun, CHEN Yan-juan, LIANG Min, WANG Jue, GONG Hui, SHEN Ru-ling(
), WANG Cheng-ji, GUO Yang, WANG Yun, CHEN Yan-juan, LIANG Min, WANG Jue, GONG Hui, SHEN Ru-ling( )
)
Received:2022-02-21
Published:2022-10-26
Online:2022-11-11
摘要:
基于CRISPR/Cas9技术构建凝血因子8(F8)基因敲除小鼠模型并对其表型进行初步验证。针对F8基因外显子1非编码区和外显子26下游序列设计sgRNA靶位点,体外转录获得sgRNA,与编码Cas9的mRNA混合后通过受精卵显微注射方法获得F0代阳性敲除小鼠,通过繁育及基因型鉴定获得F8基因敲除纯合子小鼠(F8 -/-小鼠);通过逆转录PCR(RT-PCR)检测主要组织中F8基因在mRNA水平的表达情况,通过酶联免疫吸附测定(ELISA)检测血浆中F8基因在蛋白水平的表达情况;通过活化部分凝血活酶时间检测(APTT)和纤维蛋白原含量(FIB)测定实验检测F8基因敲除后小鼠凝血功能情况。PCR及测序结果表明F8基因在小鼠基因组中被成功敲除;RT-PCR 和ELISA检测结果显示,F8 -/-小鼠中F8在mRNA和蛋白水平上表达均显著低于野生型小鼠(WT小鼠)(P<0.000 1,P<0.01);APTT、FIB和滴血实验检测结果显示,F8 -/-小鼠血浆凝固时间显著高于WT小鼠(P<0.05),F8 -/-小鼠存在严重凝血障碍表型,给予人凝血因子Ⅷ后凝血可恢复到正常水平。利用 CRISPR/Cas9技术成功构建F8基因敲除小鼠模型并初步验证其表型,证实该小鼠F8基因的缺失可以导致凝血功能障碍。
王海杰, 王成稷, 郭洋, 王云, 陈艳娟, 梁敏, 王珏, 龚慧, 沈如凌. 基于CRSIPR/Cas9技术构建凝血因子8基因敲除小鼠模型及表型验证[J]. 生物技术通报, 2022, 38(10): 273-280.
WANG Hai-jie, WANG Cheng-ji, GUO Yang, WANG Yun, CHEN Yan-juan, LIANG Min, WANG Jue, GONG Hui, SHEN Ru-ling. Construction of Coagulation Factor 8 Gene Knockout Mouse Model Based on CRSIPR/Cas9 Technique and Verification of Phenotype[J]. Biotechnology Bulletin, 2022, 38(10): 273-280.
| sgRNA靶序列 sgRNA target sequence | 序列信息 Sequence information(5'-3') |
|---|---|
| sgRNA 1 | AGGCTTAACCCATTTCCTGC TGG |
| sgRNA 2 | TCTGGATCCACAGATATAGC AGG |
表1 sgRNA序列信息
Table 1 sgRNA sequence information
| sgRNA靶序列 sgRNA target sequence | 序列信息 Sequence information(5'-3') |
|---|---|
| sgRNA 1 | AGGCTTAACCCATTTCCTGC TGG |
| sgRNA 2 | TCTGGATCCACAGATATAGC AGG |
| 寡核苷酸OligoDNA | 序列信息Sequence information(5'-3') |
|---|---|
| sgRNA 1 正义链 | ACTTATTAGTTTGCAAAACG |
| sgRNA 1 反义链 | GTGGCCTTCGATTAGGCGGA |
| sgRNA 2 正义链 | CGGTCAGAGCTGCACATACA |
| sgRNA 2 反义链 | GCCAAATTGTGATCTCAGTT |
表2 寡核苷酸链序列信息
Table 2 Oligonucleotide chain sequence information
| 寡核苷酸OligoDNA | 序列信息Sequence information(5'-3') |
|---|---|
| sgRNA 1 正义链 | ACTTATTAGTTTGCAAAACG |
| sgRNA 1 反义链 | GTGGCCTTCGATTAGGCGGA |
| sgRNA 2 正义链 | CGGTCAGAGCTGCACATACA |
| sgRNA 2 反义链 | GCCAAATTGTGATCTCAGTT |
| 引物Primer | 序列信息Sequence information(5'-3') |
|---|---|
| P1 | TTTTGGCTTTCTAACAGGTATCG |
| P2 | CCAGAAAAGGGCATCAGGT |
表3 引物序列信息
Table 3 Primer sequence information
| 引物Primer | 序列信息Sequence information(5'-3') |
|---|---|
| P1 | TTTTGGCTTTCTAACAGGTATCG |
| P2 | CCAGAAAAGGGCATCAGGT |
| 基因 Gene | 上游引物信息Upstream primer information(5'-3') | 下游引物信息Downstream primer information(5'-3') |
|---|---|---|
| F8 | AGGCCTCATTGGAGCTCTGCTA | CCATTGGGATTCCTCAGGGCA |
| β-actin | CGTTGACATCCGTAAAGACC | AACAGTCCGCCTAGAAGCAC |
表4 逆转录PCR引物序列
Table 4 Reverse transcription PCR primer sequences
| 基因 Gene | 上游引物信息Upstream primer information(5'-3') | 下游引物信息Downstream primer information(5'-3') |
|---|---|---|
| F8 | AGGCCTCATTGGAGCTCTGCTA | CCATTGGGATTCCTCAGGGCA |
| β-actin | CGTTGACATCCGTAAAGACC | AACAGTCCGCCTAGAAGCAC |
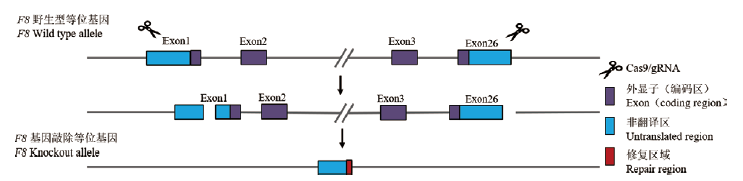
图1 F8 -/-小鼠基因敲除策略 Cas9/sgRNA复合体通过识别外显子1-26(exon1-26)区域靶点将F8基因相应片段切除
Fig.1 Knockout strategy of F8 -/- mice The Cas9/sgRNA complex removed the corresponding fragments of the F8 gene by recognizing targets in the exon1-26 region
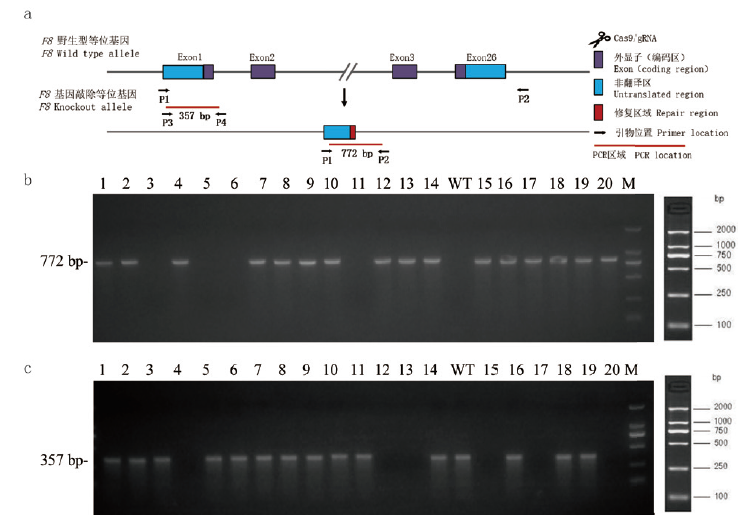
图2 F8-/-基因敲除小鼠基因型鉴定结果 (a)F8-/-基因敲除小鼠的鉴定策略,通过两对引物P1/P2和P3/P4进行PCR鉴定,确定小鼠基因型,引物位置图;(b)和(c)为基因型鉴定电泳图,(b)中利用P1/P2引物对进行PCR检测,WT小鼠不能获得条带,F8-/+杂合子小鼠与F8-/-纯合子小鼠获得单一772bp片段,(c)中P3/P4引物对进行PCR检测,WT小鼠与F8-/+杂合子小鼠可获得357bp片段,F8-/-纯合子小鼠不能获得条带。经电泳鉴定,(b)和(c)中WT小鼠编号为3,5,6,11,F8-/+杂合子小鼠编号为1,2,7,8,9,10,14,16,18,19,F8-/-纯合子小鼠编号为4,12,13,15,17,20。WT:野生型;M:1kb DNA maker
Fig.2 Genotype results of F8-/- gene knockout mice (a)The identification strategy of F8-/- knockout mice. Two pairs of primers P1/P2 and P3/P4 were used for PCR identification to determine the mice genotype. The primer positions were shown in the figure.(b)and(c)were genotype identification electrophoresis diagram,with P1/P2 primer pair for PCR detection in(b),bands were obtained in WT mice,F8-/+ heterozygous mice and F8-/- homozygous mice obtained a single 772 bp fragment.(c)The P3/P4 primer pair was used for the PCR detection,WT mice and F8-/+ heterozygous mice could obtain a 357 bp fragment,F8-/- homozygous mice could not obtain a band. According to electrophoresis identification,WT mice in(b)and(c)were numbered 3,5,6,11,and F8-/+ heterozygous mice were numbered 1,2,7,8,9,10,14,16,18,19,F8-/- homozygous mice were numbered 4,12,13,15,17,20. WT:Wild type mice. M:1 kb DNA maker
| 类型Index | 序列信息Sequence information(5'-3') |
|---|---|
| 野生型小鼠 WT mice | AGGATTCAAACTTGTTAGGATGCACCCAGCAGGA- AATGGGTTAAGCCTTAGCTCAGCCACTCTTCCTA- TTCCAGTT……GAGAAGTTGCTGAGAGTTCTATA- TCTGGATCCACAGATATAGCAGGAAGAGAAAGA- CACTGGGACTGACTTGGG |
| F8-/-小鼠序列 F8-/-mice | AGGATTCAAACTTGTTAGGATGCACCCAGCAGGAA …(207293 bp缺失)…GAGAAAGACACTGGGAC- TGACTTGGG |
表5 WT小鼠和F8-/-敲除小鼠的序列信息
Table 5 Sequence information of wild type and F8-/-mice genotype
| 类型Index | 序列信息Sequence information(5'-3') |
|---|---|
| 野生型小鼠 WT mice | AGGATTCAAACTTGTTAGGATGCACCCAGCAGGA- AATGGGTTAAGCCTTAGCTCAGCCACTCTTCCTA- TTCCAGTT……GAGAAGTTGCTGAGAGTTCTATA- TCTGGATCCACAGATATAGCAGGAAGAGAAAGA- CACTGGGACTGACTTGGG |
| F8-/-小鼠序列 F8-/-mice | AGGATTCAAACTTGTTAGGATGCACCCAGCAGGAA …(207293 bp缺失)…GAGAAAGACACTGGGAC- TGACTTGGG |
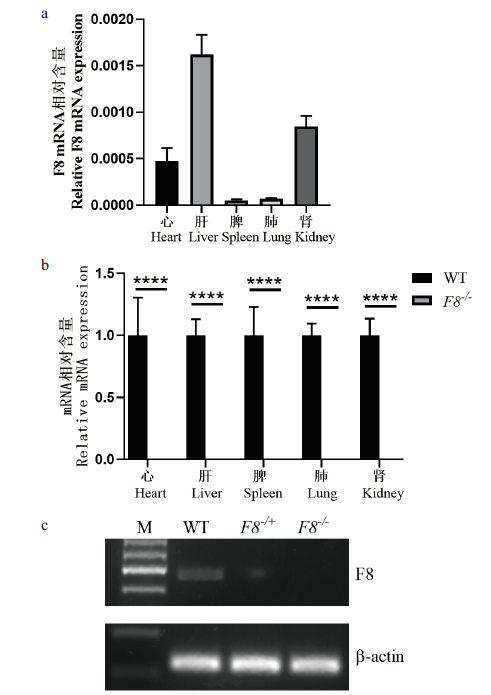
图3 F8 -/-小鼠F8 mRNA 相对表达水平 (a)F8基因在WT小鼠各个主要组织中的表达情况;(b)F8 -/-和WT小鼠心、肝、脾、肺和肾组织中F8 mRNA相对表达水平检测,每组3 只小鼠(****P <0.0001);(c)RT-PCR代表性电泳鉴定结果(肝组织);WT-野生型,F8 -/+-杂合子,F8 -/--纯合子
Fig.3 Analysis of relative mRNA expression in F8-/- mice (a)F8 gene expression in multiple tissues of WT mice.(b)F8 mRNA relative expression level detection in heart,liver,spleen,lung and kidney tissues of F8 -/- and WT mice,3 mice per group(****P <0.0001).(c)RT-PCR representative identification result of electrophoresis(liver tissue). WT:Wild type mice,F8 -/+-heterozygous,F8 -/--homozygous mice
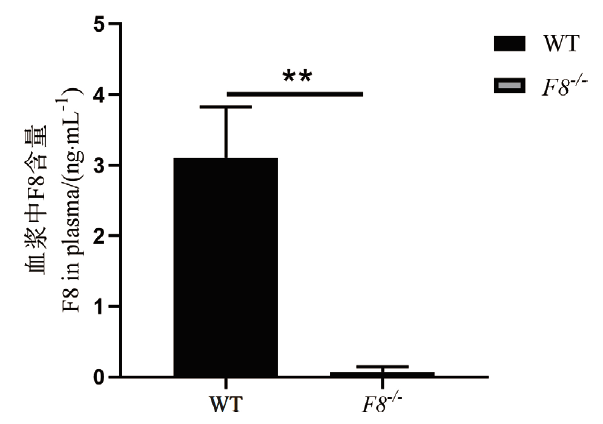
图4 F8-/-小鼠F8蛋白相对表达水平 F8 -/-小鼠和WT小鼠血浆中F8蛋白的含量结果,每组5 只小鼠(**P <0.01),WT-野生型,F8 -/--纯合子
Fig.4 Relative expression of F8 protein in F8-/- mice F8 -/- protein content in plasma of F8-/- and WT mice,5 mice per group(**P <0.01),WT-wild type,F8-/-- homozygous
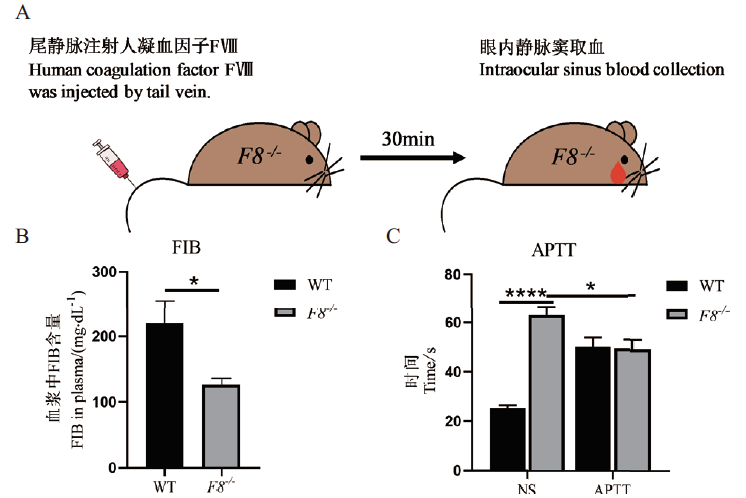
图5 F8 -/-小鼠的APTT A:小鼠凝血功能检测给药过程示意图;B:F8 -/-小鼠与WT小鼠血浆中FIB含量比较;C:F8 -/-小鼠与WT小鼠给予人凝血因子Ⅷ前后APTT值比较;每组5 只小鼠(*P <0.05,****P <0.0001),NS-尾静脉注射生理盐水,APTT-尾静脉注射人凝血因子Ⅷ,WT-野生型,F8 -/--纯合子
Fig.5 APTT results of F8-/- mice A:Schematic diagram of the administration process of blood coagulation in mice. B:Comparison of FIB in plasma of F8 -/- and WT mice. C:Comparison of APTT values before and after administration of human coagulation factor Ⅷ between F8 -/- and WT mice,5 mice per group(*P <0.05,****P <0.0001),to NS-tail vein injected with saline,to APTT-tail vein injected with human coagulation factor Ⅷ,WT-wild type,and F8 -/-- homozygous
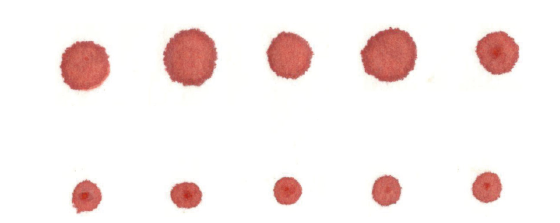
图6 F8-/-小鼠滴血实验 A:F8 -/-小鼠注射生理盐水;B:F8 -/-小鼠注射凝血因子
Fig. 6 F8 -/- mice blood drop test A:F8 -/-mice injection of saline. B:F8 -/-mice injection of clotting factor
| [1] |
Jankowska KI, Chattopadhyay M, Sauna ZE, et al. A foundational study for normal F8-containing mouse models for the miRNA regulation of hemophilia A:identification and analysis of mouse miRNAs that downregulate the murine F8 gene[J]. Int J Mol Sci, 2020, 21(16):5621.
doi: 10.3390/ijms21165621 URL |
| [2] | 王典文, 涂传清. 血友病A发病分子机制的研究现状[J]. 医学综述, 2013, 19(24):4430-4433. |
| Wang DW, Tu CQ. Research status of molecular pathogenesis of hemophilia A[J]. Med Recapitul, 2013, 19(24):4430-4433. | |
| [3] | 许正平, 王恩多. 凝血因子Ⅷ[J]. 生物化学与生物物理进展, 1991(4):8-11. |
| Xu ZP, Wang ED. Coagulation factor Ⅷ[J]. Prog Biochem Biophys, 1991(4):8-11. | |
| [4] | 侯玉香, 肖小璞. 血友病基因治疗概述[J]. 中国输血杂志, 2007, 20(1):71-74. |
| Hou YX, Xiao XP. Summary of gene therapy for hemophilia[J]. Chin J Blood Transfus, 2007, 20(1):71-74. | |
| [5] |
Yamaguti-Hayakawa GG, Ozelo MC. Gene therapy:paving new roads in the treatment of hemophilia[J]. Semin Thromb Hemost, 2019, 45(7):743-750.
doi: 10.1055/s-0039-1688445 pmid: 31096314 |
| [6] |
Rangarajan S, Walsh L, Lester W, et al. AAV5-factor VIII gene transfer in severe hemophilia A[J]. N Engl J Med, 2017, 377(26):2519-2530.
doi: 10.1056/NEJMoa1708483 URL |
| [7] |
Batty P, Lillicrap D. Advances and challenges for hemophilia gene therapy[J]. Hum Mol Genet, 2019, 28(R1):R95-R101.
doi: 10.1093/hmg/ddz157 |
| [8] |
Monahan PE, Samulski RJ, Tazelaar J, et al. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia[J]. Gene Ther, 1998, 5(1):40-49.
pmid: 9536263 |
| [9] |
Peyvandi F, Garagiola I. Clinical advances in gene therapy updates on clinical trials of gene therapy in haemophilia[J]. Haemophilia, 2019, 25(5):738-746.
doi: 10.1111/hae.13816 pmid: 31282050 |
| [10] |
Chen HN, Shi M, Gilam A, et al. Hemophilia a ameliorated in mice by CRISPR-based in vivo genome editing of human factor VIII[J]. Sci Rep, 2019, 9(1):16838.
doi: 10.1038/s41598-019-53198-y URL |
| [11] |
Guo T, Feng YL, Xiao JJ, et al. Harnessing accurate non-homologous end joining for efficient precise deletion in CRISPR/Cas9-mediated genome editing[J]. Genome Biol, 2018, 19(1):170.
doi: 10.1186/s13059-018-1518-x pmid: 30340517 |
| [12] | Hooper ML. Embryonic stem cells:introducing planned changes into the animal germline[M]. Chur: Harwood Academic Publishers, 1992. |
| [13] |
Lin HF, Maeda N, Smithies O, et al. A coagulation factor IX-deficient mouse model for human hemophilia B[J]. Blood, 1997, 90(10):3962-3966.
pmid: 9354664 |
| [14] | 陈永昌, 牛昱宇, 季维智. 通过CRISPR/Cas9和TALENs介导的基因打靶技术获得基因修饰的猴模型[J]. 中国细胞生物学学报, 2014, 36(5):557-560. |
| Chen YC, Niu YY, Ji WZ. The genetically modified monkey model was obtained by gene targeting mediated by CRISPR/ Cas9 and TALENs[J]. Chin J Cell Biol, 2014, 36(5):557-560. | |
| [15] |
Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems[J]. Science, 2013, 339(6121):819-823.
doi: 10.1126/science.1231143 pmid: 23287718 |
| [16] | 梁振伟, 饶书权, 沈岩, 等. 通过CRISPR/Cas9系统敲除人源PDE10A基因[J]. 基础医学与临床, 2014, 34(4):439-443. |
| Liang ZW, Rao SQ, Shen Y, et al. Knocking out human PDE10A gene by CRISPR/Cas9 system[J]. Basic Clin Med, 2014, 34(4):439-443. | |
| [17] | 郑武, 谷峰. CRISPR/Cas9的应用及脱靶效应研究进展[J]. 遗传, 2015, 37(10):1003-1010. |
| Zheng W, Gu F. Progress of application and off-target effects of CRISPR/Cas9[J]. Hereditas, 2015, 37(10):1003-1010. | |
| [18] |
Ma YW, Chen W, Zhang X, et al. Increasing the efficiency of CRISPR/Cas9-mediated precise genome editing in rats by inhibiting NHEJ and using Cas9 protein[J]. RNA Biol, 2016, 13(7):605-612.
doi: 10.1080/15476286.2016.1185591 pmid: 27163284 |
| [19] | Du J, Yin NR, Xie T, et al. Quantitative assessment of HR and NHEJ activities via CRISPR/Cas9-induced oligodeoxynucleotide-mediated DSB repair[J]. DNA Repair(Amst), 2018, 70:67-71. |
| [20] | 汪启翰, 怀聪, 孙瑞林, 等. 利用CRISPR/Cas系统快速高效构建血友病乙小鼠模型[J]. 遗传, 2015, 37(11):1143-1148. |
| Wang QH, Huai C, Sun RL, et al. A quick and efficient method to generate hemophilia B mouse models by the CRISPR/Cas system[J]. Hereditas, 2015, 37(11):1143-1148. |
| [1] | 陈小玲, 廖东庆, 黄尚飞, 陈英, 芦志龙, 陈东. 利用CRISPR/Cas9系统改造酿酒酵母的研究进展[J]. 生物技术通报, 2023, 39(8): 148-158. |
| [2] | 杨玉梅, 张坤晓. 应用CRISPR/Cas9技术建立ERK激酶相分离荧光探针定点整合的稳定细胞株[J]. 生物技术通报, 2023, 39(8): 159-164. |
| [3] | 施炜涛, 姚春鹏, 魏文康, 王蕾, 房元杰, 仝钰洁, 马晓姣, 蒋文, 张晓爱, 邵伟. 利用CRISPR/Cas9技术构建MDH2敲除细胞株及抗呕吐毒素效应研究[J]. 生物技术通报, 2023, 39(7): 307-315. |
| [4] | 刘晓燕, 祝振亮, 史广宇, 华梓宇, 杨晨, 张涌, 刘军. 乳腺生物反应器的表达优化策略[J]. 生物技术通报, 2023, 39(5): 77-91. |
| [5] | 程静雯, 曹磊, 张艳敏, 叶倩, 陈敏, 谭文松, 赵亮. CHO细胞多基因工程改造策略的建立及应用[J]. 生物技术通报, 2023, 39(2): 283-291. |
| [6] | 黄文莉, 李香香, 周炆婷, 罗莎, 姚维嘉, 马杰, 张芬, 沈钰森, 顾宏辉, 王建升, 孙勃. 利用CRISPR/Cas9技术靶向编辑青花菜BoZDS[J]. 生物技术通报, 2023, 39(2): 80-87. |
| [7] | 王兵, 赵会纳, 余婧, 陈杰, 骆梅, 雷波. 利用CRISPR/Cas9系统研究REVOLUTA参与烟草叶芽发育的调控[J]. 生物技术通报, 2023, 39(10): 197-208. |
| [8] | 李双喜, 华进联. 抗猪繁殖与呼吸障碍综合征基因编辑猪研究进展[J]. 生物技术通报, 2023, 39(10): 50-57. |
| [9] | 林蓉, 郑月萍, 徐雪珍, 李丹丹, 郑志富. 拟南芥ACOL8基因在乙烯合成与响应中的功能分析[J]. 生物技术通报, 2023, 39(1): 157-165. |
| [10] | 史亚楠, 王德培, 王一川, 周昊, 薛鲜丽. 敲除msn2对米曲霉生长和发酵产曲酸的影响[J]. 生物技术通报, 2022, 38(8): 188-197. |
| [11] | 刘静静, 刘晓蕊, 李琳, 王盈, 杨海元, 戴一凡. 利用CRISPR/Cas9技术建立OXTR基因敲除猪胎儿成纤维细胞系[J]. 生物技术通报, 2022, 38(6): 272-278. |
| [12] | Olalekan Amoo, 胡利民, 翟云孤, 范楚川, 周永明. 利用基因编辑技术研究BRANCHED1参与油菜分枝过程的调控[J]. 生物技术通报, 2022, 38(4): 97-105. |
| [13] | 丁亚群, 丁宁, 谢深民, 黄梦娜, 张昱, 张勤, 姜力. Vps28基因敲除小鼠模型的构建及其对泌乳和免疫性状影响的研究[J]. 生物技术通报, 2022, 38(3): 164-172. |
| [14] | 燕炯, 冯晨毅, 高学坤, 许祥, 杨佳敏, 陈朝阳. 基于CRISPR/Cas9技术构建Plin1基因敲除小鼠模型及表型分析[J]. 生物技术通报, 2022, 38(3): 173-180. |
| [15] | 钟菁, 孙玲玲, 张姝, 蒙园, 支怡飞, 涂黎晴, 徐天鹏, 濮黎萍, 陆阳清. 应用CRISPR/Cas9技术敲除Mda5基因对新城疫及传染性法氏囊病毒复制的影响[J]. 生物技术通报, 2022, 38(11): 90-96. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||