








生物技术通报 ›› 2025, Vol. 41 ›› Issue (2): 175-186.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0597
• 研究报告 • 上一篇
颜伟( ), 陈慧婷, 叶青, 刘广超, 刘新(
), 陈慧婷, 叶青, 刘广超, 刘新( ), 侯丽霞(
), 侯丽霞( )
)
收稿日期:2024-06-21
出版日期:2025-02-26
发布日期:2025-02-28
通讯作者:
侯丽霞,女,博士,教授,研究方向 :葡萄抗性生理与分子机制;E-mail: houlixia78@163.com作者简介:颜伟,男,硕士研究生,研究方向 :葡萄抗性生理与分子机制;E-mail: 17860729919@163.com
基金资助:
YAN Wei( ), CHEN Hui-ting, YE Qing, LIU Guang-chao, LIU Xin(
), CHEN Hui-ting, YE Qing, LIU Guang-chao, LIU Xin( ), HOU Li-xia(
), HOU Li-xia( )
)
Received:2024-06-21
Published:2025-02-26
Online:2025-02-28
摘要:
目的 羟基肉桂酰转移酶(hydroxycinnamoyl-CoA: shikimate hydroxycinnamoyl transferase, HCT)是双子叶植物中催化绿原酸合成的关键酶,广泛参与植物非生物胁迫应答。在葡萄全基因组中鉴定VvHCT基因家族成员,分析相关基因低温胁迫下表达模式,为后续研究提供理论基础。 方法 基于葡萄全基因组信息,利用生物信息学方法对VvHCT家族进行鉴定,并对家族成员理化性质、染色体分布、基因结构、蛋白保守结构、系统进化、启动子顺式作用元件以及组织表达特性进行分析,进一步利用荧光定量PCR检测VvHCT基因在低温胁迫下表达模式,最后通过瞬时转化方法对VvHCT8功能进行验证。 结果 在葡萄全基因组中鉴定到VvHCT的11个家族成员,编码398-457个氨基酸,分布于4条染色体上;按系统发育特征分为3个亚族;启动子顺式作用元件分析显示,VvHCT家族成员启动子上含有光响应元件、激素响应元件、应激响应元件、组织特异性元件和昼夜节律响应元件等;不同VvHCT家族成员表达模式存在较大差异;低温胁迫处理后VvHCT5表达量下调,其他10个家族成员表达量均上调;瞬时过表达葡萄叶片结果显示,VvHCT8能通过降低丙二醛和活性氧含量,缓解低温对葡萄叶片叶绿素和细胞膜的损伤。 结论 VvHCT8参与葡萄抵御低温过程,可作为葡萄抗寒候选基因。
颜伟, 陈慧婷, 叶青, 刘广超, 刘新, 侯丽霞. 葡萄HCT基因家族鉴定及其对低温胁迫的响应[J]. 生物技术通报, 2025, 41(2): 175-186.
YAN Wei, CHEN Hui-ting, YE Qing, LIU Guang-chao, LIU Xin, HOU Li-xia. Identification of the Grape HCT Gene Family and Their Responses to Low-temperature Stress[J]. Biotechnology Bulletin, 2025, 41(2): 175-186.
引物名称 Primer name | 引物序列 Primer sequence (5′-3′) |
|---|---|
| VvActin-F | ATAGAAGCAGCAAGGGA |
| VvActin-R | TGAGGCTCTTACTAATG |
| VvHCT1-F | CCACCTTCGCCATTTCCAAC |
| VvHCT1-R | TAGGGAACACCCTGGTCAGT |
| VvHCT2-F | CCTCTCCAACCCCACATTCC |
| VvHCT2-R | CCCCTTATCATTGCATGGGTT |
| VvHCT3-F | TGAGTGCAACGATGAGGGAG |
| VvHCT3-R | TGCCGTGGAGATCAGAAACC |
| VvHCT4-F | GGAGGTTTGTGTTTGATGGGG |
| VvHCT4-R | TGGGCGCAAGTTTATGCTGT |
| VvHCT5-F | ACTTCCCTTGCGGTCCATTC |
| VvHCT5-R | TTATCGCAGTCGGGAACACA |
| VvHCT6-F | GGTTGTAAGCCGAGCGAAAC |
| VvHCT6-R | TTGCCACTGTCCGCCATAAA |
| VvHCT7-F | CTGCCAACTTCTTCGACCCT |
| VvHCT7-R | GTAGGGGCGAAATCACCGAA |
| VvHCT8-F | CGACAGGCAATAGAGAAGGCA |
| VvHCT8-R | AGATGGGAAGTCTAGCCCAG |
| VvHCT9-F | CTAGCGGGATGGTTGCACT |
| VvHCT9-R | CATCAGTCATGATATGCAATGCTC |
| VvHCT10-F | AGCAATTGGCTACGATGGGA |
| VvHCT10-R | AGCAATTGGCTACGATGGGA |
| VvHCT11-F | ATCGGAGCCCACTTTCGATG |
| VvHCT11-R | AGCGGGTAGAAATGCACCAA |
表1 引物序列
Table 1 Primer sequences
引物名称 Primer name | 引物序列 Primer sequence (5′-3′) |
|---|---|
| VvActin-F | ATAGAAGCAGCAAGGGA |
| VvActin-R | TGAGGCTCTTACTAATG |
| VvHCT1-F | CCACCTTCGCCATTTCCAAC |
| VvHCT1-R | TAGGGAACACCCTGGTCAGT |
| VvHCT2-F | CCTCTCCAACCCCACATTCC |
| VvHCT2-R | CCCCTTATCATTGCATGGGTT |
| VvHCT3-F | TGAGTGCAACGATGAGGGAG |
| VvHCT3-R | TGCCGTGGAGATCAGAAACC |
| VvHCT4-F | GGAGGTTTGTGTTTGATGGGG |
| VvHCT4-R | TGGGCGCAAGTTTATGCTGT |
| VvHCT5-F | ACTTCCCTTGCGGTCCATTC |
| VvHCT5-R | TTATCGCAGTCGGGAACACA |
| VvHCT6-F | GGTTGTAAGCCGAGCGAAAC |
| VvHCT6-R | TTGCCACTGTCCGCCATAAA |
| VvHCT7-F | CTGCCAACTTCTTCGACCCT |
| VvHCT7-R | GTAGGGGCGAAATCACCGAA |
| VvHCT8-F | CGACAGGCAATAGAGAAGGCA |
| VvHCT8-R | AGATGGGAAGTCTAGCCCAG |
| VvHCT9-F | CTAGCGGGATGGTTGCACT |
| VvHCT9-R | CATCAGTCATGATATGCAATGCTC |
| VvHCT10-F | AGCAATTGGCTACGATGGGA |
| VvHCT10-R | AGCAATTGGCTACGATGGGA |
| VvHCT11-F | ATCGGAGCCCACTTTCGATG |
| VvHCT11-R | AGCGGGTAGAAATGCACCAA |
基因名称 Gene name | 基因 ID Gene ID | 染色体位置 Chromosome location | 编码区 CDS/bp | 氨基酸 Amino acid/bp | 分子量 Molecular weigh/kD | 等电点 Theoretical isoelectric points | 不稳定系数 Instability index | 总平均亲水性 Grand average of hydropathicity | KEGG | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|---|---|---|---|
| VvHCT1 | Vitvi01g01514 | Chr1:20494476:20495771 | 1 296 | 431 | 48.45 | 6.04 | 38.66 | -0.018 | 2.3.1.133 | 细胞质 |
| VvHCT2 | Vitvi01g01517 | Chr1:20532349:20533650 | 1 197 | 398 | 44.78 | 5.76 | 41.1 | -0.096 | 2.3.1.133 | 细胞质 |
| VvHCT3 | Vitvi01g02214 | Chr1:20566690:20568324 | 1 290 | 429 | 47.97 | 6.87 | 40.5 | -0.139 | 2.3.1.133 | 细胞质 |
| VvHCT4 | Vitvi03g00077 | Chr3:1057145:1058434 | 1 290 | 429 | 47.75 | 6.84 | 42.89 | -0.257 | 2.3.1.133 | 细胞质 |
| VvHCT5 | Vitvi03g01800 | Chr3:16887244:16888554 | 1 311 | 436 | 47.96 | 6.39 | 35.16 | -0.128 | 2.3.1.133 | 细胞质 |
| VvHCT6 | Vitvi03g01816 | Chr3:17430346:17432051 | 1 284 | 427 | 47.51 | 7.6 | 44.02 | -0.171 | 2.3.1.133 | 细胞质 |
| VvHCT7 | Vitvi09g01229 | Chr9:19655320:19657669 | 1 290 | 429 | 47.86 | 6.13 | 47.54 | -0.166 | 2.3.1.133 | 细胞质 |
| VvHCT8 | Vitvi11g00730 | Chr11:8730745:8732578 | 1 338 | 445 | 49.41 | 6.33 | 44.21 | -0.273 | 2.3.1.133 | 细胞质 |
| VvHCT9 | Vitvi11g00735 | Chr11:8783989:8786081 | 1 374 | 457 | 50.73 | 6.51 | 49.25 | -0.224 | 2.3.1.133 | 细胞质 |
| VvHCT10 | Vitvi11g00742 | Chr11:8868521:8869858 | 1 338 | 445 | 49.63 | 7.67 | 46.99 | -0.252 | 2.3.1.133 | 细胞质 |
| VvHCT11 | Vitvi11g01099 | Chr11:15978906:15980276 | 1 371 | 456 | 50.25 | 5.33 | 44.68 | -0.163 | 2.3.1.133 | 细胞质 |
表2 VvHCT基因家族成员理化性质分析
Table 2 Physicochemical properties analysis of VvHCT gene family members
基因名称 Gene name | 基因 ID Gene ID | 染色体位置 Chromosome location | 编码区 CDS/bp | 氨基酸 Amino acid/bp | 分子量 Molecular weigh/kD | 等电点 Theoretical isoelectric points | 不稳定系数 Instability index | 总平均亲水性 Grand average of hydropathicity | KEGG | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|---|---|---|---|
| VvHCT1 | Vitvi01g01514 | Chr1:20494476:20495771 | 1 296 | 431 | 48.45 | 6.04 | 38.66 | -0.018 | 2.3.1.133 | 细胞质 |
| VvHCT2 | Vitvi01g01517 | Chr1:20532349:20533650 | 1 197 | 398 | 44.78 | 5.76 | 41.1 | -0.096 | 2.3.1.133 | 细胞质 |
| VvHCT3 | Vitvi01g02214 | Chr1:20566690:20568324 | 1 290 | 429 | 47.97 | 6.87 | 40.5 | -0.139 | 2.3.1.133 | 细胞质 |
| VvHCT4 | Vitvi03g00077 | Chr3:1057145:1058434 | 1 290 | 429 | 47.75 | 6.84 | 42.89 | -0.257 | 2.3.1.133 | 细胞质 |
| VvHCT5 | Vitvi03g01800 | Chr3:16887244:16888554 | 1 311 | 436 | 47.96 | 6.39 | 35.16 | -0.128 | 2.3.1.133 | 细胞质 |
| VvHCT6 | Vitvi03g01816 | Chr3:17430346:17432051 | 1 284 | 427 | 47.51 | 7.6 | 44.02 | -0.171 | 2.3.1.133 | 细胞质 |
| VvHCT7 | Vitvi09g01229 | Chr9:19655320:19657669 | 1 290 | 429 | 47.86 | 6.13 | 47.54 | -0.166 | 2.3.1.133 | 细胞质 |
| VvHCT8 | Vitvi11g00730 | Chr11:8730745:8732578 | 1 338 | 445 | 49.41 | 6.33 | 44.21 | -0.273 | 2.3.1.133 | 细胞质 |
| VvHCT9 | Vitvi11g00735 | Chr11:8783989:8786081 | 1 374 | 457 | 50.73 | 6.51 | 49.25 | -0.224 | 2.3.1.133 | 细胞质 |
| VvHCT10 | Vitvi11g00742 | Chr11:8868521:8869858 | 1 338 | 445 | 49.63 | 7.67 | 46.99 | -0.252 | 2.3.1.133 | 细胞质 |
| VvHCT11 | Vitvi11g01099 | Chr11:15978906:15980276 | 1 371 | 456 | 50.25 | 5.33 | 44.68 | -0.163 | 2.3.1.133 | 细胞质 |
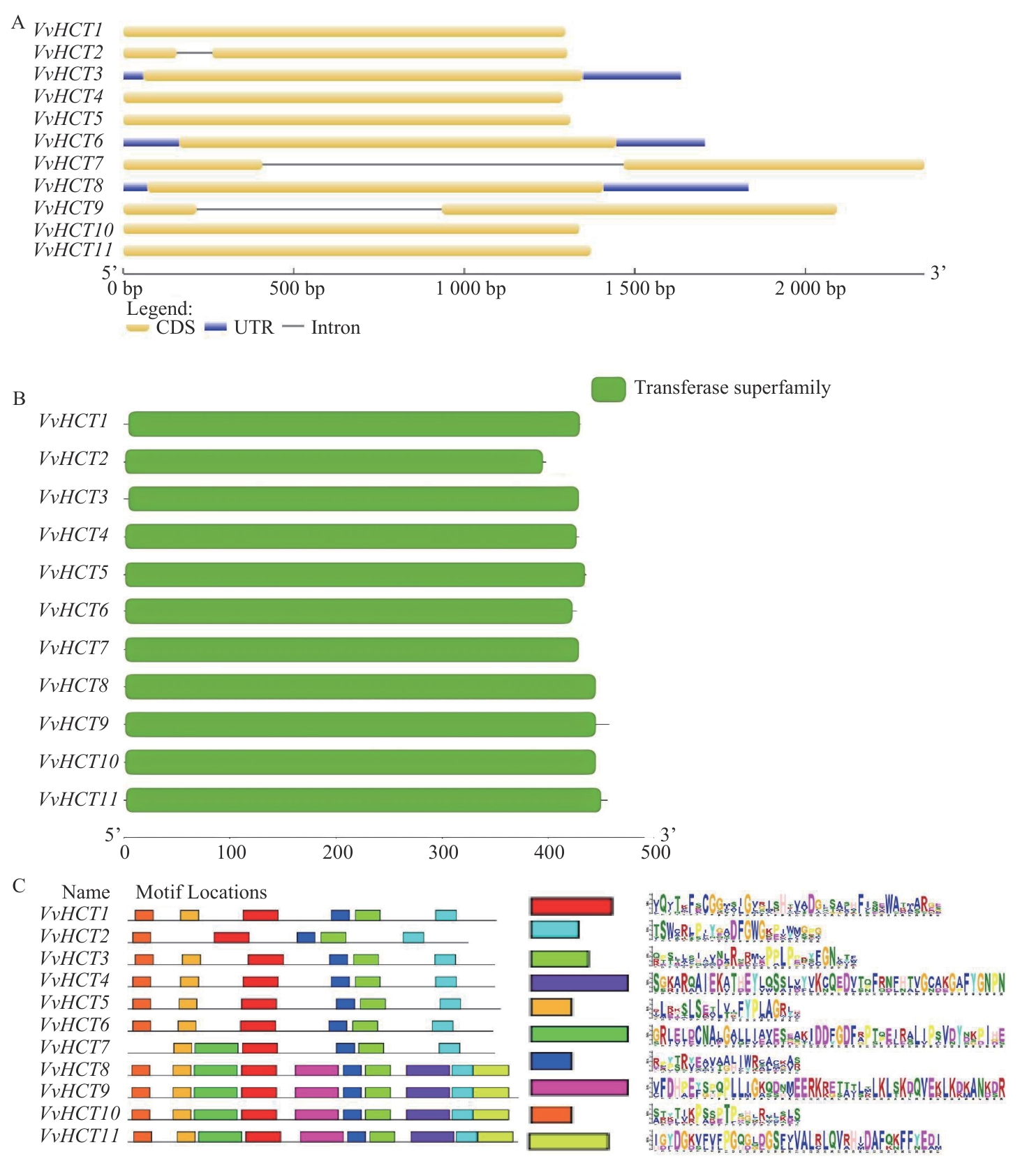
图2 VvHCT基因家族成员的基因结构(A)、保守结构域(B)和保守基序(C)分析
Fig. 2 Analysis of gene structure (A), conserved domains (B), and conserved motifs (C) of VvHCT gene family members

图4 VvHCT基因启动子顺式作用元件分析A:顺式作用元件类型和数量;B:顺式作用元件分布模式
Fig. 4 Analysis of cis-acting elements in VvHCT gene promotersA: Types and quantity of cis-acting elements. B: Distribution pattern of cis-acting elements
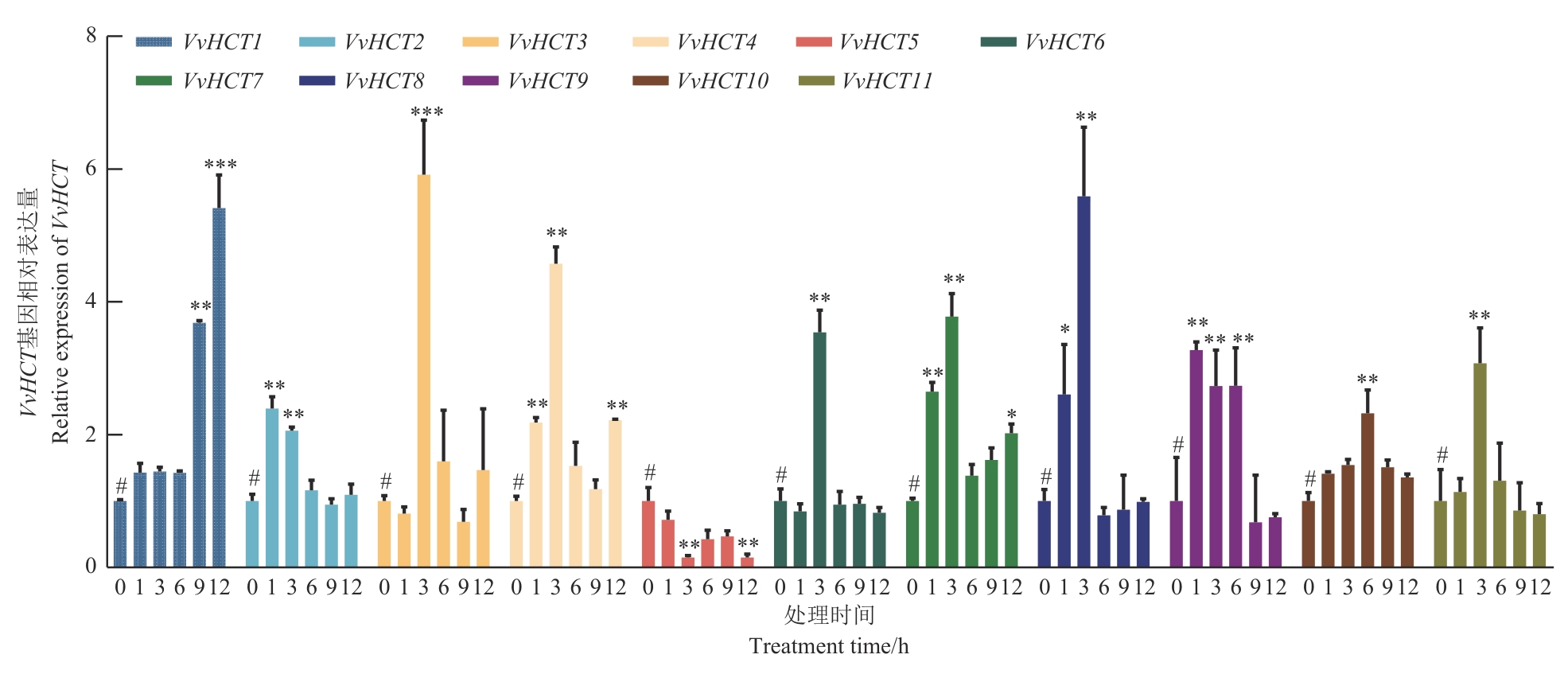
图6 VvHCT基因低温胁迫下表达模式分析与0 h对照相比,*P<0.05,**P<0.01,***P<0.001
Fig. 6 Analysis of the expression patterns of VvHCT genes under low-temperature stressCompared with 0 h control, *P<0.05, **P<0.01, ***P<0.001

图7 pSuper1300-VvHCT8过表达载体的构建A:VvHCT8基因CDS序列PCR产物;B:pSuper1300-VvHCT8转化DH5α菌落PCR;C:pSuper1300-VvHCT8转化GV3101菌落PCR
Fig. 7 Construction of pSuper1300-VvHCT8 over-expression vectorA: PCR product of the VvHCT8 gene CDS sequence. B: Colony PCR of DH5α transformed with pSuper1300-VvHCT8. C: Colony PCR of GV3101 transformed with pSuper1300-VvHCT8

图8 低温胁迫下VvHCT8瞬时过表达对葡萄叶片表型和光合指标的影响A:表型;B:VvHCT8表达量;C:叶绿素含量;D:Fv/Fm;E:ΦPS Ⅱ;*P<0.05,**P<0.01,***P<0.001;#代表对照。下同
Fig. 8 Effects of transient overexpression of VvHCT8 on the leaf phenotype and photosynthetic indexes of grapeA: Phenotype. B: Relative expression of VvHCT8. C: Chlorophyll content. D: Fv/Fm. E: ΦPS Ⅱ. *P<0.05, **P<0.01, ***P<0.001. #indicate control. The same below
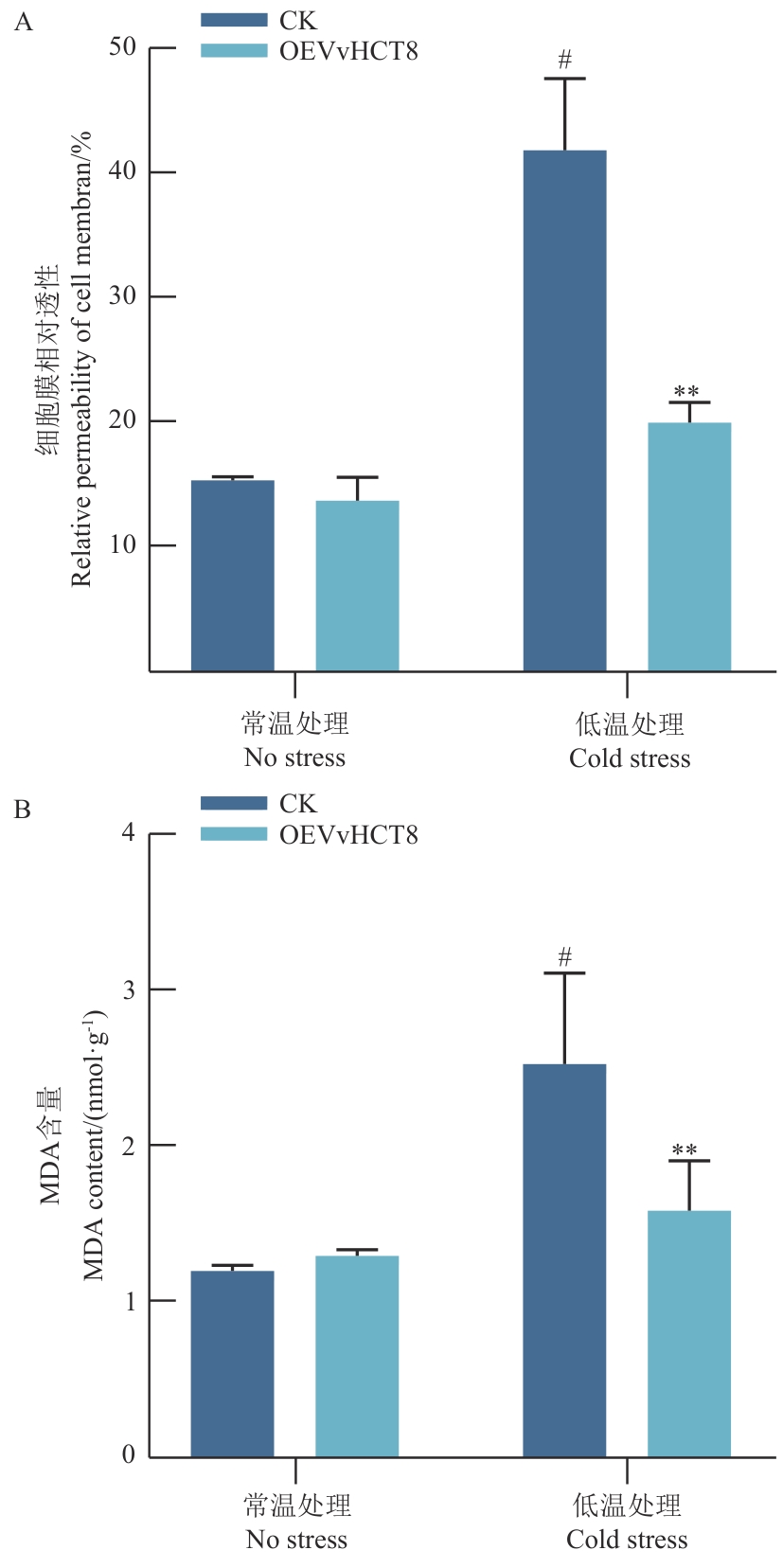
图9 VvHCT8瞬时过表达对葡萄叶片细胞膜相对透性和丙二醛含量的影响
Fig. 9 Effects of transient overexpression of VvHCT8 on relative membrane permeability and malondialdehyde content in grape leaf cells
| 1 | 李阳昱, 李庆蓉, 陈孝红, 等. 绿原酸抗菌作用及机制的研究进展 [J]. 中国抗生素杂志, 2024, 49(2): 141-150. |
| Li YY, Li QR, Chen XH, et al. Advances in research on the antibacterial effects and mechanism of chlorogenic acid [J]. Chin J Antibiot, 2024, 49(2): 141-150. | |
| 2 | 何雅静, 张群琳, 谷利伟, 等. 柑橘中酚酸类化合物及其生物活性与机理的研究进展 [J]. 食品与发酵工业, 2020, 46(15): 301-306. |
| He YJ, Zhang QL, Guli W, et al. Research progress on phenolic acids in citrus and their biological activities and mechanisms [J]. Food Ferment Ind, 2020, 46(15): 301-306. | |
| 3 | 张豫丹, 马晓寒, 李俊领, 等. 绿原酸对烟草疫霉的抑制作用及对烟草黑胫病的防治效果研究 [J]. 作物杂志, 2022(2): 230-236. |
| Zhang YD, Ma XH, Li JL, et al. Inhibitory effect of chlorogenic acid on Phytophthora nicotiana and its control effect on tobacco black shank disease [J]. Crops, 2022(2): 230-236. | |
| 4 | Singh AK, Singla RK, Pandey AK. Chlorogenic acid: a dietary phenolic acid with promising pharmacotherapeutic potential [J]. Curr Med Chem, 2023, 30(34): 3905-3926. |
| 5 | 刘凤之, 王海波, 胡成志. 我国主要果树产业现状及“十四五”发展对策 [J]. 中国果树, 2021(1): 1-5. |
| Liu FZ, Wang HB, Hu CZ. Current situation of main fruit tree industry in China and it's development countermeasure during the “14th five-year plan” period [J]. China Fruits, 2021(1): 1-5. | |
| 6 | Li RL, Xu J, Qi ZX, et al. High-resolution genome mapping and functional dissection of chlorogenic acid production in Lonicera maackii [J]. Plant Physiol, 2023, 192(4): 2902-2922. |
| 7 | Negruk V, Yang P, Subramanian M, et al. Molecular cloning and characterization of the CER2 gene of Arabidopsis thaliana [J]. Plant J, 1996, 9(2): 137-145. |
| 8 | Medison MB, Pan R, Peng Y, et al. Identification of HQT gene family and their potential function in CGA synthesis and abiotic stresses tolerance in vegetable sweet potato [J]. Physiol Mol Biol Plants, 2023, 29(3): 361-376. |
| 9 | Su ZW, Sun M, Cai ZX, et al. Identification and expression analysis of chlorogenic acid biosynthesis key gene PpHCT in peach [J]. Hortic Plant J, 2023, 9(4): 670-680. |
| 10 | Cheevarungnapakul K, Khaksar G, Panpetch P, et al. Identification and functional characterization of genes involved in the biosynthesis of caffeoylquinic acids in sunflower (Helianthus annuus L.) [J]. Front Plant Sci, 2019, 10: 968. |
| 11 | Yang YJ, Cui SQ, Zhang YL, et al. PbHCT4 regulates growth through affecting chlorogenic acid (CGA) content in pear [J]. Sci Hortic, 2022, 303: 111225. |
| 12 | Liu CF, Yang N, Teng RM, et al. Exogenous methyl jasmonate and cytokinin antagonistically regulate lignin biosynthesis by mediating CsHCT expression in Camellia sinensis [J]. Protoplasma, 2023, 260(3): 869-884. |
| 13 | Wang H, Zheng XB, Wu Y, et al. Transcriptome analysis identifies genes associated with chlorogenic acid biosynthesis during apple fruit development [J]. Horticulturae, 2023, 9(2): 217. |
| 14 | Sun CH, Yang CY, Tzen JTC. Molecular identification and characterization of hydroxycinnamoyl transferase in tea plants (Camellia sinensis L.) [J]. Int J Mol Sci, 2018, 19(12): 3938. |
| 15 | Chao N, Qi Q, Li S, et al. Characterization and functional analysis of the hydroxycinnamoyl-CoA: shikimate hydroxycinnamoyl transferase (HCT) gene family in poplar [J]. PeerJ, 2021, 9: e10741. |
| 16 | Zhao L, Wang DJ, Liu J, et al. Transcriptomic analysis of key genes involved in chlorogenic acid biosynthetic pathway and characterization of MaHCT from Morus alba L [J]. Protein Expr Purif, 2019, 156: 25-35. |
| 17 | Park YJ, Kwon DY, Koo SY, et al. Identification of drought-responsive phenolic compounds and their biosynthetic regulation under drought stress in Ligularia fischeri [J]. Front Plant Sci, 2023, 14: 1140509. |
| 18 | Zhou SQ, Chen L, Chen G, et al. Molecular mechanisms through which short-term cold storage improves the nutritional quality and sensory characteristics of postharvest sweet potato tuberous roots: a transcriptomic study [J]. Foods, 2021, 10(9): 2079. |
| 19 | Liu LJ, Pu YY, Niu ZX, et al. Corrigendum: Transcriptomic insights into root development and overwintering transcriptional memory of Brassica rapa L. grown in the field [J] . Front in Plant Sci, 2023, 12(14): 1195912. |
| 20 | Xu JY, Chen Z, Wang FZ, et al. Combined transcriptomic and metabolomic analyses uncover rearranged gene expression and metabolite metabolism in tobacco during cold acclimation [J]. Sci Rep, 2020, 10(1): 5242. |
| 21 | 刘新, 刘洪庆. 植物生理学实验 [M]. 北京: 高等教育出版社, 2017. |
| Liu X, Liu HQ. Plant physiology experiment [M]. Beijing: Higher Education Press, 2017. | |
| 22 | 陈治民, 李翠, 韦继天, 等. 绿原酸生物合成调控及其应用研究进展 [J]. 生物技术通报, 2024, 40(1): 57-71. |
| Chen ZM, Li C, Wei JT, et al. Research progress in the regulation of chlorogenic acid biosynthesis and its application [J]. Biotechnol Bull, 2024, 40(1): 57-71. | |
| 23 | Ma C, Zhang HP, Li JM, et al. Genome-wide analysis and characterization of molecular evolution of the HCT gene family in pear (Pyrus bretschneideri) [J]. Plant Syst Evol, 2017, 303(1): 71-90. |
| 24 | Delporte M, Bernard G, Legrand G, et al. A BAHD neofunctionalization promotes tetrahydroxycinnamoyl spermine accumulation in the pollen coat of the Asteraceae family [J]. J Exp Bot, 2018, 69(22): 5355-5371. |
| 25 | 李濯雪, 陈信波. 植物诱导型启动子及相关顺式作用元件研究进展 [J]. 生物技术通报, 2015, 31(10): 8-15. |
| Li ZX, Chen XB. Research advances on plant inducible promoters and related cis-acting elements [J]. Biotechnol Bull, 2015, 31(10): 8-15. | |
| 26 | Timerbaev V, Dolgov S. Functional characterization of a strong promoter of the early light-inducible protein gene from tomato [J]. Planta, 2019, 250(4): 1307-1323. |
| 27 | Chen YC, Xu N, Du LH, et al. Light plays a critical role in the accumulation of chlorogenic acid in Lonicera macranthoides Hand.-Mazz [J]. Plant Physiol Biochem, 2023, 196: 793-806. |
| 28 | Wang Q, Huang D, Tu WY, et al. Overexpression of auxin/indole-3-acetic acid gene MdIAA24 enhances Glomerella leaf spot resistance in apple (Malus domestica) [J]. Hortic Plant J, 2024, 10(1): 15-24. |
| 29 | Mansouri S, Koushesh Saba M, Sarikhani H. Exogenous melatonin delays strawberry fruit ripening by suppressing endogenous ABA signaling [J]. Sci Rep, 2023, 13(1): 14209. |
| 30 | Jie HD, He PL, Zhao L, et al. Molecular mechanisms regulating phenylpropanoid metabolism in exogenously-sprayed ethylene forage ramie based on transcriptomic and metabolomic analyses [J]. Plants, 2023, 12(22): 3899. |
| 31 | Gusain S, Joshi S, Joshi R. Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants [J]. Plant Physiol Biochem, 2023, 197: 107646. |
| 32 | Chen YF, Yi N, Yao SB, et al. Cs HCT-mediated lignin synthesis pathway involved in the response of tea plants to biotic and abiotic stresses [J]. J Agric Food Chem, 2021, 69(35): 10069-10081. |
| [1] | 杜品廷, 吴国江, 王振国, 李岩, 周伟, 周亚星. 高粱CPP基因家族鉴定及表达分析[J]. 生物技术通报, 2025, 41(1): 132-142. |
| [2] | 王子傲, 田瑞, 崔永梅, 白羿雄, 姚晓华, 安立昆, 吴昆仑. 青稞HvnJAZ4的生物信息学和表达模式分析[J]. 生物技术通报, 2025, 41(1): 173-185. |
| [3] | 孔青洋, 张晓龙, 李娜, 张晨洁, 张雪云, 于超, 张启翔, 罗乐. 单叶蔷薇GRAS转录因子家族鉴定及表达分析[J]. 生物技术通报, 2025, 41(1): 210-220. |
| [4] | 文静, 李倩倩, 张明达, 谭茗月, 金博阳, 沈秀丽, 杜志强. Duox 2调控克氏原螯虾肠组织抗细菌先天免疫的分子机制[J]. 生物技术通报, 2025, 41(1): 324-332. |
| [5] | 袁兰, 黄娅楠, 张贝妮, 熊雨萌, 王洪洋. 基于流式细胞仪鉴定马铃薯倍性的高通量样品制备方法[J]. 生物技术通报, 2024, 40(9): 141-147. |
| [6] | 谭博文, 张懿, 张鹏, 王振宇, 马秋香. 木薯镁离子转运蛋白家族基因的鉴定及生物信息学分析[J]. 生物技术通报, 2024, 40(9): 20-32. |
| [7] | 满全财, 孟姿诺, 李伟, 蔡心汝, 苏润东, 付长青, 高顺娟, 崔江慧. 马铃薯AQP基因家族鉴定及表达分析[J]. 生物技术通报, 2024, 40(9): 51-63. |
| [8] | 吴娟, 武小娟, 王沛捷, 谢锐, 聂虎帅, 李楠, 马艳红. 彩色马铃薯花青素合成相关ERF基因筛选及表达分析[J]. 生物技术通报, 2024, 40(9): 82-91. |
| [9] | 宋兵芳, 柳宁, 程新艳, 徐晓斌, 田文茂, 高悦, 毕阳, 王毅. 马铃薯G6PDH基因家族鉴定及其在损伤块茎的表达分析[J]. 生物技术通报, 2024, 40(9): 104-112. |
| [10] | 车建美, 赖恭梯, 李思雨, 郭奥琳, 陈冰星, 陈杏, 刘波, 赖呈纯. 复合微生物菌剂对葡萄生长、品质及根际土壤环境的影响[J]. 生物技术通报, 2024, 40(8): 264-274. |
| [11] | 周冉, 王兴平, 李彦霞, 罗仍卓么. 金黄色葡萄球菌型乳房炎奶牛乳腺组织的lncRNA差异表达分析[J]. 生物技术通报, 2024, 40(8): 320-328. |
| [12] | 武帅, 辛燕妮, 买春海, 穆晓娅, 王敏, 岳爱琴, 赵晋忠, 吴慎杰, 杜维俊, 王利祥. 大豆GS基因家族全基因组鉴定及胁迫响应分析[J]. 生物技术通报, 2024, 40(8): 63-73. |
| [13] | 杨巍, 赵丽芬, 唐兵, 周麟笔, 杨娟, 莫传园, 张宝会, 李飞, 阮松林, 邓英. 芥菜SRO基因家族全基因组鉴定与表达分析[J]. 生物技术通报, 2024, 40(8): 129-141. |
| [14] | 周麟, 黄顺满, 苏文坤, 姚响, 屈燕. 滇山茶bHLH基因家族鉴定及花色形成相关基因筛选[J]. 生物技术通报, 2024, 40(8): 142-151. |
| [15] | 张明亚, 庞胜群, 刘玉东, 苏永峰, 牛博文, 韩琼琼. 番茄FAD基因家族的鉴定与表达分析[J]. 生物技术通报, 2024, 40(7): 150-162. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||