








生物技术通报 ›› 2025, Vol. 41 ›› Issue (5): 186-196.doi: 10.13560/j.cnki.biotech.bull.1985.2024-1174
• 研究报告 • 上一篇
刘鑫1,2( ), 王嘉雯2, 李进伟2,3, 牟策1,2, 杨盼盼2, 明军2, 徐雷锋2(
), 王嘉雯2, 李进伟2,3, 牟策1,2, 杨盼盼2, 明军2, 徐雷锋2( )
)
收稿日期:2024-12-05
出版日期:2025-05-26
发布日期:2025-06-05
通讯作者:
徐雷锋,男,博士,副研究员,研究方向 :百合种质资源与遗传育种 ;E-mail: xuleifeng@caas.cn作者简介:刘鑫,女,硕士研究生,研究方向 :百合种质资源与遗传育种 ;E-mail: 14763795646@163.com基金资助:
LIU Xin1,2( ), WANG Jia-wen2, LI Jin-wei2,3, MOU Ce1,2, YANG Pan-pan2, MING Jun2, XU Lei-feng2(
), WANG Jia-wen2, LI Jin-wei2,3, MOU Ce1,2, YANG Pan-pan2, MING Jun2, XU Lei-feng2( )
)
Received:2024-12-05
Published:2025-05-26
Online:2025-06-05
摘要:
目的 B-box(BBX)第Ⅳ亚组的许多成员在光调控花青素苷合成中扮演着重要角色,克隆兰州百合BBX基因并分析其对不同光照时间的应答及对鳞片变红的影响,为培育见光不变色兰州百合品种提供重要的候选基因。 方法 克隆从兰州百合转录组鉴定获得的3个BBX第Ⅳ亚组成员(LdBBX21、LdBBX22、LdBBX24),并对其编码的蛋白进行生物信息学分析和亚细胞定位,基于实时荧光定量PCR研究其在兰州百合不同组织及鳞片光处理后的表达模式。 结果 LdBBX21、LdBBX22、LdBBX24均含有2个B-box保守结构域,属于第Ⅳ亚组的BBX蛋白。亚细胞定位结果显示,LdBBX21、LdBBX22、LdBBX24均定位于细胞核。组织特异性表达分析表明,LdBBX22和LdBBX24在花中的表达量最高,在鳞片中的表达量最低,LdBBX21与之相反。RT-qPCR分析表明,在不同光照时长处理的鳞片中,LdBBX21的基因表达量随光照时长的增加总体呈下降趋势;LdBBX22的基因表达量随光照时长的增加而上升,与花青素苷含量的变化趋势基本相同;LdBBX24随光照时长的增加,表达量总体呈先上升后下降趋势。 结论 LdBBX21、LdBBX22、LdBBX24均可能在光调控兰州百合鳞片花青素苷合成中起着重要功能,本研究为后续研究兰州百合BBX基因功能验证奠定基础。
刘鑫, 王嘉雯, 李进伟, 牟策, 杨盼盼, 明军, 徐雷锋. 兰州百合三个LdBBXs基因的克隆与表达分析[J]. 生物技术通报, 2025, 41(5): 186-196.
LIU Xin, WANG Jia-wen, LI Jin-wei, MOU Ce, YANG Pan-pan, MING Jun, XU Lei-feng. Cloning and Expression Analysis of Three LdBBXs in Lilium davidii var. willmottiae[J]. Biotechnology Bulletin, 2025, 41(5): 186-196.
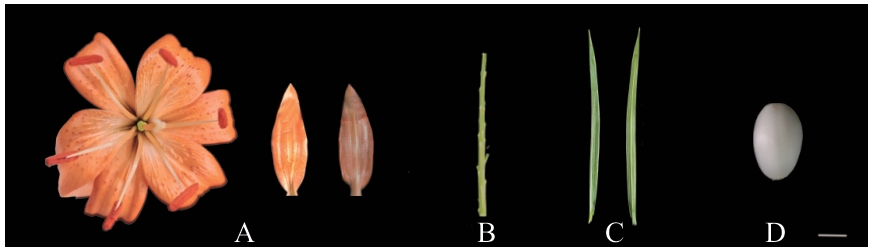
图1 兰州百合的不同组织A:花和内花被片;B:茎;C:叶;D:鳞片。标尺=1 cm
Fig. 1 Different tissues of L. davidii var. willmottiaeA: Flower and inner tepal. B: Stem. C: Leaf. D: Scale. The scale is 1 cm
| 引物 Primer | 序列 Sequence (5′‒3′) | 退火温度 Annealing temperature (℃) |
|---|---|---|
| LdBBX21-F | GGAGCCAGATCGGAGAG | 55.5 |
| LdBBX21-R | GCTTGAGGAAACAGAAAGAGG | |
| LdBBX22-F | GAGAGAGAGGAGAGAGAGA | 51.5 |
| LdBBX22-R | TCGACCCATAATAACACATC | |
| LdBBX24-F | GGGGATTGTCCAACTATATCCC | 54.8 |
| LdBBX24-R | TTCTCCGTTCATGTTTTGTGC | |
| LdBBX21-qRT-F | CTCCATCCACAGGGCCAAC | 59.0 |
| LdBBX21-qRT-R | CAAACCGTCGAGGATGAGACG | |
| LdBBX22-qRT-F | GGAGGCGACGGTGATGTG | 60.0 |
| LdBBX22-qRT-R | CGGAAGCGTTGCCAGAGATT | |
| LdBBX24-qRT-F | CAACACAGCAGTCGCCTTC | 57.5 |
| LdBBX24-qRT-R | CCACCAGAAAACTCCTTCTGC | |
| LilyActin-F | GCACCTGAAGAGCACCCT | 60.0 |
| LilyActin-R | TGGCGTAAAGCGACAAAA | |
| LdBBX21-2300-F | ATTTGGAGAGGACAGGGTACGGAGCCAGATCGGAGAG | 55.5 |
| LdBBX21-2300-R | CACCATGGTACTAGTGTCGAGCTTGAGGAAACAGAAAGAGG | |
| LdBBX22-2300-F | ATTTGGAGAGGACAGGGTAC AGAGAGAGATCTTGTCGGC | 55.0 |
| LdBBX22-2300-R | CACCATGGTACTAGTGTCGACATACTGATAGACATGTACAGCAG | |
| LdBBX24-2300-F | ATTTGGAGAGGACAGGGTACGGGGATTGTCCAACTATATCCC | 54.8 |
| LdBBX24-2300-R | CACCATGGTACTAGTGTCGATTCTCCGTTCATGTTTTGTGC | |
| Detect-2300-F | ATTTGGAGAGGACAGGGTAC | 55.0 |
| Detect-2300-R | CACCATGGTACTAGTGTCGA |
表1 所用引物信息
Table 1 Information of the primers used in this study
| 引物 Primer | 序列 Sequence (5′‒3′) | 退火温度 Annealing temperature (℃) |
|---|---|---|
| LdBBX21-F | GGAGCCAGATCGGAGAG | 55.5 |
| LdBBX21-R | GCTTGAGGAAACAGAAAGAGG | |
| LdBBX22-F | GAGAGAGAGGAGAGAGAGA | 51.5 |
| LdBBX22-R | TCGACCCATAATAACACATC | |
| LdBBX24-F | GGGGATTGTCCAACTATATCCC | 54.8 |
| LdBBX24-R | TTCTCCGTTCATGTTTTGTGC | |
| LdBBX21-qRT-F | CTCCATCCACAGGGCCAAC | 59.0 |
| LdBBX21-qRT-R | CAAACCGTCGAGGATGAGACG | |
| LdBBX22-qRT-F | GGAGGCGACGGTGATGTG | 60.0 |
| LdBBX22-qRT-R | CGGAAGCGTTGCCAGAGATT | |
| LdBBX24-qRT-F | CAACACAGCAGTCGCCTTC | 57.5 |
| LdBBX24-qRT-R | CCACCAGAAAACTCCTTCTGC | |
| LilyActin-F | GCACCTGAAGAGCACCCT | 60.0 |
| LilyActin-R | TGGCGTAAAGCGACAAAA | |
| LdBBX21-2300-F | ATTTGGAGAGGACAGGGTACGGAGCCAGATCGGAGAG | 55.5 |
| LdBBX21-2300-R | CACCATGGTACTAGTGTCGAGCTTGAGGAAACAGAAAGAGG | |
| LdBBX22-2300-F | ATTTGGAGAGGACAGGGTAC AGAGAGAGATCTTGTCGGC | 55.0 |
| LdBBX22-2300-R | CACCATGGTACTAGTGTCGACATACTGATAGACATGTACAGCAG | |
| LdBBX24-2300-F | ATTTGGAGAGGACAGGGTACGGGGATTGTCCAACTATATCCC | 54.8 |
| LdBBX24-2300-R | CACCATGGTACTAGTGTCGATTCTCCGTTCATGTTTTGTGC | |
| Detect-2300-F | ATTTGGAGAGGACAGGGTAC | 55.0 |
| Detect-2300-R | CACCATGGTACTAGTGTCGA |

图2 LdBBX21、LdBBX22、LdBBX24扩增电泳图及其翻译的氨基酸序列A、C、E:LdBBX21、LdBBX22、LdBBX24扩增电泳图;B、D、F:LdBBX21、LdBBX22、LdBBX24的CDS序列及其翻译的氨基酸序列
Fig. 2 LdBBX21, LdBBX22, and LdBBX24 amplification electropherogram and their translated amino acid sequencesA, C, E: Amplification electropherogram of LdBBX21, LdBBX22, LdBBX24; B, D, F: CDS sequence of LdBBX21, LdBBX22, LdBBX24 and its translated amino acid sequence
| 蛋白 Protein | 分子式 Molecular formula | 分子量 Molecular weight (kD) | 等电点 pI | 不稳定系数 Instability index |
|---|---|---|---|---|
| LdBBX21 | C948H1477N273O303S14 | 22.00 | 5.87 | 60.39 |
| LdBBX22 | C1425H2185N393O456S18 | 32.70 | 5.28 | 61.55 |
| LdBBX24 | C1151H1792N312O357S19 | 26.32 | 4.91 | 60.48 |
表2 LdBBX21、LdBBX22、LdBBX24蛋白基本理化性质分析结果
Table 2 Basic physicochemical properties of LdBBX21, LdBBX22, and LdBBX24 proteins
| 蛋白 Protein | 分子式 Molecular formula | 分子量 Molecular weight (kD) | 等电点 pI | 不稳定系数 Instability index |
|---|---|---|---|---|
| LdBBX21 | C948H1477N273O303S14 | 22.00 | 5.87 | 60.39 |
| LdBBX22 | C1425H2185N393O456S18 | 32.70 | 5.28 | 61.55 |
| LdBBX24 | C1151H1792N312O357S19 | 26.32 | 4.91 | 60.48 |
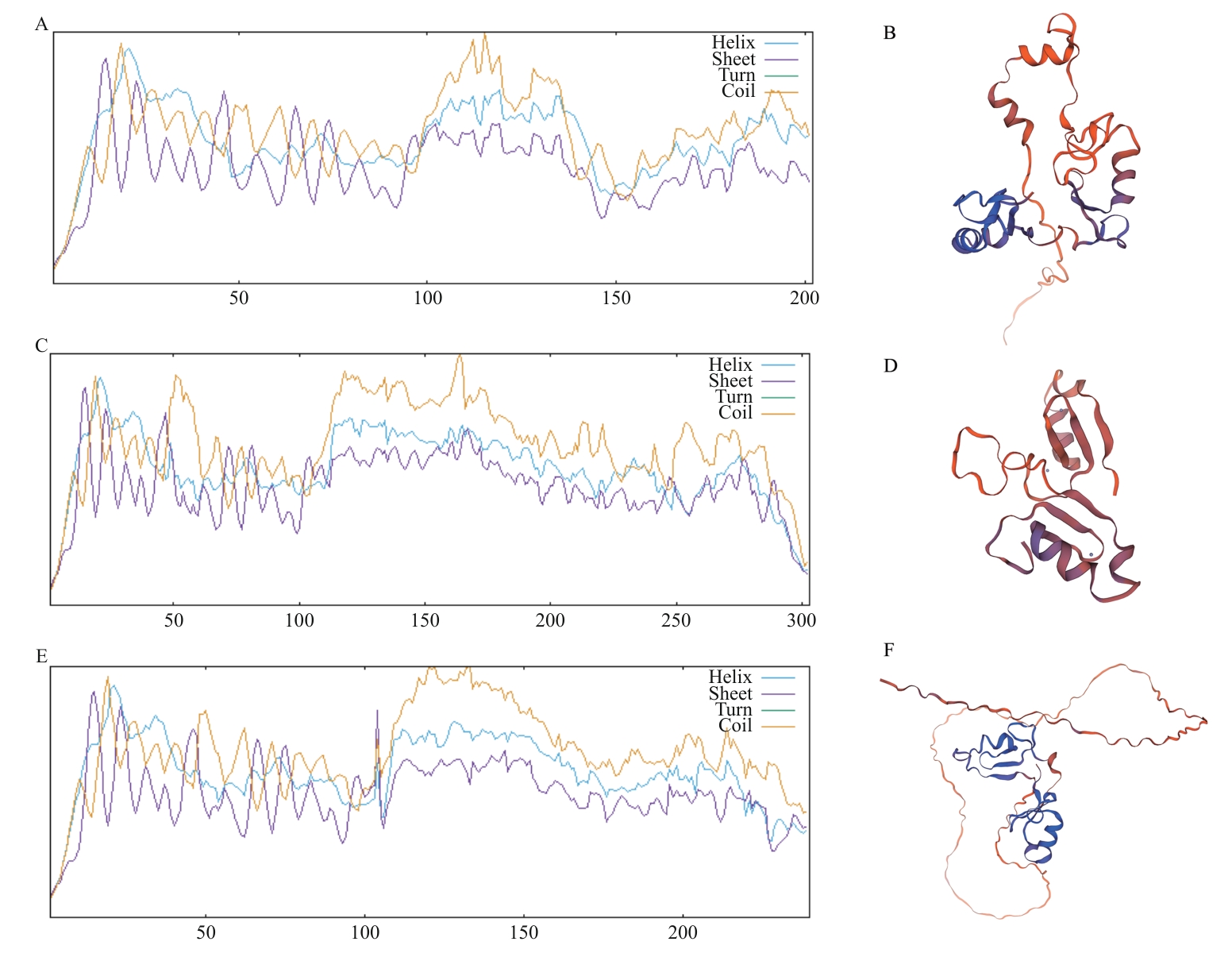
图4 LdBBX21、LdBBX22、LdBBX24蛋白二、三级结构预测A、C、E:LdBBX21、LdBBX22、LdBBX24蛋白的二级结构预测;B、D、F:LdBBX21、LdBBX22、LdBBX24蛋白的三级结构预测
Fig. 4 Prediction of secondary and tertiary structures of LdBBX21, LdBBX22, and LdBBX24 proteinsA, C, E: Secondary structure prediction of LdBBX21, LdBBX22, and LdBBX24 protein. B, D, F: Tertiary structure prediction of LdBBX21, LdBBX22, and LdBBX24 protein
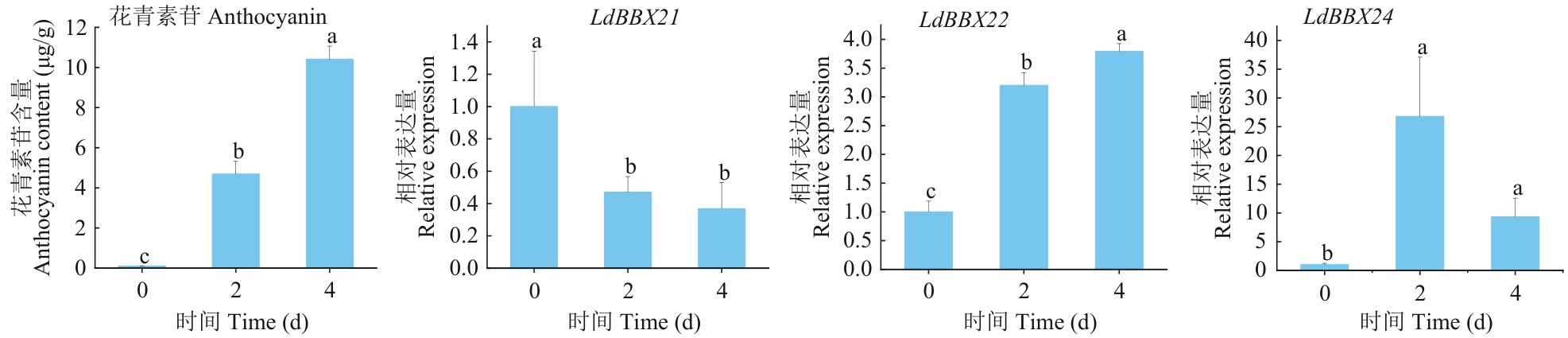
图9 光照处理0、2、4 d下LdBBX21、LdBBX22、LdBBX24基因的相对表达量及花青素苷含量变化不同字母表示在P<0.05水平差异显著。下同
Fig. 9 Relative expressions and anthocyanin contents of LdBBX21, LdBBX22, and LdBBX24 genes under light treatment for 0, 2, and 4 dDifferent letters above columns indicate significant differences at P<0.05 level. The same below
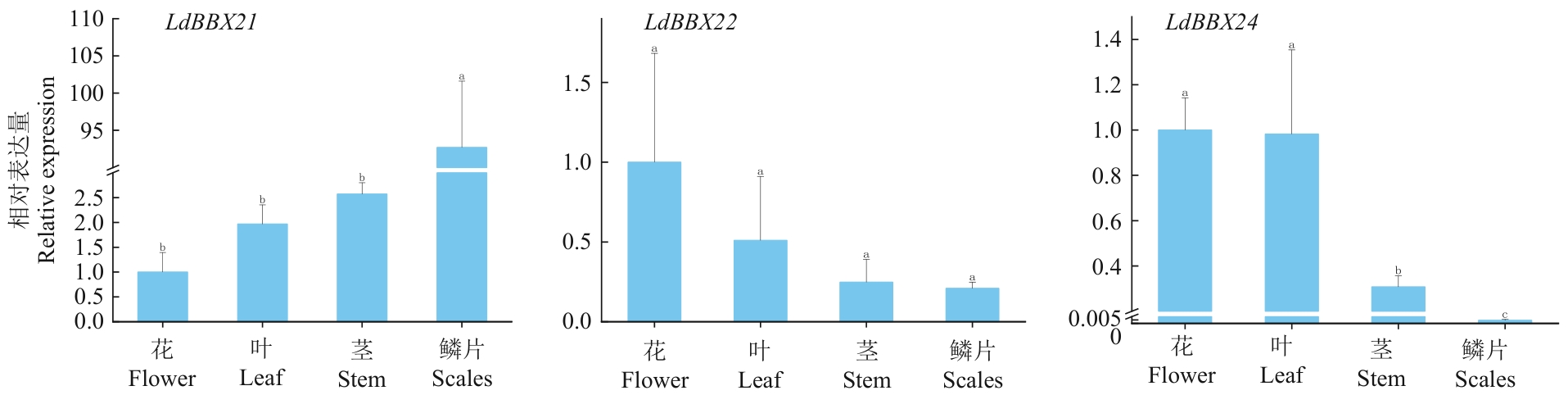
图10 兰州百合不同组织中LdBBX21、LdBBX22、LdBBX24基因的表达
Fig. 10 Expressions of LdBBX21, LdBBX22, and LdBBX24 genes in different tissues of L. davidii var. willmottiae
| 1 | 徐丽, 朱田田, 晋玲, 等. 基于感官评价和化学计量学的兰州百合品质分析 [J]. 食品工业科技, 2024, 45(18): 219-227. |
| Xu L, Zhu TT, Jin L, et al. Comprehensive evaluation of Lilium davidii var. willmottiae (E. H. Wilson) Raffill quality based on sensory evaluation and chemometrics [J]. Sci Technol Food Ind, 2024, 45(18): 219-227. | |
| 2 | 杨慧勤, 王佳丽, 李思蕤, 等. 茄科蔬菜花青素苷分子调控研究进展 [J]. 生物工程学报, 2022, 38(5): 1738-1752. |
| Yang HQ, Wang JL, Li SR, et al. Advances in the molecular regulation of anthocyanins in solanaceous vegetables [J]. Chin J Biotechnol, 2022, 38(5): 1738-1752. | |
| 3 | 范文广, 柴佳靖, 李保豫, 等. 百合花青苷分子调控研究进展 [J]. 植物遗传资源学报, 2023, 24(5): 1236-1247. |
| Fan WG, Chai JJ, Li BY, et al. Advances in molecular regulation of anthocyanin biosynthesis in Lilium [J]. J Plant Genet Resour, 2023, 24(5): 1236-1247. | |
| 4 | Fan WG, Bai P, Chen RR, et al. Postharvest light irradiation induces anthocyanin accumulation in fresh-cut lily Bulb (Lilium davidii var. unicolor) scales [J]. J Food Process Preserv, 2024, 2024(1): 7984106. |
| 5 | 毕蒙蒙, 曹雨薇, 宋蒙, 等. 百合花色研究进展 [J]. 园艺学报, 2021, 48(10): 2073-2086. |
| Bi MM, Cao YW, Song M, et al. Advances in flower color research of Lilium [J]. Acta Hortic Sin, 2021, 48(10): 2073-2086. | |
| 6 | 路喻丹, 刘晓驰, 冯新, 等. 猕猴桃BBX基因家族成员鉴定与转录特征分析 [J]. 生物技术通报, 2024, 40(2): 172-182. |
| Lu YD, Liu XC, Feng X, et al. Identification of the kiwifruit BBX gene family and analysis of their transcriptional characteristics [J]. Biotechnol Bull, 2024, 40(2): 172-182. | |
| 7 | Gangappa SN, Botto JF. The BBX family of plant transcription factors [J]. Trends Plant Sci, 2014, 19(7): 460-470. |
| 8 | Zou ZY, Wang RH, Wang R, et al. Genome-wide identification, phylogenetic analysis, and expression profiling of the BBX family genes in pear [J]. J Hortic Sci Biotechnol, 2018, 93(1): 37-50. |
| 9 | Yadav A, Ravindran N, Singh D, et al. Role of Arabidopsis BBX proteins in light signaling [J]. J Plant Biochem Biotechnol, 2020, 29(4): 623-635. |
| 10 | Bursch K, Niemann ET, Nelson DC, et al. Karrikins control seedling photomorphogenesis and anthocyanin biosynthesis through a HY5-BBX transcriptional module [J]. Plant J, 2021, 107(5): 1346-1362. |
| 11 | Yadukrishnan P, Job N, Johansson H, et al. Opposite roles of group IV BBX proteins: exploring missing links between structural and functional diversity [J]. Plant Signal Behav, 2018: e1462641. |
| 12 | Sarmiento F. The BBX subfamily IV: additional cogs and sprockets to fine-tune light-dependent development [J]. Plant Signal Behav, 2013, 8(4): e23831. |
| 13 | Cao J, Yuan JL, Zhang YL, et al. Multi-layered roles of BBX proteins in plant growth and development [J]. Stress Biol, 2023, 3(1): 1. |
| 14 | Bursch K, Toledo-Ortiz G, Pireyre M, et al. Identification of BBX proteins as rate-limiting cofactors of HY5 [J]. Nat Plants, 2020, 6(8): 921-928. |
| 15 | Gangappa SN, Crocco CD, Johansson H, et al. The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis [J]. Plant Cell, 2013, 25(4): 1243-1257. |
| 16 | Xu DQ, Jiang Y, Li J, et al. The B-box domain protein BBX21 promotes photomorphogenesis [J]. Plant Physiol, 2018, 176(3): 2365-2375. |
| 17 | Crocco CD, Holm M, Yanovsky MJ, et al. AtBBX21 and COP1 genetically interact in the regulation of shade avoidance [J]. Plant J, 2010, 64(4): 551-562. |
| 18 | Podolec R, Wagnon TB, Leonardelli M, et al. Arabidopsis B-box transcription factors BBX20-22 promote UVR8 photoreceptor-mediated UV-B responses [J]. Plant J, 2022, 111(2): 422-439. |
| 19 | Zhang B, Zhu ZZ, Qu D, et al. MdBBX21, a B-box protein, positively regulates light-induced anthocyanin accumulation in apple peel [J]. Front Plant Sci, 2021, 12: 774446. |
| 20 | Xie YX, Miao TT, Lyu SH, et al. Arabidopsis ERD15 regulated by BBX24 plays a positive role in UV-B signaling [J]. Plant Sci, 2024, 343: 112077. |
| 21 | Singh A, Ram H, Abbas N, et al. Molecular interactions of GBF1 with HY5 and HYH proteins during light-mediated seedling development in Arabidopsis thaliana [J]. J Biol Chem, 2012, 287(31): 25995-26009. |
| 22 | Job N, Yadukrishnan P, Bursch K, et al. Two B-box proteins regulate photomorphogenesis by oppositely modulating HY5 through their diverse C-terminal domains [J]. Plant Physiol, 2018, 176(4): 2963-2976. |
| 23 | Jakoby M, Weisshaar B, Dröge-Laser W, et al. bZIP transcription factors in Arabidopsis [J]. Trends Plant Sci, 2002, 7(3): 106-111. |
| 24 | Bai SL, Saito T, Honda C, et al. An apple B-box protein, MdCOL11, is involved in UV-B- and temperature-induced anthocyanin biosynthesis [J]. Planta, 2014, 240(5): 1051-1062. |
| 25 | Fang HC, Dong YH, Yue XX, et al. The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature [J]. Plant Cell Environ, 2019, 42(7): 2090-2104. |
| 26 | An JP, Wang XF, Zhang XW, et al. MdBBX22 regulates UV-B-induced anthocyanin biosynthesis through regulating the function of MdHY5 and is targeted by MdBT2 for 26S proteasome-mediated degradation [J]. Plant Biotechnol J, 2019, 17(12): 2231-2233. |
| 27 | Zhang B, Yang HJ, Qu D, et al. The MdBBX22-miR858-MdMYB9/11/12 module regulates proanthocyanidin biosynthesis in apple peel [J]. Plant Biotechnol J, 2022, 20(9): 1683-1700. |
| 28 | Bai SL, Tao RY, Yin L, et al. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit [J]. Plant J, 2019, 100(6): 1208-1223. |
| 29 | 曹雨薇, 徐雷锋, 杨盼盼, 等. 百合花青素苷呈色类型中3种R2R3-MYBs基因的差异表达 [J]. 园艺学报, 2019, 46(5): 955-963. |
| Cao YW, Xu LF, Yang PP, et al. Differential expression of three R2R3-MYBs genes regulating anthocyanin pigmentation patterns in Lilium spp [J]. Acta Hortic Sin, 2019, 46(5): 955-963. | |
| 30 | Chang CJ, Maloof JN, Wu SH. COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis [J]. Plant Physiol, 2011, 156(1): 228-239. |
| 31 | Xu DQ, Jiang Y, Li JG, et al. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation [J]. Proc Natl Acad Sci U S A, 2016, 113(27): 7655-7660. |
| 32 | Lyu GZ, Li DB, Li SS. Bioinformatics analysis of BBX family genes and its response to UV-B in Arabidopsis thaliana [J]. Plant Signal Behav, 2020, 15(9): 1782647. |
| 33 | Huang YW, Xiong H, Xie YX, et al. BBX24 interacts with DELLA to regulate UV-B-induced photomorphogenesis in Arabidopsis thaliana [J]. Int J Mol Sci, 2022, 23(13): 7386. |
| 34 | Gangappa SN, Holm M, Botto JF. Molecular interactions of BBX24 and BBX25 with HYH, HY5 HOMOLOG, to modulate Arabidopsis seedling development [J]. Plant Signal Behav, 2013, 8(8): e25208. |
| 35 | Hao XL, Zhong YJ, Tzmann HN, et al. Light-induced artemisinin biosynthesis is regulated by the bZIP transcription factor AaHY5 in Artemisia annua [J]. Plant Cell Physiol, 2019, 60(8): 1747-1760. |
| 36 | He WZ, Liu H, Wu Z, et al. The AaBBX21-AaHY5 module mediates light-regulated artemisinin biosynthesis in Artemisia annua L [J]. J Integr Plant Biol, 2024, 66(8): 1735-1751. |
| 37 | Yang GQ, Zhang CL, Dong HX, et al. Activation and negative feedback regulation of SlHY5 transcription by the SlBBX20/21-SlHY5 transcription factor module in UV-B signaling [J]. Plant Cell, 2022, 34(5): 2038-2055. |
| 38 | Li SR, Ou CQ, Wang F, et al. Ppbbx24-del mutant positively regulates light-induced anthocyanin accumulation in the ‘Red Zaosu’ pear (Pyrus pyrifolia White Pear Group) [J]. J Integr Agric, 2024. . |
| [1] | 李志强, 罗正乾, 徐琳黎, 周国慧, 屈丝雨, 刘恩良, 顼东婷. 基于T2T基因组鉴定大豆R2R3-MYB基因家族及干旱和盐胁迫下的表达分析[J]. 生物技术通报, 2025, 41(5): 141-152. |
| [2] | 赵婧, 郭茜, 李睿琦, 雷滢炀, 岳爱琴, 赵晋忠, 殷丛丛, 杜维俊, 牛景萍. 大豆GmGST基因簇基因序列分析及诱导表达分析[J]. 生物技术通报, 2025, 41(5): 129-140. |
| [3] | 刘涛, 王志淇, 吴文博, 石文婷, 王超楠, 杜崇, 杨中敏. 马铃薯GRAM基因家族鉴定与表达分析[J]. 生物技术通报, 2025, 41(4): 145-155. |
| [4] | 孙天国, 衣兰, 秦旭洋, 乔梦雪, 谷新颖, 韩艺, 沙伟, 张梅娟, 马天意. 大白菜DABB基因家族的全基因组鉴定及盐碱胁迫下的表达分析[J]. 生物技术通报, 2025, 41(4): 156-165. |
| [5] | 王田田, 常雪瑞, 黄婉洋, 黄嘉欣, 苗如意, 梁燕平, 王静. 辣椒GASA基因家族的鉴定及分析[J]. 生物技术通报, 2025, 41(4): 166-175. |
| [6] | 黄金恒, 黄茜, 张家燕, 周新裕, 廖沛然, 杨全. 广金钱草C3H基因家族鉴定及不同品种表达分析[J]. 生物技术通报, 2025, 41(4): 243-255. |
| [7] | 班秋艳, 赵鑫月, 迟文静, 黎俊生, 王琼, 夏瑶, 梁丽云, 贺巍, 李叶云, 赵广山. 茶树光敏色素互作因子CsPIF3a的克隆及其与光温逆境的响应[J]. 生物技术通报, 2025, 41(4): 256-265. |
| [8] | 王琛, 刘国梅, 陈畅, 张晋龙, 姚琳, 孙璇, 杜春芳. 白菜型油菜CCDs家族全基因组鉴定及表达分析[J]. 生物技术通报, 2025, 41(3): 161-170. |
| [9] | 彭婷, 林颖, 谭圆圆, 饶英, 黄覃, 张文娥, 汪波, 田瑞丰, 刘国锋. 多星韭AwANSs基因的克隆与表达分析[J]. 生物技术通报, 2025, 41(3): 230-239. |
| [10] | 马天意, 许家佳, 路文婧, 吴艳, 沙伟, 张梅娟, 彭疑芳. ‘金小童’大白菜BrcGASA3基因在盐碱胁迫下的表达分析及抗性鉴定[J]. 生物技术通报, 2025, 41(2): 127-138. |
| [11] | 许圆梦, 毛娇, 王梦瑶, 王数, 任江陵, 刘宇涵, 刘思辰, 乔治军, 王瑞云, 曹晓宁. 糜子PmDEP1和PmEP3基因的克隆与表达特征分析[J]. 生物技术通报, 2025, 41(2): 150-162. |
| [12] | 贾子健, 王宝强, 陈立飞, 王义真, 魏小红, 赵颖. 响应NO的藜麦CHX基因家族在盐碱胁迫下的表达模式[J]. 生物技术通报, 2025, 41(2): 163-174. |
| [13] | 焦小雨, 吴琼, 刘丹丹, 孙明慧, 阮旭, 王雷刚, 王文杰. 茶树CsWAK8克隆及其在响应冷胁迫过程中的功能分析[J]. 生物技术通报, 2025, 41(2): 210-220. |
| [14] | 钱政毅, 吴绍芳, 曹舒怡, 宋雅欣, 潘鑫峰, 李兆伟, 范凯. 睡莲NAC转录因子的鉴定及其表达分析[J]. 生物技术通报, 2025, 41(2): 234-247. |
| [15] | 向春繁, 李勒松, 王娟, 梁艳丽, 杨生超, 栗孟飞, 赵艳. 当归肉桂醇脱氢酶AsCAD功能鉴定及表达分析[J]. 生物技术通报, 2025, 41(2): 295-308. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||