








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (7): 80-89.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1289
Previous Articles Next Articles
CHEN Hong-yan( ), LI Xiao-er, LI Zhong-guang(
), LI Xiao-er, LI Zhong-guang( )
)
Received:2021-10-11
Online:2022-07-26
Published:2022-08-09
Contact:
LI Zhong-guang
E-mail:hongyan_chen@163.com;zhongguang_li@163.com
CHEN Hong-yan, LI Xiao-er, LI Zhong-guang. Sugar Signaling and Its Role in Plant Response to Environmental Stress[J]. Biotechnology Bulletin, 2022, 38(7): 80-89.
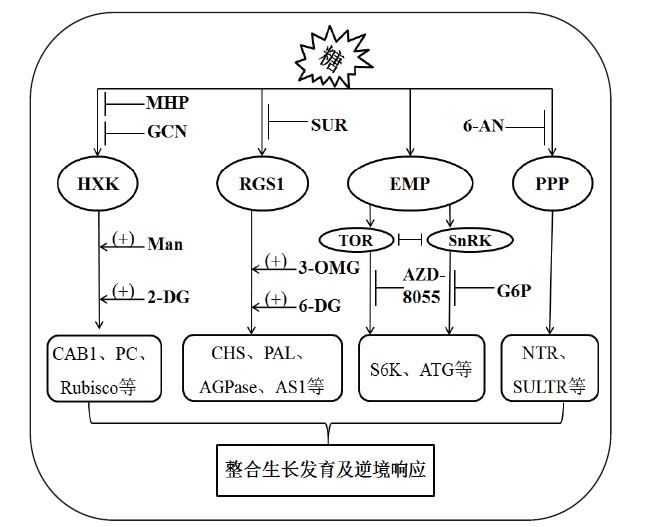
Fig. 1 Sugar signaling pathways in plants Plants can transduce sugar signaling by hexokinase(HXK)-,regulator of G protein signaling1(RGS1)-,glycolysis(EMP)-,and pentose phosphate pathway(PPP)-dependent signaling pathways,which in turn up-regulate gene expression of chlorophyll a/b-binding protein1(CAB1)、plastocyanin(PC),ribulose-1, 5-bisphosphate carboxylase/oxygenase(Rubisco),chalcone synthase(CHS),phenylalanine amonia-lyase(PAL),adenosine diphosphate-glucose pyrophosphorylase(AGPase),asparagine synthetase1(AS1),protein S6 kinase(S6K),autophagy-related protein(ATG),nitrate transporter(NTR),and sulfate transporter(SULTR),and then integrate plant growth,development,and response to environmental stress. The HXK-,RGS1-,EMP- and PPP-dependent sugar signaling pathways can be activated by 2-deoxymannose(Man),2-deoxyglucose(2-DG),3-O-methyl-D-glucose(3-OMG)and 6-deoxyglucose(6-DG),respectively,while inactivated by mannoheptulose(MHP),glucosamine(GCN),suramin(SUR),AZD-8055,glucose-6-phosphate(G6P)and 6-aminonicotinamide(6-AN),respectively.(+)represents promotion,while(├)represents inhibiton

Fig. 2 Role of sugar signaling in the response of plants to environmental stress Heat,cold,drought,salt,and heavy metal stress as well as exogenous application of sugar increase the level of endogenous sugar,and which triggers sugar signaling pathways and/or exerts direct action,followed by enhancement in the activity of plant hormone,heat shock proteins,osmoregulation system,antioxidant system,and methylglyoxal(MG)detoxification system,thus plants respond to environmental stress
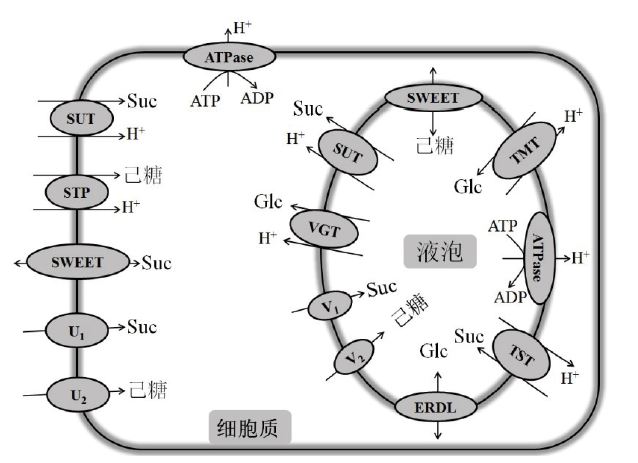
Fig.3 Sugar transport in plant cells Plant cells can transport sugar by sucrose transporter(SUT),sugar transport protein(STP),sugar will eventually be exported transporter(SWEET),sucrose import protein(U1),and hexose import protein(U2)locating in plasma membrane,as well as sucrose transporter(SUT),vacuole glucose transporter(VGT),SWEET,sucrose import protein(V1),hexose import protein(V2),early response dehydration protein-like(ERDL),tonoplast sucrose transporter(TST),and tonoplast monosaccharide transporter(TMT)locating in tonoplast,to response to growth,development environmental stress
| [1] |
Yoon J, Cho LH, Tun W, et al. Sucrose signaling in higher plants[J]. Plant Sci, 2021, 302:110703.
doi: 10.1016/j.plantsci.2020.110703 URL |
| [2] | Shah D, Sajjad N, Ali R, et al. Sugar regulates plant growth and development under in vitro conditions[M]// Plant signal molecules: role and regulation under stressful environments. Cambridge:Elsevier, 2019:257-267. |
| [3] |
Sakr S, Wang M, Dédaldéchamp F, et al. The sugar-signaling hub:overview of regulators and interaction with the hormonal and metabolic network[J]. Int J Mol Sci, 2018, 19(9):2506.
doi: 10.3390/ijms19092506 URL |
| [4] | Ahmad IZ. Role of sugars in abiotic stress signaling in plants[M]// Plant signal molecules: role and regulation under stressful environments. Cambridge:Elsevier, 2019:207-217. |
| [5] |
Liu W, Gendron JM. Same concept different outcomes:sugars determine circadian clock protein fate in animals and plants[J]. Mol Plant, 2020, 13(3):360-362.
doi: 10.1016/j.molp.2020.02.013 URL |
| [6] | Banerjee A, Roychoudhury A. Role of sugars in mediating abiotic stress tolerance in legumes[M]//Abiotic stress and legumes. Amsterdam:Elsevier, 2021:93-103. |
| [7] | Sharma P, Arora P, Kapoor D, et al. The role of sugars in improving plant abiotic stress tolerance[M]// Improving abiotic stress tolerance in plants. Boca Raton: CRC Press, 2020:31-48. |
| [8] |
Zhao Y, Wang XQ. The hot issue:TOR signalling network in plants[J]. Funct Plant Biol, 2020, 48(1):1-7.
doi: 10.1071/FP20071 URL |
| [9] | Ahmad F, Singh A, Kamal A. Osmoprotective role of sugar in mitigating abiotic stress in plants[M]// Protective chemical agents in the amelioration of plant abiotic stress: biochemical and molecular perspectives. Hoboken:Wiley, 2020:53-70. |
| [10] | Gangola MP, Ramadoss BR. Sugars play a critical role in abiotic stress tolerance in plants[M]//Biochemical, physiological and molecular avenues for combating abiotic stress tolerance in plants. Amsterdam:Elsevier, 2018:17-38. |
| [11] |
Li L, Sheen J. Dynamic and diverse sugar signaling[J]. Curr Opin Plant Biol, 2016, 33:116-125.
doi: 10.1016/j.pbi.2016.06.018 URL |
| [12] | 陈清帅. 拟南芥糖信号快速响应的机理研究[D]. 泰安: 山东农业大学, 2019. |
| Chen QS. Studies on mechanism of the rapid response to sugar signal in Arabidopsis thaliana[D]. Tai’an: Shandong Agricultural University, 2019. | |
| [13] | Martínez-Noël GMA, Tognetti JA. Sugar signaling under abiotic stress in plants[M]//Plant metabolites and regulation under environmental stress. Amsterdam:Elsevier, 2018:397-406. |
| [14] |
Rodriguez M, Parola R, Andreola S, et al. TOR and SnRK1 signaling pathways in plant response to abiotic stresses:do they always act according to the “Yin-Yang” model?[J]. Plant Sci, 2019, 288:110220.
doi: 10.1016/j.plantsci.2019.110220 URL |
| [15] |
Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants:conserved and novel mechanisms[J]. Annu Rev Plant Biol, 2006, 57:675-709.
pmid: 16669778 |
| [16] | Morgutti S, Negrini N, Pucciariello C, et al. Role of trehalose and regulation of its levels as a signal molecule to abiotic stresses in plants[M]//Plant signaling molecules. Amsterdam:Elsevier, 2019:235-255. |
| [17] | Poonam, Bhardwaj R, Handa N, et al. Sugar signalling in plants:a novel mechanism for drought stress management[M]// Water stress and crop plants: a sustainable approach. Chichester:Wiley, 2016:287-302. |
| [18] | Bhattacharya S, Kundu A. Sugars and sugar polyols in overcoming environmental stresses[M]// Protective chemical agents in the amelioration of plant abiotic stress: biochemical and molecular perspectives. Hoboken:Wiley, 2020:71-101. |
| [19] | Amist N, Singh NB. The role of sugars in the regulation of environmental stress[M]//Plant life under changing environment. Amsterdam:Elsevier, 2020:497-512. |
| [20] |
Dong YH, Silbermann M, Speiser A, et al. Sulfur availability regulates plant growth via glucose-TOR signaling[J]. Nat Commun, 2017, 8(1):1174.
doi: 10.1038/s41467-017-01224-w URL |
| [21] |
Sharma M, Banday ZZ, Shukla BN, et al. Glucose-regulated HLP1 acts as a key molecule in governing thermomemory[J]. Plant Physiol, 2019, 180(2):1081-1100.
doi: 10.1104/pp.18.01371 pmid: 30890662 |
| [22] |
Saksena HB, Sharma M, Singh D, et al. The versatile role of glucose signalling in regulating growth, development and stress responses in plants[J]. J Plant Biochem Biotechnol, 2020, 29(4):687-699.
doi: 10.1007/s13562-020-00614-4 URL |
| [23] | Cisse A. 蔗糖影响水稻耐热性的作用机理研究[D]. 北京: 中国农业科学院, 2019. |
| Cisse A. Role of sucrose in heat response in rice plants[D]. Beijing: Chinese Academy of Agricultural Sciences. 2019. | |
| [24] |
Li ZG, Luo LJ, Zhu LP. Involvement of trehalose in hydrogen sulfide donor sodium hydrosulfide-induced the acquisition of heat tolerance in maize(Zea mays L.)seedlings[J]. Bot Stud, 2014, 55(1):20.
doi: 10.1186/1999-3110-55-20 URL |
| [25] |
Suzuki N, Bajad S, Shuman J, et al. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana[J]. J Biol Chem, 2008, 283(14):9269-9275.
doi: 10.1074/jbc.M709187200 URL |
| [26] |
Kaur H, Manna M, Thakur T, et al. Imperative role of sugar signaling and transport during drought stress responses in plants[J]. Physiol Plant, 2021, 171(4):833-848.
doi: 10.1111/ppl.13364 URL |
| [27] | 李忠光, 龙维彪, 杨仕忠, 等. 硫化氢:从有毒气体到植物信号分子[J]. 云南师范大学学报:自然科学版, 2022, 42(1):1-10. |
| Li ZG, Long WB, Yang SZ, et al. Hydrogen sulfide:From toxic gas to plant signaling molecule. Journal of Yunnan Normal University:Natural Sciences Edition, 2022, 42(1):1-10. | |
| [28] |
Melvin P, Bankapalli K, D’Silva P, et al. Methylglyoxal detoxification by a DJ-1 family protein provides dual abiotic and biotic stress tolerance in transgenic plants[J]. Plant Mol Biol, 2017, 94(4/5):381-397.
doi: 10.1007/s11103-017-0613-9 URL |
| [29] | Yuanyuan M, Yali Z, Jiang L, et al. Roles of plant soluble sugars and their responses to plant cold stress[J]. Afr J Biotechnol, 2009, 8(10):2004-2010. |
| [30] |
Garg AK, Kim JK, Owens TG, et al. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses[J]. Proc Natl Acad Sci USA, 2002, 99(25):15898-15903.
doi: 10.1073/pnas.252637799 URL |
| [31] |
Livingston DP, Hincha DK, Heyer AG. Fructan and its relationship to abiotic stress tolerance in plants[J]. Cell Mol Life Sci, 2009, 66(13):2007-2023.
doi: 10.1007/s00018-009-0002-x pmid: 19290476 |
| [32] |
Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks[J]. J Exp Bot, 2012, 63(4):1593-1608.
doi: 10.1093/jxb/err460 pmid: 22291134 |
| [33] |
Fernandez O, Béthencourt L, Quero A, et al. Trehalose and plant stress responses:friend or foe?[J]. Trends Plant Sci, 2010, 15(7):409-417.
doi: 10.1016/j.tplants.2010.04.004 pmid: 20494608 |
| [34] |
Li YH, Lee KK, Walsh S, et al. Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a relevance vector machine[J]. Genome Res, 2006, 16(3):414-427.
doi: 10.1101/gr.4237406 URL |
| [35] |
Eastmond PJ, Graham IA. Trehalose metabolism:a regulatory role for trehalose-6-phosphate?[J]. Curr Opin Plant Biol, 2003, 6(3):231-235.
doi: 10.1016/s1369-5266(03)00037-2 pmid: 12753972 |
| [36] |
Anchordoguy TJ, Rudolph AS, Carpenter JF, et al. Modes of interaction of cryoprotectants with membrane phospholipids during freezing[J]. Cryobiology, 1987, 24(4):324-331.
pmid: 3621976 |
| [37] |
Crowe JH, Crowe LM, Carpenter JF, et al. Interactions of sugars with membranes[J]. Biochim Biophys Acta, 1988, 947(2):367-384.
pmid: 3285894 |
| [38] |
Schrier AA, Hoffmann-Thoma G, van Bel AJE. Temperature effects on symplasmic and apoplasmic phloem loading and loading-associated carbohydrate processing[J]. Funct Plant Biol, 2000, 27(9):769.
doi: 10.1071/PP99166 URL |
| [39] |
Patzke K, Prananingrum P, Klemens PAW, et al. The plastidic sugar transporter pSuT influences flowering and affects cold responses[J]. Plant Physiol, 2019, 179(2):569-587.
doi: 10.1104/pp.18.01036 URL |
| [40] |
Klemens PAW, Patzke K, Trentmann O, et al. Overexpression of a proton-coupled vacuolar glucose exporter impairs freezing tolerance and seed germination[J]. New Phytol, 2014, 202(1):188-197.
doi: 10.1111/nph.12642 URL |
| [41] |
Saddhe AA, Manuka R, Penna S. Plant sugars:Homeostasis and transport under abiotic stress in plants[J]. Physiol Plant, 2021, 171(4):739-755.
doi: 10.1111/ppl.13283 URL |
| [42] |
Eom JS, Chen LQ, Sosso D, et al. SWEETs, transporters for intracellular and intercellular sugar translocation[J]. Curr Opin Plant Biol, 2015, 25:53-62.
doi: 10.1016/j.pbi.2015.04.005 URL |
| [43] |
Breia R, Conde A, Badim H, et al. Plant SWEETs:from sugar transport to plant-pathogen interaction and more unexpected physiological roles[J]. Plant Physiol, 2021, 186(2):836-852.
doi: 10.1093/plphys/kiab127 URL |
| [44] |
Pelleschi S, Rocher JP, Prioul JL. Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves[J]. Plant Cell Environ, 1997, 20(4):493-503.
doi: 10.1046/j.1365-3040.1997.d01-89.x URL |
| [45] |
Li NN, Qian WJ, Wang L, et al. Isolation and expression features of hexose kinase genes under various abiotic stresses in the tea plant(Camellia sinensis)[J]. J Plant Physiol, 2017, 209:95-104.
doi: 10.1016/j.jplph.2016.11.007 URL |
| [46] |
Yang YT, Fu ZW, Su YC, et al. A cytosolic glucose-6-phosphate dehydrogenase gene, ScG6PDH, plays a positive role in response to various abiotic stresses in sugarcane[J]. Sci Rep, 2014, 4:7090.
doi: 10.1038/srep07090 URL |
| [47] |
Trouverie J, Chateau-Joubert S, Thévenot C, et al. Regulation of vacuolar invertase by abscisic acid or glucose in leaves and roots from maize plantlets[J]. Planta, 2004, 219(5):894-905.
pmid: 15179513 |
| [48] |
Dai N, Schaffer A, Petreikov M, et al. Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence[J]. Plant Cell, 1999, 11(7):1253-1266.
pmid: 10402427 |
| [49] |
Suwa R, Fujimaki S, Suzui N, et al. Use of positron-emitting tracer imaging system for measuring the effect of salinity on temporal and spatial distribution of 11C tracer and coupling between source and sink organs[J]. Plant Sci, 2008, 175(3):210-216.
doi: 10.1016/j.plantsci.2008.03.022 URL |
| [50] |
Rosa M, Prado C, Podazza G, et al. Soluble sugars[J]. Plant Signal Behav, 2009, 4(5):388-393.
doi: 10.4161/psb.4.5.8294 URL |
| [51] |
Hu MY, Shi ZG, Zhang ZB, et al. Effects of exogenous glucose on seed germination and antioxidant capacity in wheat seedlings under salt stress[J]. Plant Growth Regul, 2012, 68(2):177-188.
doi: 10.1007/s10725-012-9705-3 URL |
| [52] | Nemati I, Moradi F, Gholizadeh S, et al. The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths and roots of rice(Oryza sativa L.)seedlings[J]. Plant Soil Environ, 2011, 57 (No. 1):26-33. |
| [53] |
Pattanagul W, Thitisaksakul M. Effect of salinity stress on growth and carbohydrate metabolism in three rice(Oryza sativa L.)cultivars differing in salinity tolerance[J]. Indian J Exp Biol, 2008, 46(10):736-742.
pmid: 19024173 |
| [54] |
Couée I, Sulmon C, Gouesbet G, et al. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants[J]. J Exp Bot, 2006, 57(3):449-459.
pmid: 16397003 |
| [55] |
Yamada K, Kanai M, Osakabe Y, et al. Monosaccharide absorption activity of Arabidopsis roots depends on expression profiles of transporter genes under high salinity conditions[J]. J Biol Chem, 2011, 286(50):43577-43586.
doi: 10.1074/jbc.M111.269712 pmid: 22041897 |
| [56] |
Hackenberg D, McKain MR, Lee SG, et al. Gα and regulator of G-protein signaling(RGS)protein pairs maintain functional compatibility and conserved interaction interfaces throughout evolution despite frequent loss of RGS proteins in plants[J]. New Phytol, 2017, 216(2):562-575.
doi: 10.1111/nph.14180 pmid: 27634188 |
| [57] |
Liu CY, Fan W, Zhu PP, et al. Mulberry RGS negatively regulates salt stress response and tolerance[J]. Plant Signal Behav, 2019, 14(12):1672512.
doi: 10.1080/15592324.2019.1672512 URL |
| [58] |
Martins LL, Mourato MP, Baptista S, et al. Response to oxidative stress induced by cadmium and copper in tobacco plants(Nicotiana tabacum)engineered with the trehalose-6-phosphate synthase gene(AtTPS1)[J]. Acta Physiol Plant, 2014, 36(3):755-765.
doi: 10.1007/s11738-013-1453-0 URL |
| [59] |
Jalmi SK, Bhagat PK, Verma D, et al. Traversing the links between heavy metal stress and plant signaling[J]. Front Plant Sci, 2018, 9:12.
doi: 10.3389/fpls.2018.00012 URL |
| [60] |
Mostofa MG, Hossain MA, Fujita M, et al. Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice[J]. Sci Rep, 2015, 5:11433.
doi: 10.1038/srep11433 URL |
| [61] |
Duman F, Aksoy A, Aydin Z, et al. Effects of exogenous glycinebetaine and trehalose on cadmium accumulation and biological responses of an aquatic plant(Lemna gibba L.)[J]. Water Air Soil Pollut, 2011, 217(1/2/3/4):545-556.
doi: 10.1007/s11270-010-0608-5 URL |
| [62] |
Richards KD, Schott EJ, Sharma YK, et al. Aluminum induces oxidative stress genes in Arabidopsis thaliana[J]. Plant Physiol, 1998, 116(1):409-418.
pmid: 9449849 |
| [63] |
Tan JK, Kong FX, Cao HS, et al. Effects of acid precipitation and aluminum on carbohydrate metabolism in mycorrhizae of Pinus massioniana[J]. Bull Environ Contam Toxicol, 2005, 74(3):614-622.
doi: 10.1007/s00128-005-0628-9 URL |
| [1] | HAN Zhi-yang, JIA Zi-miao, LIANG Qiu-ju, WANG Ke, TANG Hua-li, YE Xing-guo, ZHANG Shuang-xi. Salt Tolerance at Seedling Stage and Analysis of Selenium and Folic Acid Content in Seeds in Two Sets of Wheat-Dasypyrum villosum Chromosom Additional Lines [J]. Biotechnology Bulletin, 2023, 39(8): 185-193. |
| [2] | LI Zhi-qi, YUAN Yue, MIAO Rong-qing, PANG Qiu-ying, ZHANG Ai-qin. Melatonin Contents in Eutrema salsugineum and Arabidopsis thaliana Under Salt Stress, and Expression Pattern Analysis of Synthesis Related Genes [J]. Biotechnology Bulletin, 2023, 39(5): 142-151. |
| [3] | LIU Kui, LI Xing-fen, YANG Pei-xin, ZHONG Zhao-chen, CAO Yi-bo, ZHANG Ling-yun. Functional Study and Validation of Transcriptional Coactivator PwMBF1c in Picea wilsonii [J]. Biotechnology Bulletin, 2023, 39(5): 205-216. |
| [4] | CHEN Yi-bo, YANG Wan-ming, YUE Ai-qin, WANG Li-xiang, DU Wei-jun, WANG Min. Construction of Soybean Genetic Map Based on SLAF Markers and QTL Mapping Analysis of Salt Tolerance at Seedling Stage [J]. Biotechnology Bulletin, 2023, 39(2): 70-79. |
| [5] | YANG Mao, LIN Yu-feng, DAI Yang-shuo, PAN Su-jun, PENG Wei-ye, YAN Ming-xiong, LI Wei, WANG Bing, DAI Liang-ying. OsDIS1 Negatively Regulates Rice Drought Tolerance Through Antioxidant Pathways [J]. Biotechnology Bulletin, 2023, 39(2): 88-95. |
| [6] | ZHU Ye-sheng, WU Guo-qiang, WEI Ming. Roles of Plasma Membrane Na+/H+ Antiporter SOS1 in Maintaining Ionic Homeostasis of Plants [J]. Biotechnology Bulletin, 2023, 39(12): 16-32. |
| [7] | LIN Rong, ZHENG Yue-ping, XU Xue-zhen, LI Dan-dan, ZHENG Zhi-fu. Functional Analysis of ACOL8 Gene in the Ethylene Synthesis and Response in Arabidopsis thaliana [J]. Biotechnology Bulletin, 2023, 39(1): 157-165. |
| [8] | LIU Jia-xin, ZHANG Hui-long, ZOU Rong-song, YANG Xiu-yan, ZHU Jian-feng, ZHANG Hua-xin. Research Progress in Na+ Antiport and Physiological Growth Mechanisms of Differernt Halophytes Adapted to Salt Stress [J]. Biotechnology Bulletin, 2023, 39(1): 59-72. |
| [9] | CHEN Guang, LI Jia, DU Rui-ying, WANG Xu. pOsHAK1:OsFLN2 Expression Enhances the Drought Tolerance by Altering Sugar Metabolism in Rice [J]. Biotechnology Bulletin, 2022, 38(8): 92-100. |
| [10] | YIPARE·Paerhati , ZULIHUMAER·Rouzi , TIAN Yong-zhi, ZHU Yan-lei, LI Yuan-ting, MA Xiao-lin. Research Progress in Diversity of Endophytes Microbial Communities Isolated from Desert Plants and Their Strengthening Effects on Drought and Salt Tolerance in Crops [J]. Biotechnology Bulletin, 2022, 38(12): 88-99. |
| [11] | LI Cai-xia, LAN Hai-yan. Research Progress in the Stress Tolerance Mechanisms of Desert Plant Tamarix spp. [J]. Biotechnology Bulletin, 2021, 37(5): 128-140. |
| [12] | MA Xu-hui, CHEN Ru-mei, LIU Xiao-qing, ZHAO Jun, ZHANG Xia. Effects of Melatonin on Root Growth and Drought Tolerance of Maize Seedlings [J]. Biotechnology Bulletin, 2021, 37(2): 1-14. |
| [13] | ZHANG Yun-chuan, LIN Yi-xuan, CAO Xin-wen, WANG Hai-nan, YAN Jie. TkDREB2 Clone from Taraxacum kok-saghyz and Drought Tolerance Analysis of Transgenic Nicotiana tabacum [J]. Biotechnology Bulletin, 2021, 37(11): 212-224. |
| [14] | HU Yu-jie, ZHU Xiu-ling, DING Yan-qin, DU Bing-hai, WANG Cheng-qiang. Research Progress on Salt Tolerance and Growth-promoting Mechanism of Bacillus [J]. Biotechnology Bulletin, 2020, 36(9): 64-74. |
| [15] | ZHANG Xiao-jia, LU Ya-jun, ZHANG Wen-jin, ZHANG Yu, CUI Gao-chang, LANG Duo-yong, ZHANG Xin-hui. Preparation of Drought-resistant and Salt-tolerant Bacteria and Its Effect on Germination of Licorice Seeds [J]. Biotechnology Bulletin, 2020, 36(9): 180-193. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||