








生物技术通报 ›› 2023, Vol. 39 ›› Issue (11): 168-181.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0625
王晨宇1( ), 周楚源1, 何堤1, 樊梓豪2, 王梦梦1, 杨柳燕1(
), 周楚源1, 何堤1, 樊梓豪2, 王梦梦1, 杨柳燕1( )
)
收稿日期:2023-06-30
出版日期:2023-11-26
发布日期:2023-12-20
通讯作者:
杨柳燕,男,博士,教授,研究方向:微生物学和湖泊生态学;E-mail: yangly@nju.edu.cn作者简介:王晨宇,男,博士研究生,研究方向:环境生物学;E-mail: 18851732535@163.com
基金资助:
WANG Chen-yu1( ), ZHOU Chu-yuan1, HE Di1, FAN Zi-hao2, WANG Meng-meng1, YANG Liu-yan1(
), ZHOU Chu-yuan1, HE Di1, FAN Zi-hao2, WANG Meng-meng1, YANG Liu-yan1( )
)
Received:2023-06-30
Published:2023-11-26
Online:2023-12-20
摘要:
抗环境胁迫是微生物提高环境适应性和增加生存机会的一个重要策略,探明微生物抗环境胁迫的过程及分子机制对于了解微生物进化和开发微生物资源具有重要意义。多聚磷酸盐(polyphosphate, polyP)在微生物抗环境胁迫中发挥重要作用。在营养限制条件下,polyP可充当微生物的能源来源和信号分子,增强微生物对低营养环境的适应能力。在微生物应对环境胁迫过程中,polyP可作为蛋白质的伴侣,通过蛋白质修饰改变蛋白质结构使其免受失活,从而维持其功能完整性。polyP具有金属螯合能力,可提高微生物对重金属胁迫的抵抗能力。微生物能通过调节polyP的合成来适应环境pH的改变,调节酸碱胁迫过程中的能量消耗。基于polyP抗环境胁迫的特性,通过转基因技术,把polyP合成相关基因转入到农作物中,可以增加农作物体内polyP含量,从而提高农作物抗环境胁迫的能力。利用含有polyP的微生物处理重金属废水,可极大地提高重金属离子的去除效率。同时,微生物中合成的polyP颗粒也能进一步开发为生物活性产品。因此,polyP在微生物抗胁迫中发挥多样化作用,通过各种分子途径提高微生物对环境胁迫的耐受性。加强polyP在微生物抗环境胁迫中的作用与机制研究,不仅丰富微生物抗环境胁迫的研究内容,而且为多聚磷酸盐类生物活性物质的工程应用提供技术支撑。
王晨宇, 周楚源, 何堤, 樊梓豪, 王梦梦, 杨柳燕. 多聚磷酸盐在微生物抗环境胁迫中的作用及机制[J]. 生物技术通报, 2023, 39(11): 168-181.
WANG Chen-yu, ZHOU Chu-yuan, HE Di, FAN Zi-hao, WANG Meng-meng, YANG Liu-yan. Role and Mechanism of Polyphosphate in the Microbial Response to Environmental Stresses[J]. Biotechnology Bulletin, 2023, 39(11): 168-181.
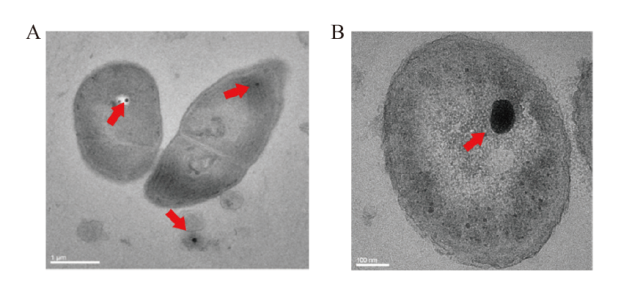
图1 微生物细胞内polyP颗粒的透射电镜图 A:泽丝藻polyP颗粒(藻体横截面);B:弗氏柠檬酸杆菌polyP颗粒
Fig. 1 Transmission electron micrograph of intracellular polyP granules in microorganism cells A: polyP granules of Limnothrix(cross-section); B: polyP granules of Citrobacter fumigatus
| 胁迫类型 Type of stress | PolyP抗胁迫机制 Mechanism of polyP-mediated resistance to stress | 文献 References |
|---|---|---|
| 高温 High temperature | (1)使不耐热的α-螺旋蛋白质转化成耐热的β-中间体Converting thermolabile α-helical proteins into heat-resistant β-intermediates (2)结合热休克蛋白,参与其折叠调节Binding heat shock proteins and regulating their folding | [ [ |
| 酸碱 Acid and alkali | 通过酶解polyP释放质子来中和碱性环境 Neutralisation of the alkaline environment by enzymolysis of polyP to release protons | [ |
| 渗透压 Osmotic pressure | (1)被碱性蛋白调控,影响细胞生长和分裂Regulated by basic proteins, affecting cell growth and division (2)结合Ca2+,改变生物膜延展性和刚性Binding Ca2+ and altering membrane ductility and rigidity | [ [ |
| 重金属离子 Heavy metal ion | (1)螯合重金属离子Chelating heavy metal ions (2)对于未知重金属配体起中介作用Mediating role for unknown heavy metal ligands | [ [ |
| 紫外线 Ultraviolet ray | (1)改变细胞壁结构,合成外膜或EPS Changing the structure of the cell wall and synthesizing the outer membrane or EPS (2)结合蛋白质,促进其折叠或防止其自我聚集 Binding proteins to promote their folding or prevents their self-aggregation (3)结合水,促进悬浮和凝胶Binding water and promoting suspension and gelation | [ [ [ |
| 贫营养 Oligotrophic | (1)积聚在蛋白质周围,防止其失活Accumulating around proteins to prevent their inactivation (2)维持ADP和ATP调节的电子流Maintenance of ADP- and ATP-regulated electron flow (3)受(p)ppGpp、RpoS和PhoB综合调节Regulated by a combination of(p)ppGpp, RpoS and PhoB | [ [ [ |
| 干旱 Droughts | 分泌并保留细胞壁外的EPS,结合大量水,增加持水性 Secreting and retaining EPS outside the cell wall, and binding a large amount of water to increase water-holding capacity | [ |
表1 微生物胞内polyP抗非生物胁迫机制
Table 1 Mechanisms of microbial intracellular polyP-mediated resistance to abiotic stresses
| 胁迫类型 Type of stress | PolyP抗胁迫机制 Mechanism of polyP-mediated resistance to stress | 文献 References |
|---|---|---|
| 高温 High temperature | (1)使不耐热的α-螺旋蛋白质转化成耐热的β-中间体Converting thermolabile α-helical proteins into heat-resistant β-intermediates (2)结合热休克蛋白,参与其折叠调节Binding heat shock proteins and regulating their folding | [ [ |
| 酸碱 Acid and alkali | 通过酶解polyP释放质子来中和碱性环境 Neutralisation of the alkaline environment by enzymolysis of polyP to release protons | [ |
| 渗透压 Osmotic pressure | (1)被碱性蛋白调控,影响细胞生长和分裂Regulated by basic proteins, affecting cell growth and division (2)结合Ca2+,改变生物膜延展性和刚性Binding Ca2+ and altering membrane ductility and rigidity | [ [ |
| 重金属离子 Heavy metal ion | (1)螯合重金属离子Chelating heavy metal ions (2)对于未知重金属配体起中介作用Mediating role for unknown heavy metal ligands | [ [ |
| 紫外线 Ultraviolet ray | (1)改变细胞壁结构,合成外膜或EPS Changing the structure of the cell wall and synthesizing the outer membrane or EPS (2)结合蛋白质,促进其折叠或防止其自我聚集 Binding proteins to promote their folding or prevents their self-aggregation (3)结合水,促进悬浮和凝胶Binding water and promoting suspension and gelation | [ [ [ |
| 贫营养 Oligotrophic | (1)积聚在蛋白质周围,防止其失活Accumulating around proteins to prevent their inactivation (2)维持ADP和ATP调节的电子流Maintenance of ADP- and ATP-regulated electron flow (3)受(p)ppGpp、RpoS和PhoB综合调节Regulated by a combination of(p)ppGpp, RpoS and PhoB | [ [ [ |
| 干旱 Droughts | 分泌并保留细胞壁外的EPS,结合大量水,增加持水性 Secreting and retaining EPS outside the cell wall, and binding a large amount of water to increase water-holding capacity | [ |
| [1] | Frank C, Jendrossek D. Acidocalcisomes and polyphosphate granules are different subcellular structures in Agrobacterium tumefaciens[J]. Appl Environ Microbiol, 2020, 86(8): e02759-e02719. |
| [2] |
Müller WEG, Schröder HC, Wang XH. Inorganic polyphosphates as storage for and generator of metabolic energy in the extracellular matrix[J]. Chem Rev, 2019, 119(24): 12337-12374.
doi: 10.1021/acs.chemrev.9b00460 pmid: 31738523 |
| [3] |
Asady B, Dick CF, Ehrenman K, et al. A single Na+-Pi cotransporter in Toxoplasma plays key roles in phosphate import and control of parasite osmoregulation[J]. PLoS Pathog, 2020, 16(12): e1009067.
doi: 10.1371/journal.ppat.1009067 URL |
| [4] | Potapenko E, Cordeiro CD, Huang GZ, et al. Pyrophosphate stimulates the phosphate-sodium symporter of Trypanosoma brucei acidocalcisomes and Saccharomyces cerevisiae vacuoles[J]. mSphere, 2019, 4(2): e00045-e00019. |
| [5] |
Nunes DCOS, Costa MS, Bispo-da-Silva LB, et al. Mitochondrial dysfunction on Leishmania(Leishmania)amazonensis induced by ketoconazole: insights into drug mode of action[J]. Mem Inst Oswaldo Cruz, 2022, 117: e210157.
doi: 10.1590/0074-02760210157 URL |
| [6] |
Gezelius K. Inorganic polyphosphates and enzymes of polyphosphate metabolism in the cellular slime mold Dictyostelium discoideum[J]. Arch Microbiol, 1974, 98(4): 311-329.
pmid: 4367840 |
| [7] |
Negreiros RS, Lander N, Huang GZ, et al. Inorganic polyphosphate interacts with nucleolar and glycosomal proteins in trypanosomatids[J]. Mol Microbiol, 2018, 110(6): 973-994.
doi: 10.1111/mmi.14131 pmid: 30230089 |
| [8] |
Werner TP, Amrhein N, Freimoser FM. Inorganic polyphosphate occurs in the cell wall of Chlamydomonas reinhardtii and accumulates during cytokinesis[J]. BMC Plant Biol, 2007, 7: 51.
doi: 10.1186/1471-2229-7-51 |
| [9] |
Das S, Lengweiler UD, Seebach D, et al. Proof for a nonproteinaceous calcium-selective channel in Escherichia coli by total synthesis from(R)-3-hydroxybutanoic acid and inorganic polyphosphate[J]. Proc Natl Acad Sci USA, 1997, 94(17): 9075-9079.
pmid: 9256437 |
| [10] | Racki LR, Tocheva EI, Dieterle MG, et al. Polyphosphate granule biogenesis is temporally and functionally tied to cell cycle exit during starvation in Pseudomonas aeruginosa[J]. Proc Natl Acad Sci USA, 2017, 114(12): E2440-E2449. |
| [11] |
Watanabe T, Kitamura Y, Aizawa H, et al. Fluorometric quantification of human platelet polyphosphate using 4', 6-diamidine-2-phenylindole dihydrochloride: applications in the Japanese population[J]. Int J Mol Sci, 2021, 22(14): 7257.
doi: 10.3390/ijms22147257 URL |
| [12] |
Kornberg A, Rao NN, Ault-Riché D. Inorganic polyphosphate: a molecule of many functions[J]. Annu Rev Biochem, 1999, 68: 89-125.
pmid: 10872445 |
| [13] |
Trilisenko L, Zvonarev A, Valiakhmetov A, et al. The reduced level of inorganic polyphosphate mobilizes antioxidant and manganese-resistance systems in Saccharomyces cerevisiae[J]. Cells, 2019, 8(5): 461.
doi: 10.3390/cells8050461 URL |
| [14] |
Baijal K, Downey M. Targeting polyphosphate kinases in the fight against Pseudomonas aeruginosa[J]. mBio, 2021, 12(4): e0147721.
doi: 10.1128/mBio.01477-21 URL |
| [15] |
Solovchenko A, Gorelova O, Karpova O, et al. Phosphorus feast and famine in cyanobacteria: is luxury uptake of the nutrient just a consequence of acclimation to its shortage?[J]. Cells, 2020, 9(9): 1933.
doi: 10.3390/cells9091933 URL |
| [16] |
Ghosh R, Barman S, Mandal NC. Phosphate deficiency induced biofilm formation of Burkholderia on insoluble phosphate granules plays a pivotal role for maximum release of soluble phosphate[J]. Sci Rep, 2019, 9(1): 5477.
doi: 10.1038/s41598-019-41726-9 |
| [17] |
Hothorn M, Neumann H, Lenherr ED, et al. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase[J]. Science, 2009, 324(5926): 513-516.
doi: 10.1126/science.1168120 pmid: 19390046 |
| [18] |
Ruiz FA, Marchesini N, Seufferheld M, et al. The polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes[J]. J Biol Chem, 2001, 276(49): 46196-46203.
doi: 10.1074/jbc.M105268200 pmid: 11579086 |
| [19] |
Docampo R, Moreno SN. The acidocalcisome[J]. Mol Biochem Parasitol, 2001, 114(2): 151-159.
doi: 10.1016/S0166-6851(01)00246-8 URL |
| [20] | 金文育, 姚炜民, 欧林坚, 等. 米氏凯伦藻胞内多聚磷酸盐对环境磷变化的响应研究[J]. 海洋与湖沼, 2022, 53(2): 340-345. |
| Jin WY, Yao WM, Ou LJ, et al. Response of intracellular polyphosphate in Karenia mikimotoi to the variation of phosphorus in the environment[J]. Oceanol Limnol Sin, 2022, 53(2): 340-345. | |
| [21] |
Sanz-Luque E, Bhaya D, Grossman AR. Polyphosphate: a multifunctional metabolite in cyanobacteria and algae[J]. Front Plant Sci, 2020, 11: 938.
doi: 10.3389/fpls.2020.00938 pmid: 32670331 |
| [22] |
Mullan A, Quinn JP, McGrath JW. Enhanced phosphate uptake and polyphosphate accumulation in Burkholderia cepacia grown under low pH conditions[J]. Microb Ecol, 2002, 44(1): 69-77.
doi: 10.1007/s00248-002-3004-x pmid: 12187377 |
| [23] |
Wacey D, Sirantoine E, Saunders M, et al. 1 billion-year-old cell contents preserved in monazite and xenotime[J]. Sci Rep, 2019, 9(1): 9068.
doi: 10.1038/s41598-019-45575-4 pmid: 31227773 |
| [24] |
Swift DT, Forciniti D. Accumulation of lead by Anabaena cylindrica: mathematical modeling and an energy dispersive X-ray study[J]. Biotechnol Bioeng, 1997, 55(2): 408-418.
pmid: 18636499 |
| [25] |
Zhu JL, Wei RP, Wang X, et al. Polyphosphate accelerates transformation of nonstructural carbohydrates to improve growth of ppk-expressing transgenic rice in phosphorus deficiency culture[J]. Rice Sci, 2023, 30(3): 235-246.
doi: 10.1016/j.rsci.2023.03.007 |
| [26] |
Wei RP, Wang X, Zhang W, et al. The improved phosphorus utilization and reduced phosphorus consumption of ppk-expressing transgenic rice[J]. Field Crops Res, 2020, 248: 107715.
doi: 10.1016/j.fcr.2020.107715 URL |
| [27] |
Zhang Y, Jing J, Liu T, et al. A molecularly engineered bioderived polyphosphate for enhanced flame retardant, UV-blocking and mechanical properties of poly(lactic acid)[J]. Chem Eng J, 2021, 411: 128493.
doi: 10.1016/j.cej.2021.128493 URL |
| [28] |
Gao JP, Chao DY, Lin HX. Understanding abiotic stress tolerance mechanisms: recent studies on stress response in rice[J]. J Integr Plant Biol, 2007, 49(6): 742-750.
doi: 10.1111/jipb.2007.49.issue-6 URL |
| [29] | Camci İY, Doruk T, Avİcan Ü, et al. Deletion of polyphosphate kinase gene(ppk)has a stimulatory effect on actinorhodin production by Streptomyces coelicolor A3(2)[J]. Turk J Biol, 2012, 36: 373-380. |
| [30] |
Cavalcanti Luna MA, Vieira ER, Okada K, et al. Copper-induced adaptation, oxidative stress and its tolerance in Aspergillus niger UCP1261[J]. Electron J Biotechnol, 2015, 18(6): 418-427.
doi: 10.1016/j.ejbt.2015.09.006 URL |
| [31] |
Cheng YY, Sun BL. Polyphosphate kinase affects oxidative stress response by modulating cAMP receptor protein and rpoS expression in Salmonella typhimurium[J]. J Microbiol Biotechnol, 2009, 19(12): 1527-1535.
doi: 10.4014/jmb URL |
| [32] | Diaz JM, Ingall ED, Snow SD, et al. Potential role of inorganic polyphosphate in the cycling of phosphorus within the hypoxic water column of Effingham Inlet, British Columbia[J]. Global Biogeochem Cycles, 2012, 26(2): 2040. |
| [33] |
Cherry DS, Guthrie RK. Effects of Flavobacterium lutescens growth on populations of Escherichia coli and Streptococcus faecalis in water following thermal loading[J]. Microb Ecol, 1975, 2(3): 186-193.
doi: 10.1007/BF02010438 pmid: 24241333 |
| [34] |
McDevitt-Irwin JM, Baum JK, Garren M, et al. Responses of coral-associated bacterial communities to local and global stressors[J]. Front Mar Sci, 2017, 4: 262.
doi: 10.3389/fmars.2017.00262 URL |
| [35] | Guthrie RK, Cherry DS, Singleton FL. Alteration of microbial populations in thermal stress[J]. J Water Pollut Control Fed, 1976, 48(5): 962-965. |
| [36] |
Guan NZ, Li JH, Shin HD, et al. Microbial response to environmental stresses: from fundamental mechanisms to practical applications[J]. Appl Microbiol Biotechnol, 2017, 101(10): 3991-4008.
doi: 10.1007/s00253-017-8264-y pmid: 28409384 |
| [37] |
Guyot S, Gervais P, Young M, et al. Surviving the heat: heterogeneity of response in Saccharomyces cerevisiae provides insight into thermal damage to the membrane[J]. Environ Microbiol, 2015, 17(8): 2982-2992.
doi: 10.1111/emi.2015.17.issue-8 URL |
| [38] |
Nicolaou SA, Gaida SM, Papoutsakis ET. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: from biofuels and chemicals, to biocatalysis and bioremediation[J]. Metab Eng, 2010, 12(4): 307-331.
doi: 10.1016/j.ymben.2010.03.004 pmid: 20346409 |
| [39] |
Gao LM, Liu YQ, Sun H, et al. Advances in mechanisms and modifications for rendering yeast thermotolerance[J]. J Biosci Bioeng, 2016, 121(6): 599-606.
doi: S1389-1723(15)00403-X pmid: 26685013 |
| [40] |
Achbergerová L, Nahálka J. Polyphosphate - an ancient energy source and active metabolic regulator[J]. Microb Cell Fact, 2011, 10: 63.
doi: 10.1186/1475-2859-10-63 pmid: 21816086 |
| [41] |
Solovchenko A, Khozin-Goldberg I, Selyakh I, et al. Phosphorus starvation and luxury uptake in green microalgae revisited[J]. Algal Res, 2019, 43: 101651.
doi: 10.1016/j.algal.2019.101651 URL |
| [42] |
Cremers CM, Knoefler D, Gates S, et al. Polyphosphate: a conserved modifier of amyloidogenic processes[J]. Mol Cell, 2016, 63(5): 768-780.
doi: 10.1016/j.molcel.2016.07.016 pmid: 27570072 |
| [43] |
Marijan D, Tse R, Elliott K, et al. Stress-specific aggregation of proteins in the amyloid bodies[J]. FEBS Lett, 2019, 593(22): 3162-3172.
doi: 10.1002/1873-3468.13597 pmid: 31512750 |
| [44] |
Gray MJ, Wholey WY, Wagner NO, et al. Polyphosphate is a primordial chaperone[J]. Mol Cell, 2014, 53(5): 689-699.
doi: 10.1016/j.molcel.2014.01.012 pmid: 24560923 |
| [45] |
Yoo NG, Dogra S, Meinen BA, et al. Polyphosphate stabilizes protein unfolding intermediates as soluble amyloid-like oligomers[J]. J Mol Biol, 2018, 430(21): 4195-4208.
doi: S0022-2836(18)30625-9 pmid: 30130556 |
| [46] | 陈成, 郑超群, 王梦梦, 等. 低浓度硝态氮促进微囊藻累积多聚磷酸盐[J]. 湖泊科学, 2022, 34(3): 766-776. |
| Chen C, Zheng CQ, Wang MM, et al. Low concentration nitrate-nitrogen improves polyphosphate accumulation in Microcystis[J]. J Lake Sci, 2022, 34(3): 766-776. | |
| [47] |
Baker-Austin C, Dopson M. Life in acid: pH homeostasis in acidophiles[J]. Trends Microbiol, 2007, 15(4): 165-171.
pmid: 17331729 |
| [48] |
Guan NZ, Liu L, Shin HD, et al. Systems-level understanding of how Propionibacterium acidipropionici respond to propionic acid stress at the microenvironment levels: mechanism and application[J]. J Biotechnol, 2013, 167(1): 56-63.
doi: 10.1016/j.jbiotec.2013.06.008 URL |
| [49] | 杨柳燕, 肖琳. 湖泊蓝藻水华暴发、危害与控制[M]. 北京: 科学出版社, 2011. |
| Yang LY, Xiao L. Outbreak, harm and control of cyanobacteria bloom in lakes[M]. Beijing: Science Press, 2011. | |
| [50] | 魏峥, 聂琰晖, 刘乐庭, 等. 多聚磷酸盐在原核和真核生物中的研究进展[J]. 生理科学进展, 2009, 40(3): 197-202. |
| Wei Z, Nie YH, Liu LT, et al. Progress in functional polyphosphate in prokaryotic and eukaryotic living organisms[J]. Prog Physiol Sci, 2009, 40(3): 197-202. | |
| [51] |
Bremer E, Krämer R. Responses of microorganisms to osmotic stress[J]. Annu Rev Microbiol, 2019, 73: 313-334.
doi: 10.1146/annurev-micro-020518-115504 pmid: 31180805 |
| [52] |
De Angelis M, Gobbetti M. Environmental stress responses in Lactobacillus: a review[J]. Proteomics, 2004, 4(1): 106-122.
pmid: 14730676 |
| [53] | 马芮, 苏莉, 宋宇昊, 等. 多聚磷酸盐: 菌体内多功能调控子和环境压力守护者[J]. 微生物学通报, 2017, 44(7): 1736-1746. |
| Ma R, Su L, Song YH, et al. Inorganic polyphosphate: the multifunctional regulator and the guardian of environmental stresses in bacteria[J]. Microbiol China, 2017, 44(7): 1736-1746. | |
| [54] |
Liu W, Li YL, Feng Y, et al. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil[J]. Sci Rep, 2020, 10(1): 858.
doi: 10.1038/s41598-020-57954-3 pmid: 31965039 |
| [55] | 姚雪丹, 付建红, 徐彤, 等. 重金属胁迫下的微生物代谢组学研究进展[J]. 生物资源, 2020, 42(6): 678-685. |
| Yao XD, Fu JH, Xu T, et al. Metabolomics of microorganisms in response to heavy metal stress[J]. Biotic Resour, 2020, 42(6): 678-685. | |
| [56] |
White RA III, Soles SA, Gavelis G, et al. The complete genome and physiological analysis of the eurythermal firmicute Exiguobacterium chiriqhucha strain RW2 isolated from a freshwater microbialite, widely adaptable to broad thermal, pH, and salinity ranges[J]. Front Microbiol, 2019, 9: 3189.
doi: 10.3389/fmicb.2018.03189 URL |
| [57] |
Xu LZJ, Wu J, Xia WJ, et al. Adaption and restoration of anammox biomass to Cd(II)stress: performance, extracellular polymeric substance and microbial community[J]. Bioresour Technol, 2019, 290: 121766.
doi: 10.1016/j.biortech.2019.121766 URL |
| [58] |
Haider FU, Farooq M, Naveed M, et al. Influence of biochar and microorganism co-application on stabilization of cadmium(Cd)and improved maize growth in Cd-contaminated soil[J]. Front Plant Sci, 2022, 13: 983830.
doi: 10.3389/fpls.2022.983830 URL |
| [59] |
Darnall DW, Greene B, Henzl MT, et al. Selective recovery of gold and other metal ions from an algal biomass[J]. Environ Sci Technol, 1986, 20(2): 206-208.
doi: 10.1021/es00144a018 URL |
| [60] |
Komine Y, Eggink LL, Park H, et al. Vacuolar Granules in Chlamydomonas reinhardtii: polyphosphate and a 70-kDa polypeptide as major components[J]. Planta, 2000, 210(6): 897-905.
pmid: 10872220 |
| [61] |
Chu FF, Chu PN, Cai PJ, et al. Phosphorus plays an important role in enhancing biodiesel productivity of Chlorella vulgaris under nitrogen deficiency[J]. Bioresour Technol, 2013, 134: 341-346.
doi: 10.1016/j.biortech.2013.01.131 URL |
| [62] |
刘沙沙, 付建平, 蔡信德, 等. 重金属污染对土壤微生物生态特征的影响研究进展[J]. 生态环境学报, 2018, 27(6): 1173-1178.
doi: 10.16258/j.cnki.1674-5906.2018.06.024 |
| Liu SS, Fu JP, Cai XD, et al. Effect of heavy metals pollution on ecological characteristics of soil microbes: a review[J]. Ecol Environ Sci, 2018, 27(6): 1173-1178. | |
| [63] |
Moura KAF, Lizieri C, Wittig Franco M, et al. Physiological and thylakoid ultrastructural changes in cyanobacteria in response to toxic manganese concentrations[J]. Ecotoxicology, 2019, 28(8): 1009-1021.
doi: 10.1007/s10646-019-02098-y pmid: 31471822 |
| [64] |
Sicko-Goad L, Jensen TE, Ayala RP. Phosphate metabolism in blue-green bacteria. V. Factors affecting phosphate uptake in Plectonema boryanum[J]. Can J Microbiol, 1978, 24(2): 105-108.
pmid: 25703 |
| [65] |
Samadani M, Dewez D. Effect of mercury on the polyphosphate level of Alga Chlamydomonas reinhardtii[J]. Environ Pollut, 2018, 240: 506-513.
doi: S0269-7491(17)33603-5 pmid: 29754100 |
| [66] |
Tsednee M, Castruita M, Salomé PA, et al. Manganese co-localizes with calcium and phosphorus in Chlamydomonas acidocalcisomes and is mobilized in manganese-deficient conditions[J]. J Biol Chem, 2019, 294(46): 17626-17641.
doi: 10.1074/jbc.RA119.009130 URL |
| [67] |
Penen F, Isaure MP, Dobritzsch D, et al. Pools of cadmium in Chlamydomonas reinhardtii revealed by chemical imaging and XAS spectroscopy[J]. Metallomics, 2017, 9(7): 910-923.
doi: 10.1039/c7mt00029d pmid: 28598481 |
| [68] |
MacFie SM, Welbourn PM. The cell wall as a barrier to uptake of metal ions in the unicellular green Alga Chlamydomonas reinhardtii(Chlorophyceae)[J]. Arch Environ Contam Toxicol, 2000, 39(4): 413-419.
doi: 10.1007/s002440010122 URL |
| [69] |
Samadani M, Dewez D. Cadmium accumulation and toxicity affect the extracytoplasmic polyphosphate level in Chlamydomonas reinhardtii[J]. Ecotoxicol Environ Saf, 2018, 166: 200-206.
doi: 10.1016/j.ecoenv.2018.09.094 URL |
| [70] |
Samadani M, El-Khoury J, Dewez D. Tolerance capacity of Chlamydomonas VHLR mutants for the toxicity of mercury[J]. Water Air Soil Pollut, 2020, 231(4): 1-12.
doi: 10.1007/s11270-019-4368-6 |
| [71] |
El Bouraie M, Masoud AA. Adsorption of phosphate ions from aqueous solution by modified bentonite with magnesium hydroxide Mg(OH)2[J]. Appl Clay Sci, 2017, 140: 157-164.
doi: 10.1016/j.clay.2017.01.021 URL |
| [72] |
Li SS, Li JH, Xia MS, et al. Adsorption of nitrogen and phosphorus by intact cells and cell wall polysaccharides of Microcystis[J]. J Appl Phycol, 2013, 25(5): 1539-1544.
doi: 10.1007/s10811-013-9992-8 URL |
| [73] |
Sheng GP, Xu J, Li WH, et al. Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances(EPS)of sludge[J]. Chemosphere, 2013, 93(7): 1436-1441.
doi: 10.1016/j.chemosphere.2013.07.076 URL |
| [74] |
Tan X, Gao WP, Duan ZP, et al. Synthesis of novel algal extracellular polymeric substances(EPS)-based hydrogels for the efficient removal and recovery of phosphorus from contaminated waters: development, characterisation, and performance[J]. J Environ Chem Eng, 2023, 11(1): 109044.
doi: 10.1016/j.jece.2022.109044 URL |
| [75] | 杨柳燕, 胡志新, 何连生. 中国湖泊水生态系统区域差异性[M]. 北京: 科学出版社, 2016. |
| Yang LY, Hu ZX, He LS. Regional differences of lake water ecosystem in China[M]. Beijing: Science Press, 2016. | |
| [76] |
Stumpf JD, Foster PL. Polyphosphate kinase regulates error-prone replication by DNA polymerase IV in Escherichia coli[J]. Mol Microbiol, 2005, 57(3): 751-761.
doi: 10.1111/mmi.2005.57.issue-3 URL |
| [77] |
Rodriguez RJ. Polyphosphate present in DNA preparations from filamentous fungal species of Colletotrichum inhibits restriction endonucleases and other enzymes[J]. Anal Biochem, 1993, 209(2): 291-297.
pmid: 8385889 |
| [78] | Kuroda A, Nomura K, Takiguchi N, et al. Inorganic polyphosphate stimulates lon-mediated proteolysis of nucleoid proteins in Escherichia coli[J]. Cell Mol Biol, 2006, 52(4): 23-29. |
| [79] | English BP, Hauryliuk V, Sanamrad A, et al. Single-molecule investigations of the stringent response machinery in living bacterial cells[J]. Proc Natl Acad Sci USA, 2011, 108(31): E365-E373. |
| [80] |
Price-Carter M, Fazzio TG, Vallbona EI, et al. Polyphosphate kinase protects Salmonella enterica from weak organic acid stress[J]. J Bacteriol, 2005, 187(9): 3088-3099.
pmid: 15838036 |
| [81] |
Kulakovskaya TV, Vagabov VM, Kulaev IS. Inorganic polyphosphate in industry, agriculture and medicine: modern state and outlook[J]. Process Biochem, 2012, 47(1): 1-10.
doi: 10.1016/j.procbio.2011.10.028 URL |
| [82] |
Paerl HW, Fulton RS Ⅲ, Moisander PH, et al. Harmful freshwater algal blooms, with an emphasis on cyanobacteria[J]. Sci World J, 2001, 1: 76-113.
pmid: 12805693 |
| [83] |
Schwarz R, Forchhammer K. Acclimation of unicellular cyanobacteria to macronutrient deficiency: emergence of a complex network of cellular responses[J]. Microbiology, 2005, 151(Pt 8): 2503-2514.
doi: 10.1099/mic.0.27883-0 pmid: 16079330 |
| [84] |
Goodenough U, Heiss AA, Roth R, et al. Acidocalcisomes: ultrastructure, biogenesis, and distribution in microbial eukaryotes[J]. Protist, 2019, 170(3): 287-313.
doi: S1434-4610(19)30003-3 pmid: 31154072 |
| [85] |
Chu FF, Shen XF, Lam PKS, et al. Polyphosphate during the regreening of Chlorella vulgaris under nitrogen deficiency[J]. Int J Mol Sci, 2015, 16(10): 23355-23368.
doi: 10.3390/ijms161023355 URL |
| [86] |
Jensen TE, Sicko LM. Phosphate metabolism in blue-green algae. I. Fine structure of the polyphosphate overplus phenomenon in Plectonema boryanum[J]. Can J Microbiol, 1974, 20(9): 1235-1239.
pmid: 4371465 |
| [87] |
Liss E, Langen P. Experiments on polyphosphate overcompensation in yeast cells after phosphate deficiency[J]. Archiv für Mikrobiologie, 1962, 41: 383-392.
doi: 10.1007/BF00422195 URL |
| [88] |
Aksoy M, Pootakham W, Grossman AR. Critical function of a Chlamydomonas reinhardtii putative polyphosphate polymerase subunit during nutrient deprivation[J]. Plant Cell, 2014, 26(10): 4214-4229.
doi: 10.1105/tpc.114.129270 URL |
| [89] | Hiraishi A, Kitamura H. Changes in the polyphosphate content of photosynthetically grown Rhodobacter sphaeroides due to nutrient limitation[J]. Agric Biol Chem, 1985, 49(11): 3343-3345. |
| [90] |
Sanz-Luque E, Saroussi S, Huang W, et al. Metabolic control of acclimation to nutrient deprivation dependent on polyphosphate synthesis[J]. Sci Adv, 2020, 6(40): eabb5351.
doi: 10.1126/sciadv.abb5351 URL |
| [91] |
Wild R, Gerasimaite R, Jung JY, et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains[J]. Science, 2016, 352(6288): 986-990.
doi: 10.1126/science.aad9858 pmid: 27080106 |
| [92] |
Gerasimaite R, Pavlovic I, Capolicchio S, et al. Inositol pyrophosphate specificity of the SPX-dependent polyphosphate polymerase VTC[J]. ACS Chem Biol, 2017, 12(3): 648-653.
doi: 10.1021/acschembio.7b00026 pmid: 28186404 |
| [93] |
Potrykus K, Cashel M. (p)ppGpp: still magical?[J]. Annu Rev Microbiol, 2008, 62: 35-51.
doi: 10.1146/annurev.micro.62.081307.162903 pmid: 18454629 |
| [94] |
Rao NN, Kornberg A. Inorganic polyphosphate regulates responses of Escherichia coli to nutritional stringencies, environmental stresses and survival in the stationary phase[J]. Prog Mol Subcell Biol, 1999, 23: 183-195.
pmid: 10448677 |
| [95] |
Alpert P. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare?[J]. J Exp Biol, 2006, 209(Pt 9): 1575-1584.
doi: 10.1242/jeb.02179 URL |
| [96] |
Wang Q, Li J, Yang J, et al. Diversity of endophytic bacterial and fungal microbiota associated with the medicinal lichen Usnea longissima at high altitudes[J]. Front Microbiol, 2022, 13: 958917.
doi: 10.3389/fmicb.2022.958917 URL |
| [97] |
Knowles EJ, Castenholz RW. Effect of exogenous extracellular polysaccharides on the desiccation and freezing tolerance of rock-inhabiting phototrophic microorganisms[J]. FEMS Microbiol Ecol, 2008, 66(2): 261-270.
doi: 10.1111/j.1574-6941.2008.00568.x pmid: 18710394 |
| [98] |
Jasso-Chávez R, Campos-García ML, Vega-Segura A, et al. Microaerophilia enhances heavy metal biosorption and internal binding by polyphosphates in photosynthetic Euglena gracilis[J]. Algal Res, 2021, 58: 102384.
doi: 10.1016/j.algal.2021.102384 URL |
| [99] | Kiyono M, Pan-Hou H. Genetic engineering of bacteria for environmental remediation of mercury[J]. J Heath Sci, 2006, 52(3): 199-204. |
| [100] |
Correa Deza MA, Grillo-Puertas M, Salva S, et al. Inorganic salts and intracellular polyphosphate inclusions play a role in the thermotolerance of the immunobiotic Lactobacillus rhamnosus CRL 1505[J]. PLoS One, 2017, 12(6): e0179242.
doi: 10.1371/journal.pone.0179242 URL |
| [101] |
Mullan A, McGrath JW, Adamson T, et al. Pilot-scale evaluation of the application of low pH-inducible polyphosphate accumulation to the biological removal of phosphate from wastewaters[J]. Environ Sci Technol, 2006, 40(1): 296-301.
doi: 10.1021/es0509782 URL |
| [102] |
Feng GX, Dong SY, Huang M, et al. Biogenic polyphosphate nanoparticles from a marine cyanobacterium Synechococcus sp. PCC 7002: production, characterization, and anti-inflammatory properties in vitro[J]. Mar Drugs, 2018, 16(9): 322.
doi: 10.3390/md16090322 URL |
| [103] |
Gao FZ, Wu HH, Zeng MY, et al. Overproduction, purification, and characterization of nanosized polyphosphate bodies from Synechococcus sp. PCC 7002[J]. Microb Cell Fact, 2018, 17(1): 27.
doi: 10.1186/s12934-018-0870-6 |
| [104] |
Christ JJ, Smith SA, Willbold S, et al. Biotechnological synthesis of water-soluble food-grade polyphosphate with Saccharomyces cerevisiae[J]. Biotechnol Bioeng, 2020, 117(7): 2089-2099.
doi: 10.1002/bit.v117.7 URL |
| [105] | 赵心清, 彭楠. 新资源微生物:微生物资源的发现和应用[J]. 微生物学报, 2022, 62(11): 4091-4094. |
| Zhao XQ, Peng N. Preface for special issue on new-resource microbes: discovery and applications of microbial resources[J]. Acta Microbiol Sin, 2022, 62(11): 4091-4094. |
| [1] | 张坤, 闫畅, 田新朋. 微生物单细胞分离方法研究进展[J]. 生物技术通报, 2023, 39(9): 1-11. |
| [2] | 赵志祥, 王殿东, 周亚林, 王培, 严婉荣, 严蓓, 罗路云, 张卓. 枯草芽孢杆菌Ya-1对辣椒枯萎病的防治及其对根际真菌群落的影响[J]. 生物技术通报, 2023, 39(9): 213-224. |
| [3] | 程亚楠, 张文聪, 周圆, 孙雪, 李玉, 李庆刚. 乳酸乳球菌生产2'-岩藻糖基乳糖的途径构建及发酵培养基优化[J]. 生物技术通报, 2023, 39(9): 84-96. |
| [4] | 江润海, 姜冉冉, 朱城强, 侯秀丽. 微生物强化植物修复铅污染土壤的机制研究进展[J]. 生物技术通报, 2023, 39(8): 114-125. |
| [5] | 李焕敏, 高峰涛, 李伟忠, 王金庆, 封佳丽. 天然生物质材料作为固定化载体的研究应用进展[J]. 生物技术通报, 2023, 39(7): 105-112. |
| [6] | 赵林艳, 徐武美, 王豪吉, 王昆艳, 魏富刚, 杨绍周, 官会林. 施用生物炭对连作三七根际真菌群落与存活率的影响[J]. 生物技术通报, 2023, 39(7): 219-227. |
| [7] | 徐汝悦, 王子霄, 沈禄, 吴蓉蓉, 姚芳婷, 谭中原, 刘恒蔚, 张文超. Cr(VI)的生物修复技术研究进展[J]. 生物技术通报, 2023, 39(6): 49-60. |
| [8] | 张晶, 张浩睿, 曹云, 黄红英, 曲萍, 张志萍. 嗜热纤维素降解菌研究进展[J]. 生物技术通报, 2023, 39(6): 73-87. |
| [9] | 余洋, 刘天海, 刘理旭, 唐杰, 彭卫红, 陈阳, 谭昊. 羊肚菌菌种生产车间气溶胶微生物群落研究[J]. 生物技术通报, 2023, 39(5): 267-275. |
| [10] | 雷彩荣, 郭晓鹏, 柴冉, 张苗苗, 任军乐, 陆栋. 组学技术在重离子辐射微生物诱变育种中的应用[J]. 生物技术通报, 2023, 39(5): 54-62. |
| [11] | 张华香, 徐晓婷, 郑云婷, 肖春桥. 溶磷微生物在钝化和植物修复重金属污染土壤中的作用[J]. 生物技术通报, 2023, 39(3): 52-58. |
| [12] | 李凯航, 王浩臣, 程可心, 杨艳, 金一, 何晓青. 全基因组关联分析研究植物与微生物组的互作遗传机制[J]. 生物技术通报, 2023, 39(2): 24-34. |
| [13] | 李昕悦, 周明海, 樊亚超, 廖莎, 张风丽, 刘晨光, 孙悦, 张霖, 赵心清. 基于转运蛋白工程提升微生物菌株耐受性和生物制造效率的研究进展[J]. 生物技术通报, 2023, 39(11): 123-136. |
| [14] | 胡锦超, 沈文琦, 徐超业, 樊雅祺, 卢浩宇, 蒋雯杰, 李世龙, 晋洪晨, 骆健美, 王敏. 微生物酸胁迫耐受性能强化的研究进展[J]. 生物技术通报, 2023, 39(11): 137-149. |
| [15] | 祝瑛萱, 李克景, 何敏, 郑道琼. 酵母模型揭示胁迫因子驱动基因组变异的研究进展[J]. 生物技术通报, 2023, 39(11): 191-204. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||