








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (10): 128-135.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0338
Previous Articles Next Articles
HOU Rui-ze BAO Yue CHEN Qi-liang MAO Gui-ling WEI Bo-lin HOU Lei-ping LI Mei-lan( )
)
Received:2023-04-11
Online:2023-10-26
Published:2023-11-28
HOU Rui-ze BAO Yue CHEN Qi-liang MAO Gui-ling WEI Bo-lin HOU Lei-ping LI Mei-lan. Cloning,Expression and Functional Identification of PRR5 Gene in Pakchoi[J]. Biotechnology Bulletin, 2023, 39(10): 128-135.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 引物用途 Primer purpose |
|---|---|---|
| C-Bra009768 | F:ATGGGAGAAGTGAGCGACGAAG | 基因克隆 |
| R:TCATTGTGGAGCTTCTTGTGTTGAG | Gene clone | |
| G-Bra009768 | F:GAGAACACGGGGGACTCTAGAATGGGAGAAGTGAGCGACGAAG | 基因克隆 Gene clone |
| R:ATAAGGGACTGACCACCCGGGTCATTGTGGAGCTTCTTGTGTTGAG | ||
| N-YZ | F:GACGTTCCAACCACGTCTTC | 菌液PCR引物 |
| R:CCAGACTGAATGCCCACAGG | Bacterial liquid PCR primer |
Table 1 Primers of Bra009768
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 引物用途 Primer purpose |
|---|---|---|
| C-Bra009768 | F:ATGGGAGAAGTGAGCGACGAAG | 基因克隆 |
| R:TCATTGTGGAGCTTCTTGTGTTGAG | Gene clone | |
| G-Bra009768 | F:GAGAACACGGGGGACTCTAGAATGGGAGAAGTGAGCGACGAAG | 基因克隆 Gene clone |
| R:ATAAGGGACTGACCACCCGGGTCATTGTGGAGCTTCTTGTGTTGAG | ||
| N-YZ | F:GACGTTCCAACCACGTCTTC | 菌液PCR引物 |
| R:CCAGACTGAATGCCCACAGG | Bacterial liquid PCR primer |
| 引物名称 Gene name | 引物序列 Primer sequence(5'-3') | 引物用途 Primer purpose |
|---|---|---|
| ACTIN | F:GTTGCTATCCAGGCTGTTCT | 大白菜内参引物 |
| R:AGCGTGAGGAAGAGCATAAC | Internal primers in Brassica pekinensis | |
| Bra009768 | F:AGTTACAGAGTCGCTGCT | 荧光定量引物 |
| R:TATTAACCGAGTCCTGTGTTG | Fluorescent quantitative primer | |
| ACT11 | F:CACACTGGAGTGATGGTTGG | 拟南芥内参引物 |
| R:ATTGGCCTTGGGGTTAAGAG | The internal reference gene of Arabidopsis |
Table 2 Primers of real time quantitative PCR
| 引物名称 Gene name | 引物序列 Primer sequence(5'-3') | 引物用途 Primer purpose |
|---|---|---|
| ACTIN | F:GTTGCTATCCAGGCTGTTCT | 大白菜内参引物 |
| R:AGCGTGAGGAAGAGCATAAC | Internal primers in Brassica pekinensis | |
| Bra009768 | F:AGTTACAGAGTCGCTGCT | 荧光定量引物 |
| R:TATTAACCGAGTCCTGTGTTG | Fluorescent quantitative primer | |
| ACT11 | F:CACACTGGAGTGATGGTTGG | 拟南芥内参引物 |
| R:ATTGGCCTTGGGGTTAAGAG | The internal reference gene of Arabidopsis |
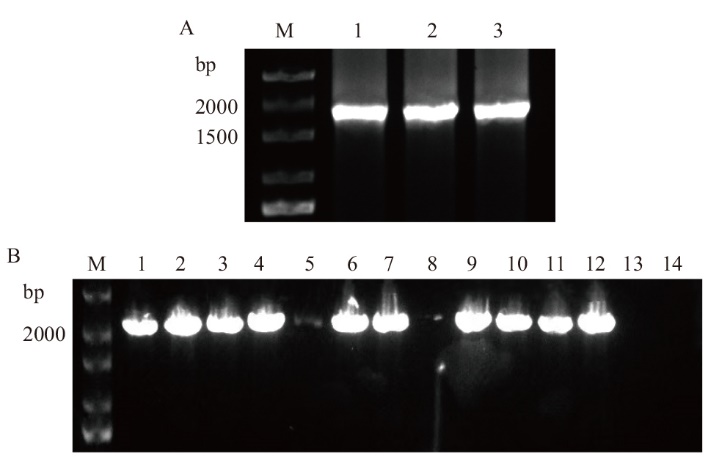
Fig. 1 Cloning of Bra009768 and detection results of its broth A: Cloning of Bra009768(M: DNA marker; 1-3: Bra009768). B: PCR detection of bacterial liquid(M: DNA marker; 1-12: Bra009768; 13-14: negative control)
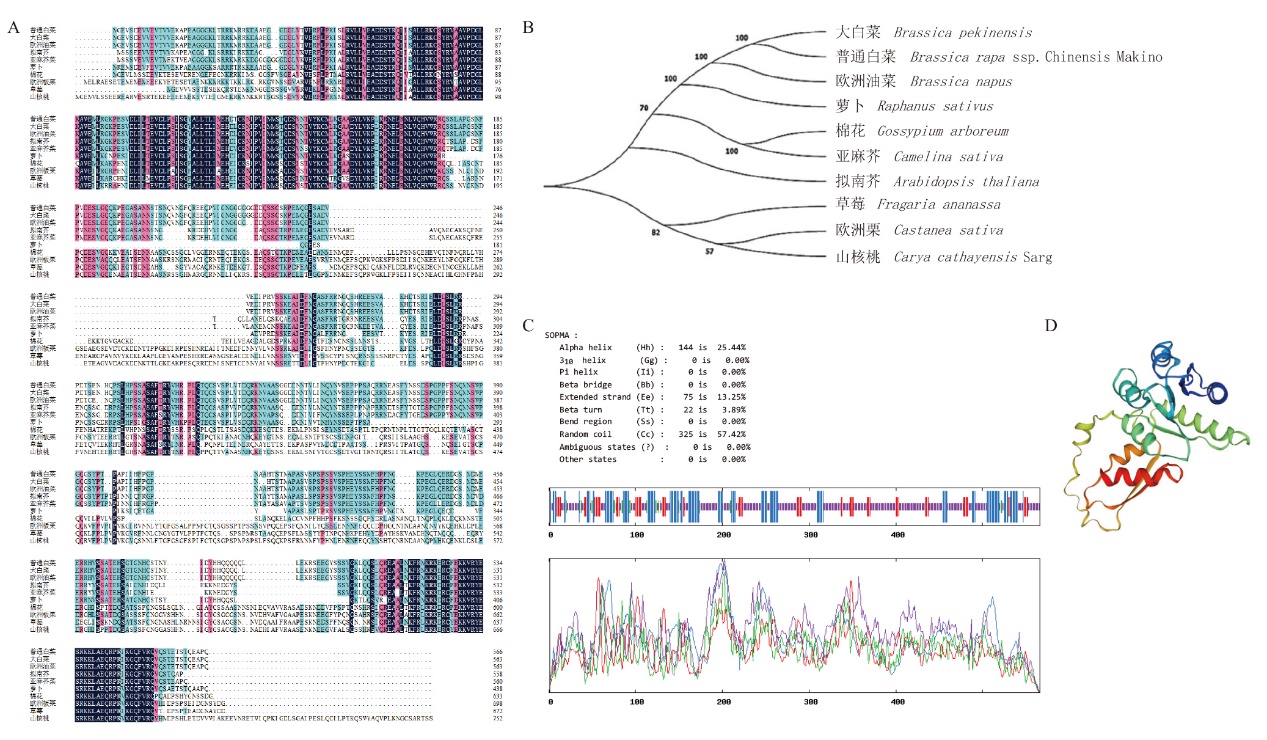
Fig. 2 Bioinformatics analysis of Bra009768 A: Multiple alignment of Bra009768 amino acid sequences. B: Phylogenetic tree of Bra009768 homologous proteins. C, D: Prediction of secondary and tertiary structures of Bra009768 proteins
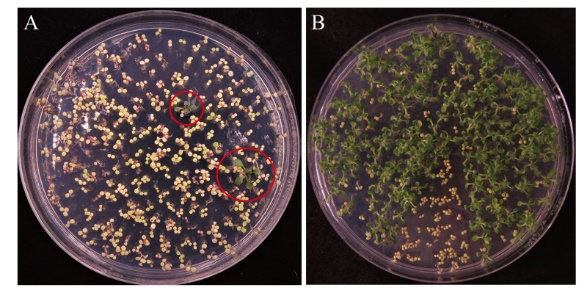
Fig. 4 Selection of transgenic Arabidopsis thaliana resistant plants A: Screening of resistant plants in T1 generation. B: Screening of resistant plants in T2 generation
| 植株 Plant | 抽薹时间 Bolting time/d | 侧薹数 Number of side bolting | 株高 Plant height/cm | 茎粗 Stem thick/mm |
|---|---|---|---|---|
| Col-1 | 13.75±0.82 | 11.50±0.31 | 25.90±0.88 | 0.45±0.04 |
| T-1 | 12.50±2.38 | 11.25±2.12 | 25.75±3.13 | 0.54±0.12 |
| Col-2 | 17.20±1.25 | 7.50±0.21 | 22.30±0.91 | 0.53±0.02 |
| T-2 | 15.72±0.78 | 8.10±0.13 | 29.80±0.53 | 0.94±0.06 |
Table 3 Phenotypic statistics of T1 and T2 transgenic plants
| 植株 Plant | 抽薹时间 Bolting time/d | 侧薹数 Number of side bolting | 株高 Plant height/cm | 茎粗 Stem thick/mm |
|---|---|---|---|---|
| Col-1 | 13.75±0.82 | 11.50±0.31 | 25.90±0.88 | 0.45±0.04 |
| T-1 | 12.50±2.38 | 11.25±2.12 | 25.75±3.13 | 0.54±0.12 |
| Col-2 | 17.20±1.25 | 7.50±0.21 | 22.30±0.91 | 0.53±0.02 |
| T-2 | 15.72±0.78 | 8.10±0.13 | 29.80±0.53 | 0.94±0.06 |
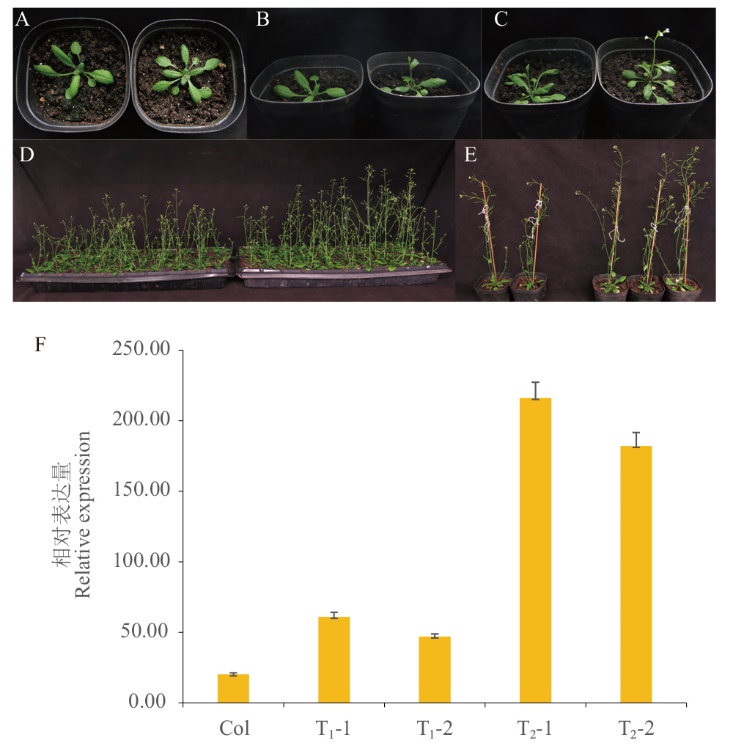
Fig. 5 Phenotypic observation of transgenic plants and RT-qPCR validation of BrcPRR5 A: Vegetative growth period. B: Bolting stage. C: Budding stage. D-E: Mature stage(left: wild type(Col); right: transgenic plants). F: RT-qPCR detection of transgenic plants
| [25] |
Imaizumi T, Tran HG, Swartz TE, et al. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis[J]. Nature, 2003, 426(6964): 302-306.
doi: 10.1038/nature02090 |
| [26] |
Imaizumi T, Schultz TF, Harmon FG, et al. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis[J]. Science, 2005, 309(5732): 293-297.
doi: 10.1126/science.1110586 pmid: 16002617 |
| [27] |
Fornara F, Panigrahi KCS, Gissot L, et al. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response[J]. Dev Cell, 2009, 17(1): 75-86.
doi: 10.1016/j.devcel.2009.06.015 pmid: 19619493 |
| [28] |
Valverde F, Mouradov A, Soppe W, et al. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering[J]. Science, 2004, 303(5660): 1003-1006.
doi: 10.1126/science.1091761 pmid: 14963328 |
| [29] |
Nakamichi N, Kita M, Niinuma K, et al. Arabidopsis clock-associated pseudo-response regulators PRR9, PRR7 and PRR5 coordinately and positively regulate flowering time through the canonical CONSTANS-dependent photoperiodic pathway[J]. Plant Cell Physiol, 2007, 48(6): 822-832.
doi: 10.1093/pcp/pcm056 pmid: 17504813 |
| [30] |
Hayama R, Sarid-Krebs L, Richter R, et al. PSEUDO RESPONSE REGULATORs stabilize CONSTANS protein to promote flowering in response to day length[J]. Embo J, 2017, 36(7): 904-918.
doi: 10.15252/embj.201693907 pmid: 28270524 |
| [1] |
Amasino R. Seasonal and developmental timing of flowering[J]. Plant J, 2010, 61(6): 1001-1013.
doi: 10.1111/tpj.2010.61.issue-6 URL |
| [2] |
Fornara F, de Montaigu A, Coupland G. SnapShot: Control of flowering in Arabidopsis[J]. Cell, 2010, 141(3): 550-550.e2.
doi: 10.1016/j.cell.2010.04.024 pmid: 20434991 |
| [3] | 刘娟, 黎黎, 陆柄辰, 等. 温度调控植物开花研究进展[J]. 应用与环境生物学报, 2020, 26(3): 713-721. |
| Liu J, Li L, Lu BC, et al. Research progress on temperature regulation of plant flowering[J]. Chin J Appl Environ Biol, 2020, 26(3): 713-721. | |
| [4] | 孙昌辉, 邓晓建, 方军, 等. 高等植物开花诱导研究进展[J]. 遗传, 2007, 29(10): 1182-1190. |
| Sun CH, Deng XJ, Fang J, et al. An overview of flowering transition in higher plants[J]. Hereditas, 2007, 29(10): 1182-1190. | |
| [5] |
Andrés F, Coupland G. The genetic basis of flowering responses to seasonal cues[J]. Nat rev Genet, 2012, 13(9): 627-639.
doi: 10.1038/nrg3291 pmid: 22898651 |
| [6] |
Mutasa-Göttgens E, Hedden P. Gibberellin as a factor in floral regulatory networks[J]. J Exp Bot, 2009, 60(7): 1979-1989.
doi: 10.1093/jxb/erp040 pmid: 19264752 |
| [7] |
Bäurle I, Dean C. The timing of developmental transitions in plants[J]. Cell, 2006, 125(4): 655-664.
doi: 10.1016/j.cell.2006.05.005 pmid: 16713560 |
| [8] |
Sablowski R. Flowering and determinacy in Arabidopsis[J]. J Exp Bot, 2007, 58(5): 899-907.
doi: 10.1093/jxb/erm002 pmid: 17293602 |
| [9] | Boss PK, Bastow RM, Mylne JS, et al. Multiple pathways in the decision to flower: Enabling, promoting, and resetting[J]. Plant Cell, 2004, 16(suppl): S18-S31. |
| [10] |
Makino S, Kiba T, Imamura A, et al. Genes encoding pseudo-response regulators: insight into His-to-Asp phosphorelay and circadian rhythm in Arabidopsis thaliana[J]. Plant Cell Physiol, 2000, 41(6): 791-803.
doi: 10.1093/pcp/41.6.791 pmid: 10945350 |
| [11] |
Nakamichi N, Kiba T, Kamioka M, et al. Transcriptional repressor PRR5 directly regulates clock-output pathways[J]. Proc Natl Acad Sci USA, 2012, 109(42): 17123-17128.
doi: 10.1073/pnas.1205156109 pmid: 23027938 |
| [12] |
Imaizumi T, Kay SA. Photoperiodic control of flowering: not only by coincidence[J]. Trends Plant Sci, 2006, 11(11): 550-558.
pmid: 17035069 |
| [13] |
Nakamichi N, Kudo T, Makita N, et al. Flowering time control in rice by introducing Arabidopsis clock-associated PSEUDO-RESPONSE REGULATOR 5[J]. Biosci, Biotechnol, Biochem, 2020, 84(5): 970-979.
doi: 10.1080/09168451.2020.1719822 URL |
| [14] |
Shang M, Wang X, Zhang J, et al. Genetic regulation of GA metabolism during vernalization, floral bud initiation and development in pak choi(Brassica rapa ssp. chinensis Makino)[J]. Front Plant Sci, 2017, 8: 1533.
doi: 10.3389/fpls.2017.01533 URL |
| [15] | 宋红霞, 付超, 侯雷平, 等. 普通白菜石蜡切片染色方法筛选及花芽分化形态学鉴定[J]. 江苏农业科学, 2018, 46(2): 88-91. |
| Song HX, Fu C, Hou LP, et al. Screening of staining methods for paraffin sections of Chinese cabbage and morphological identification of flower bud differentiation[J]. Jiangsu Agric Sci, 2018, 46(2): 88-91. | |
| [16] |
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta CT)method[J]. Methods, 2001, 25(4): 402-408.
doi: 10.1006/meth.2001.1262 pmid: 11846609 |
| [17] |
Strayer C, Oyama T, Schultz TF, et al. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog[J]. Science, 2000, 289(5480): 768-771.
doi: 10.1126/science.289.5480.768 pmid: 10926537 |
| [18] |
Ito S, Matsushika A, Yamada H, et al. Characterization of the APRR9 pseudo-response regulator belonging to the APRR1/TOC1 quintet in Arabidopsis thaliana[J]. Plant Cell Physiol, 2003, 44(11): 1237-1245.
doi: 10.1093/pcp/pcg136 URL |
| [19] |
Yamamoto Y, Sato E, Shimizu T, et al. Comparative genetic studies on the APRR5 and APRR7 genes belonging to the APRR1/TOC1 quintet implicated in circadian rhythm, control of flowering time, and early photomorphogenesis[J]. Plant Cell Physiol, 2003, 44(11): 1119-1130.
pmid: 14634148 |
| [20] |
Murakami M, Yamashino T, Mizuno T. Characterization of circadian-associated APRR3 pseudo-response regulator belonging to the APRR1/TOC1 quintet in Arabidopsis thaliana[J]. Plant Cell Physiol, 2004, 45(5): 645-650.
pmid: 15169947 |
| [21] |
Para A, Farré EM, Imaizumi T, et al. PRR3 is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock[J]. Plant Cell, 2007, 19(11): 3462-3473.
doi: 10.1105/tpc.107.054775 URL |
| [22] |
Nakamichi N, Takao SR, Kudo T, et al. Improvement of Arabidopsis biomass and cold, drought and salinity stress tolerance by modified circadian clock-associated PSEUDO-RESPONSE REGULATORs[J]. Plant Cell Physiol, 2016, 57(5): 1085-1097.
doi: 10.1093/pcp/pcw057 pmid: 27012548 |
| [23] |
Matsushika A, Murakami M, Ito S, et al. Characterization of Circadian-associated pseudo-response regulators: I. Comparative studies on a series of transgenic lines misexpressing five distinctive PRR Genes in Arabidopsis thaliana[J]. Biosci, Biotechnol, Biochem, 2007, 71(2): 527-534.
doi: 10.1271/bbb.60583 URL |
| [24] |
Beales J, Turner A, Griffiths S, et al. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat(Triticum aestivum L.)[J]. Theor Appl Genet, 2007, 115(5): 721-733.
doi: 10.1007/s00122-007-0603-4 pmid: 17634915 |
| [1] | LYU Qiu-yu, SUN Pei-yuan, RAN Bin, WANG Jia-rui, CHEN Qing-fu, LI Hong-you. Cloning, Subcellular Localization and Expression Analysis of the Transcription Factor Gene FtbHLH3 in Fagopyrum tataricum [J]. Biotechnology Bulletin, 2023, 39(8): 194-203. |
| [2] | WANG Jia-rui, SUN Pei-yuan, KE Jin, RAN Bin, LI Hong-you. Cloning and Expression Analyses of C-glycosyltransferase Gene FtUGT143 in Fagopyrum tataricum [J]. Biotechnology Bulletin, 2023, 39(8): 204-212. |
| [3] | SUN Ming-hui, WU Qiong, LIU Dan-dan, JIAO Xiao-yu, WANG Wen-jie. Cloning and Expression Analysis of CsTMFs Gene in Tea Plant [J]. Biotechnology Bulletin, 2023, 39(7): 151-159. |
| [4] | LIU Si-jia, WANG Hao-nan, FU Yu-chen, YAN Wen-xin, HU Zeng-hui, LENG Ping-sheng. Cloning and Functional Analysis of LiCMK Gene in Lilium ‘Siberia’ [J]. Biotechnology Bulletin, 2023, 39(3): 196-205. |
| [5] | YANG Xu-yan, ZHAO Shuang, MA Tian-yi, BAI Yu, WANG Yu-shu. Cloning of Three Cabbage WRKY Genes and Their Expressions in Response to Abiotic Stress [J]. Biotechnology Bulletin, 2023, 39(11): 261-269. |
| [6] | YANG Min, LONG Yu-qing, ZENG Juan, ZENG Mei, ZHOU Xin-ru, WANG Ling, FU Xue-sen, ZHOU Ri-bao, LIU Xiang-dan. Cloning and Function Analysis of Gene UGTPg17 and UGTPg36 in Lonicera macranthoides [J]. Biotechnology Bulletin, 2023, 39(10): 256-267. |
| [7] | LI Xiu-qing, HU Zi-yao, LEI Jian-feng, DAI Pei-hong, LIU Chao, DENG Jia-hui, LIU Min, SUN Ling, LIU Xiao-dong, LI Yue. Cloning and Functional Analysis of Gene GhTIFY9 Related to Cotton Verticillium Wilt Resistance [J]. Biotechnology Bulletin, 2022, 38(8): 127-134. |
| [8] | WANG Nan, ZHANG Rui, PAN Yang-yang, HE Hong-hong, WANG Jing-lei, CUI Yan, YU Si-jiu. Cloning of Bos grunniens TGF-β1 Gene and Its Expression in Major Organs of Female Reproductive System [J]. Biotechnology Bulletin, 2022, 38(6): 279-290. |
| [9] | LI Yang, ZHANG Xiao-tian, PIAO Jing-zi, ZHOU Ru-jun, LI Zi-bo, GUAN Hai-wen. Cloning and Bioinformatics Analysis of Blue-light Receptor EaWC 1 Gene in Elsinoë arachidis [J]. Biotechnology Bulletin, 2022, 38(5): 93-99. |
| [10] | HU Qi, HOU Yu-xiang, LI Rui, LI Mei-lan. Cloning and Expression of CYP79B2 Homologous Genes in Brassica rapa ssp. chinensis [J]. Biotechnology Bulletin, 2022, 38(12): 168-174. |
| [11] | GAN Cheng-yan, ZHANG Xin-hui, WANG Sha, FAN Yao-yu-wei, ZHAO Xue-qing, YUAN Zhao-he. Cloning and Functional Study of the PgSPL2 Gene Related to the Development of Pomegranate Flowers [J]. Biotechnology Bulletin, 2022, 38(12): 194-203. |
| [12] | SHENG Xue-qing, ZHAO Nan, LIN Ya-qiu, CHEN Ding-shuang, WANG Rui-long, LI Ao, WANG Yong, LI Yan-yan. Cloning and Expression Analysis of ZNF32 Gene in Goat [J]. Biotechnology Bulletin, 2022, 38(12): 300-311. |
| [13] | FU Wei-jie, KUANG Jie-hua, LUO Jun, HUANG Jian-sheng, CHEN You-ming, CHEN Gang. Gene Cloning of Galectin-8 in Epinephelus fuscoguttatus♀×E. polyphekadion♂ and Its Expression Responses Under Different of Ferulic Acid Level [J]. Biotechnology Bulletin, 2022, 38(12): 312-323. |
| [14] | ZHANG Lin, WEI Zhen-zhen, SONG Cheng-wei, GUO Li-li, GUO Qi, HOU Xiao-gai, WANG Hua-fang. Cloning and Expression Analysis of PoFD Gene from Paeonia ostii ‘Fengdan’ [J]. Biotechnology Bulletin, 2022, 38(11): 104-111. |
| [15] | DANG Yuan, LI Wei, MIAO Xiang, XIU Yu, LIN Shan-zhi. Cloning of Oleosin Gene PsOLE4 from Prunus sibirica and Its Regulatory Function Analysis for Oil Accumulation [J]. Biotechnology Bulletin, 2022, 38(11): 151-161. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||