








生物技术通报 ›› 2025, Vol. 41 ›› Issue (11): 134-142.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0223
• 未来食品工程专题 • 上一篇
收稿日期:2025-03-04
出版日期:2025-11-26
发布日期:2025-12-09
通讯作者:
崔文璟,男,副教授,研究方向 :微生物合成生物学和蛋白质工程;E-mail: wjcui@jiangnan.edu.cn作者简介:叶妍,女,硕士研究生,研究方向 :生物催化和酶工程;E-mail: 1815143307@qq.com
基金资助:
YE Yan( ), WU Yu-xuan, ZHOU Zhe-min, CUI Wen-jing(
), WU Yu-xuan, ZHOU Zhe-min, CUI Wen-jing( )
)
Received:2025-03-04
Published:2025-11-26
Online:2025-12-09
摘要:
目的 通过挖掘高性能转氨酶和引入丙酮酸代谢酶形成催化级联体系抑制逆反应活性,提升产物L-2-氨基丁酸转化水平。 方法 利用基因挖掘技术在数据库中对转氨酶进行大规模挖掘,以2-酮丁酸作为底物,筛选高效转氨酶,并对新酶进行生化分析,表征酶学性质,通过建立全细胞生物转化和催化级联体系调控反应平衡,减缓逆反应水平,提升产物转化率。 结果 从数据库中发现了源自大肠杆菌的Ec4a转氨酶,以2-酮丁酸为底物,Ec4a的最适温度为45 ℃,最适pH值为9.0,比酶活1.25 U/mg,酶蛋白的熔融温度(Tm值)为68.2 ℃。酶蛋白在55 ℃和70 ℃下的酶活半衰期分别为321 min和150 min。在pH 8.5的条件下孵育6 h相对酶活剩余59%。在全细胞催化体系中两种底物最佳的浓度比为1∶1。分别以30 mmol/L的2-酮丁酸和30 mmol/L的L-Ala为底物,在菌体为OD600=10的条件下,2-氨基丁酸的转化率为37.5%。引入枯草芽胞杆菌乙酰乳酸合成酶(Bsalss)可以消耗副产物丙酮酸抑制逆反应,形成体外级联后相同全细胞催化体系下,转化率提高至61.4%。 结论 挖掘到稳定性好的转氨酶Ec4a,建立并优化2-氨基丁酸的全细胞生物转化体系,并通过引入Bsalss构建级联体系提高转化率至61.4%。
叶妍, 吴雨萱, 周哲敏, 崔文璟. 转氨酶新酶的挖掘、表征及在2-氨基丁酸生物转化中的应用[J]. 生物技术通报, 2025, 41(11): 134-142.
YE Yan, WU Yu-xuan, ZHOU Zhe-min, CUI Wen-jing. Exploration, Characterization, and Application of Transaminase New Enzymes in the Biocatalytic Conversion of 2-aminobutyric Acid[J]. Biotechnology Bulletin, 2025, 41(11): 134-142.
菌株与质粒 Strain and plasmid | 性质 Properties | 来源 Source |
|---|---|---|
| E.coli BL21(DE3) | 表达宿主 | 实验室保存 |
| E.coli JM109 | 克隆宿主 | 实验室保存 |
| pET-28a-EsRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-Ec4a | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-Bs | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-CbRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-AtRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-TsRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-TsRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| Pbad-Bsalss | AmpR 抗性、araBAD 启动子 | 本实验构建 |
表1 本研究中使用的主要菌株和质粒
Table 1 Main strains and plasmids used in this study
菌株与质粒 Strain and plasmid | 性质 Properties | 来源 Source |
|---|---|---|
| E.coli BL21(DE3) | 表达宿主 | 实验室保存 |
| E.coli JM109 | 克隆宿主 | 实验室保存 |
| pET-28a-EsRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-Ec4a | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-Bs | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-CbRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-AtRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-TsRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| pET-28a-TsRTA | His 标签、Kan 抗性、T7 启动子 | 本实验构建 |
| Pbad-Bsalss | AmpR 抗性、araBAD 启动子 | 本实验构建 |

图1 转氨酶的结构聚类和序列同源性分析以及重组表达SDS-PAGEA:结构聚类; B:同源性分析; C:重组表达SDS-PAGE (b代表菌液,s代表破碎菌液上清)
Fig. 1 Structural clustering and sequence homology analysis of transaminases and recombinant expression of SDS-PAGEA: Structural clustering; B: sequence homology analysis; C: recombinant expression of SDS-PAGE (b indicates bacterial liquid, s indicates supernatant of lysed bacterial liquid)

图3 Ec4a的最适温度,最适pH,温度稳定性和pH稳定性A:最适温度;B:最适pH;C:温度稳定性;D:pH稳定性
Fig. 3 Optimal temperature, optimal pH, temperature stability and pH stability of Ec4aA: Optimal temperature for Ec4a. B: Optimal pH for Ec4a. C: Temperature stability of Ec4a. D: pH stability of Ec4a
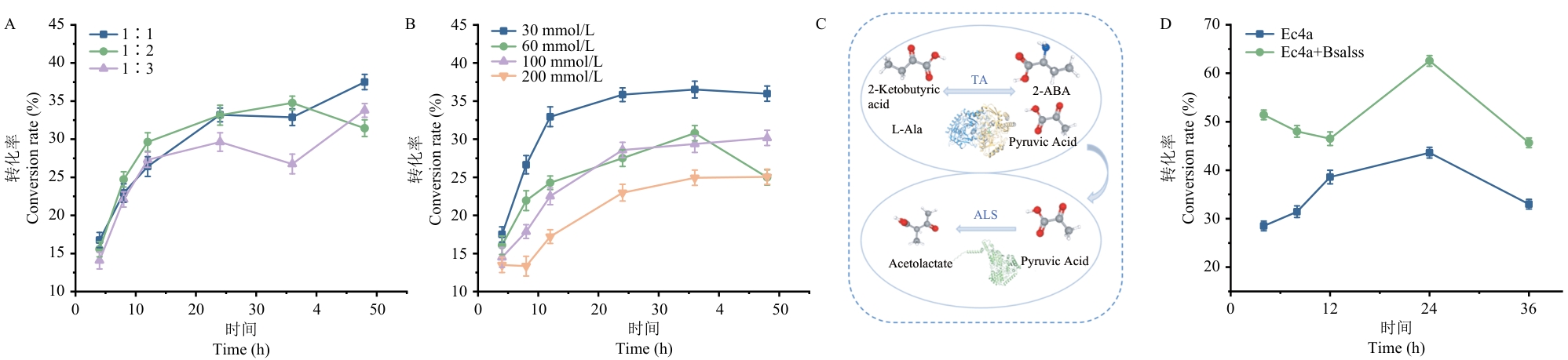
图5 全细胞催化体系优化和生物催化级联体系A:不同底物配比转化率; B:不同底物浓度转化率; C:级联体系示意图; D:对照组催化转化率
Fig. 5 Optimization of whole-cell catalytic systems and biocatalysis cascade systemsA: Conversion rates under different substrate ratios. B: Conversion rates under different substrate concentrations. C: Schematic diagram of the cascading system. D: Catalytic conversion rate of the control group
| [1] | Kelly SA, Pohle S, Wharry S, et al. Application of ω-transaminases in the pharmaceutical industry [J]. Chem Rev, 2018, 118(1): 349-367. |
| [2] | 蔡雪, 孙晨阳, 翟增春, 等. 磷酸吡哆醛依赖型酶的研究进展及其应用[J]. 生物工程学报, 2024, 40(9): 2771-2785. |
| Cai X, Sun CY, Zhai ZC, et al. Research progress and applications of pyridoxal phosphate-dependent enzymes [J]. Chinese Journal of Biotechnology, 2024, 40(9): 2771-2785. | |
| [3] | Slabu I, Galman JL, Lloyd RC, et al. Discovery, engineering, and synthetic application of transaminase biocatalysts [J]. ACS Catalysis, 2017, 7(12): 8263-8284. |
| [4] | Farkas E, Sátorhelyi P, Szakács Z, et al. Transaminase-catalysis to produce trans-4-substituted cyclohexane-1-amines including a key intermediate towards cariprazine [J]. Commun Chem, 2024, 7(1): 86. |
| [5] | 夏温娜, 孙雨, 闵聪, 等. 转氨酶催化不对称合成芳香族L-氨基酸 [J]. 生物工程学报, 2012, 28(11): 1346-1358. |
| Xia WN, Sun Y, Min C, et al. Asymmetric synthesis of aromatic L-amino acids catalyzed by transaminase [J]. Chin J Biotechnol, 2012, 28(11): 1346-1358. | |
| [6] | Park ES, Dong JY, Shin JS. ω-Transaminase-catalyzed asymmetric synthesis of unnatural amino acids using isopropylamine as an amino donor [J]. Org Biomol Chem, 2013, 11(40): 6929-6933. |
| [7] | Babu KC, Reddy RB, Mukkanti K, et al. Enantioselective synthesis of antiepileptic agent, (-)-levetiracetam, through Evans Asymmetric strategy [J]. Journal of Chemistry, 2013, 72(64): 13846-13855. |
| [8] | Chen JJ, Zhu RS, Zhou JP, et al. Efficient single whole-cell biotransformation for L-2-aminobutyric acid production through engineering of leucine dehydrogenase combined with expression regulation [J]. Bioresour Technol, 2021, 326: 124665. |
| [9] | 张利坤, 肖延铭, 杨卫华, 等. 亮氨酸脱氢酶偶联NADH再生体系合成L-2-氨基丁酸 [J]. 生物工程学报, 2020, 36(5): 992-1001. |
| Zhang LK, Xiao YM, Yang WH, et al. Synthesis of L-2-aminobutyric acid by leucine dehydrogenase coupling with an NADH regeneration system [J]. Chin J Biotechnol, 2020, 36(5): 992-1001. | |
| [10] | 奚强, 丁友友, 林丫丫, 等. 2-氨基丁酸的酶拆分[J]. 武汉工程大学学报, 2009, 31(3): 26-29. |
| Xi Q, Ding YY, Lin YY, et al. Enzymatic resolution of 2-aminobutyric acid [J]. Journal of Wuhan Institute of Technology, 2009, 31(3): 26-29. | |
| [11] | Liu YF, Han LC, Cheng ZY, et al. Enzymatic biosynthesis of L-2-aminobutyric acid by glutamate mutase coupled with L-Aspartate-β-decarboxylase using L-glutamate as the sole substrate [J]. ACS Catalysis, 2020, 10(23): 13913-13917. |
| [12] | Shin JS, Kim BG. Transaminase-catalyzed asymmetric synthesis of L-2-aminobutyric acid from achiral reactants [J]. Biotechnol Lett, 2009, 31(10): 1595-1599. |
| [13] | Tao RS, Jiang Y, Zhu FY, et al. A one-pot system for production of L-2-aminobutyric acid from L-threonine by L-threonine deaminase and a NADH-regeneration system based on L-leucine dehydrogenase and formate dehydrogenase [J]. Biotechnol Lett, 2014, 36(4): 835-841. |
| [14] | 周俊平. L-2-氨基丁酸的微生物高效制备及关键酶的理性改造 [D]. 无锡:江南大学, 2019. |
| Zhou JP, Microbial high-efficiency preparation of L-2-aminobutyric acid and rational modification of key enzymes [D]. Wuxi: Jiangnan University, 2019. | |
| [15] | Zhang ZW, Liu Y, Zhao J, et al. Active-site engineering of ω- transaminase from Ochrobactrum anthropi for preparation of L-2-aminobutyric acid [J]. BMC Biotechnol, 2021, 21(1): 55. |
| [16] | Luo W, Hu JG, Lu JP, et al. One pot cascade synthesis of L-2-aminobutyric acid employing ω-transaminase from Paracoccus pantotrophus [J]. Mol Catal, 2021, 515: 111890. |
| [17] | Cui YM, Gao YD, Yang LC. Transaminase catalyzed asymmetric synthesis of active pharmaceutical ingredients [J]. Green Synth Catal, 2024. |
| [18] | Gomm A, O'Reilly E. Transaminases for chiral amine synthesis [J]. Curr Opin Chem Biol, 2018, 43: 106-112. |
| [19] | Pinto A, Contente ML, Tamborini L. Advances on whole-cell biocatalysis in flow [J]. Curr Opin Green Sustain Chem, 2020, 25: 100343. |
| [20] | Lin BX, Tao Y. Whole-cell biocatalysts by design [J]. Microb Cell Fact, 2017, 16(1): 106. |
| [21] | 胡佳桂. ω-转氨酶基因挖掘、级联反应体系构建及其应用 [D]. 无锡: 江南大学, 2021. |
| Hu JG. Gene mining of ω-aminotransferase, construction of cascade reaction system and its application [D]. Wuxi: Jiangnan University, 2021. | |
| [22] | Jiang JJ, Chen X, Zhang DL, et al. Characterization of (R)-selective amine transaminases identified by in silico motif sequence blast [J]. Appl Microbiol Biotechnol, 2015, 99(6): 2613-2621. |
| [23] | Kelly SA, Mix S, Moody TS, et al. Transaminases for industrial biocatalysis: novel enzyme discovery [J]. Appl Microbiol Biotechnol, 2020, 104(11): 4781-4794. |
| [24] | 蒙丽钧. 转氨酶在大肠杆菌中的克隆表达及应用 [D]. 杭州: 浙江大学, 2019. |
| Meng LJ. Cloning and expression of transaminase in Escherichia coli and its application [D]. Hangzhou: Zhejiang University, 2019. | |
| [25] | 蔡婷婷, 曹佳仁, 邱帅, 等. 半理性设计进化土曲霉来源的ω-转氨酶AtTA热稳定性 [J]. 生物工程学报, 2023, 39(6): 2126-2140. |
| Cai TT, Cao JR, Qiu S, et al. Semi-rational evolution of ω-transaminase from Aspergillus terreus for enhancing the thermostability [J]. Chin J Biotechnol, 2023, 39(6): 2126-2140. | |
| [26] | Meng QL, Ramírez-Palacios C, Wijma HJ, et al. Protein engineering of amine transaminases [J]. Frontiers in Catalysis, 2022, 2. DOI: 10.3389/fctls.2022.1049179 |
| [27] | Khatik AG, Muley AB, More PR, et al. Transaminase-mediated chiral selective synthesis of (1R)-(3-methylphenyl)ethan-1-amine from 1-(3-methylphenyl)ethan-1-one: process minutiae, optimization, characterization and ‘What If studies’ [J]. Bioprocess Biosyst Eng, 2023, 46(2): 207-225. |
| [28] | 郑裕国, 程峰, 金利群, 等. 一种转氨酶突变体及其生产L-草铵膦的应用:中国,CN201810540686.1 [P]. 2018-05-30. |
| Zheng YG, Cheng F, Jin LQ, et al. A transaminase mutant and its application in the production of L-phosphinothricin: China, CN201810540686.1 [P]. 2018-05-30. | |
| [29] | 李仲霞, 刘妍, 罗泉, 等. 从专利角度分析ω-转氨酶在我国手性胺生物合成应用中的研究进展 [J]. 生物工程学报, 2023, 39(8): 3169-3187. |
| Li ZX, Liu Y, Luo Q, et al. The advance of ω-transaminase in chiral amine biosynthesis in China from the perspective of patents [J]. Chin J Biotechnol, 2023, 39(8): 3169-3187. | |
| [30] | Cárdenas-Fernández M, Sinclair O, Ward JM. Novel transaminases from thermophiles: from discovery to application [J]. Microb Biotechnol, 2022, 15(1): 305-317. |
| [31] | Wang ZC, Xu ML, Xie YY, et al. One-pot two-stage biocatalytic cascade to produce l-phosphinothricin by two enantioselective complementary aminotransferases at high substrate loading via a deracemization process [J]. J Agric Food Chem, 2024. |
| [32] | Höhne M, Kühl S, Robins K, et al. Efficient asymmetric synthesis of chiral amines by combining transaminase and pyruvate decarboxylase [J]. Chembiochem, 2008, 9(3): 363-365. |
| [33] | Guo F, Berglund P. Transaminase biocatalysis: optimization and application [J]. Green Chem, 2017, 19(2): 333-360. |
| [1] | 王碧成, 景海青, 万坤, 张莹莹, 丁家豪, 李润植, 薛金爱, 张海平. 大豆BCAT基因家族鉴定及GmBCAT3在干旱胁迫中的功能分析[J]. 生物技术通报, 2025, 41(10): 196-209. |
| [2] | 张曼玉, 董嘉诚, 苟福凡, 弓朝晖, 刘倩, 孙文良, 孔臻, 郝捷, 王敏, 田朝光. 嗜热毁丝霉果胶酯酶MtCE12-1的克隆表达及其酶学性质和应用研究[J]. 生物技术通报, 2024, 40(9): 291-300. |
| [3] | 殷亮, 王代玮, 刘悦莹, 刘海燕, 罗光宏. 蛋白酶SpP1基因克隆、表达及酶学性质的表征[J]. 生物技术通报, 2024, 40(4): 278-286. |
| [4] | 郑菲, 杨俊钊, 牛羽丰, 李蕊麟, 赵国柱. 嗜热毁丝菌裂解性多糖单加氧酶TtLPMO9I的酶学性质及其功能研究[J]. 生物技术通报, 2024, 40(2): 289-299. |
| [5] | 郝楠, 耿珊, 赵雨薇, 侯智涵, 赵斌, 刘颖超. 拟轮枝镰孢丙氨酸转氨酶FvALT的克隆与表达分析[J]. 生物技术通报, 2024, 40(12): 256-263. |
| [6] | 曲戈, 孙周通. 催化混杂性驱动的酶功能重塑[J]. 生物技术通报, 2023, 39(4): 1-9. |
| [7] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [8] | 王慕镪, 陈琦, 马薇, 李春秀, 欧阳鹏飞, 许建和. 机器学习方法在酶定向进化中的应用进展[J]. 生物技术通报, 2023, 39(4): 38-48. |
| [9] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [10] | 魏婷柳, 苗华彪, 吴倩, 黄遵锡. 漆酶BmLac的异源表达、酶学特性及棉酚降解的研究[J]. 生物技术通报, 2023, 39(12): 320-328. |
| [11] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [12] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [13] | 常晴, 束月蓉, 王文韬, 蒋昊, 延泉德, 钱政, 高雪纯, 吴金鸿, 张勇. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131. |
| [14] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [15] | 岑潇龙, 雷曦, 马诗云, 陈倩茹, 黄遵锡, 周峻沛, 张蕊. 金黄色葡萄球菌透明质酸裂解酶HylS的异源表达与特性研究[J]. 生物技术通报, 2022, 38(1): 157-167. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||