








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (12): 328-341.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0491
Previous Articles Next Articles
ZHAO Yi-fan1( ), WANG Tong1(
), WANG Tong1( ), YE Lan2, ZHAO Le1, ZHANG Bao1, DU Peng-qiang3(
), YE Lan2, ZHAO Le1, ZHANG Bao1, DU Peng-qiang3( ), HE Hai-rong1(
), HE Hai-rong1( )
)
Received:2025-05-13
Online:2025-12-26
Published:2026-01-06
Contact:
DU Peng-qiang, HE Hai-rong
E-mail:xi040122@163.com;dupengq@163.com;hhirong@163.com
ZHAO Yi-fan, WANG Tong, YE Lan, ZHAO Le, ZHANG Bao, DU Peng-qiang, HE Hai-rong. Screening, Identification and Whole Genome Analysis of a Paenibacillus Strain Resistant to Root Rot of Rehmannia glutinosa[J]. Biotechnology Bulletin, 2025, 41(12): 328-341.
病原真菌 Pathogenic fungi | 抑菌率 Fungal inhibition rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| QH-1 | QH-9 | QH-16 | QH-33 | QH-39 | QH-41 | ||
| 褐斑病菌 C. cassiicola | 59.07±5.45cd | 45.73±0.84b | 40.13 1.75e | 35.7±2.65d | 49.63±3.65c | NA | |
| 茎基腐病菌 F. graminearum Schw | 88.84±3.49b | NA | 35.76±2.78d | 38.65±2.07c | NA | 47.55±3.98b | |
| 炭疽病菌 C. orbiculare | 51.44±3.50d | 65.51±3.03a | NA | NA | 49.78±4.97d | 40.20±3.69c | |
| 枯萎病菌 F. oxysporum | 15.47±5.11e | NA | 20.36±1.06c | NA | NA | NA | |
| 叶斑病菌 F. solani | 80.28±5.00c | NA | NA | NA | NA | 55.65±6.01d | |
| 黄萎病菌 V. dahliae Kleb | 51.80±3.78d | NA | 42.76±6.7b | 38.95±4.88b | NA | NA | |
| 轮纹病菌 P. herbarum | 89.27±5.25c | 34.77±4.32c | NA | 58.76±5.68a | 42.65±2.09a | NA | |
| 根腐病菌 Fusarium sp. Rf7 | 89.57±2.97a | NA | 60.23±3.54a | NA | 75.23±6.35a | 65.30±5.07a | |
Table 1 Antifungal activity of 6 bacterial strains against plant pathogenic fungi
病原真菌 Pathogenic fungi | 抑菌率 Fungal inhibition rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| QH-1 | QH-9 | QH-16 | QH-33 | QH-39 | QH-41 | ||
| 褐斑病菌 C. cassiicola | 59.07±5.45cd | 45.73±0.84b | 40.13 1.75e | 35.7±2.65d | 49.63±3.65c | NA | |
| 茎基腐病菌 F. graminearum Schw | 88.84±3.49b | NA | 35.76±2.78d | 38.65±2.07c | NA | 47.55±3.98b | |
| 炭疽病菌 C. orbiculare | 51.44±3.50d | 65.51±3.03a | NA | NA | 49.78±4.97d | 40.20±3.69c | |
| 枯萎病菌 F. oxysporum | 15.47±5.11e | NA | 20.36±1.06c | NA | NA | NA | |
| 叶斑病菌 F. solani | 80.28±5.00c | NA | NA | NA | NA | 55.65±6.01d | |
| 黄萎病菌 V. dahliae Kleb | 51.80±3.78d | NA | 42.76±6.7b | 38.95±4.88b | NA | NA | |
| 轮纹病菌 P. herbarum | 89.27±5.25c | 34.77±4.32c | NA | 58.76±5.68a | 42.65±2.09a | NA | |
| 根腐病菌 Fusarium sp. Rf7 | 89.57±2.97a | NA | 60.23±3.54a | NA | 75.23±6.35a | 65.30±5.07a | |
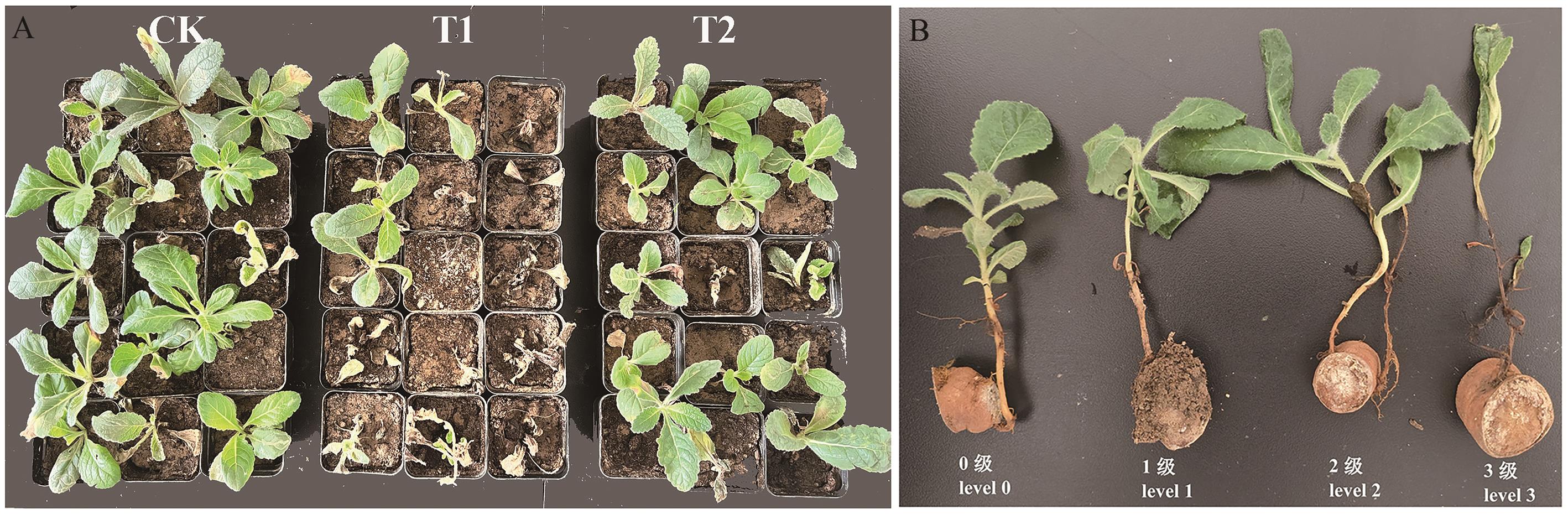
Fig. 2 Pot cultivation conditions of R. glutinosa in the QH-1 against R. glutinosa root rot (A) and disease severity level of R. glutinosa root rot (B)
| 处理 Treatment | 病情指数 Disease index | 发病率 Incidence (%) | 防病效果 Prevention effect (%) |
|---|---|---|---|
| CK(无菌水处理) Treatment with sterile water | 10.12 ± 3.16c | 15.56 ± 4.71c | 46.82 ± 11.75 |
| T1(Rf7处理) Treatment with pathogen Rf7 | 54.32 ± 4.45a | 74.07 ± 9.69a | |
| T2(Rf7+QH-1处理) Treatment with Rf7 and QH-1 | 28.64 ± 5.82b | 49.63 ± 5.88b |
Table 2 Control effect of QH-1 against Fusarium root rot of R. glutinosa
| 处理 Treatment | 病情指数 Disease index | 发病率 Incidence (%) | 防病效果 Prevention effect (%) |
|---|---|---|---|
| CK(无菌水处理) Treatment with sterile water | 10.12 ± 3.16c | 15.56 ± 4.71c | 46.82 ± 11.75 |
| T1(Rf7处理) Treatment with pathogen Rf7 | 54.32 ± 4.45a | 74.07 ± 9.69a | |
| T2(Rf7+QH-1处理) Treatment with Rf7 and QH-1 | 28.64 ± 5.82b | 49.63 ± 5.88b |
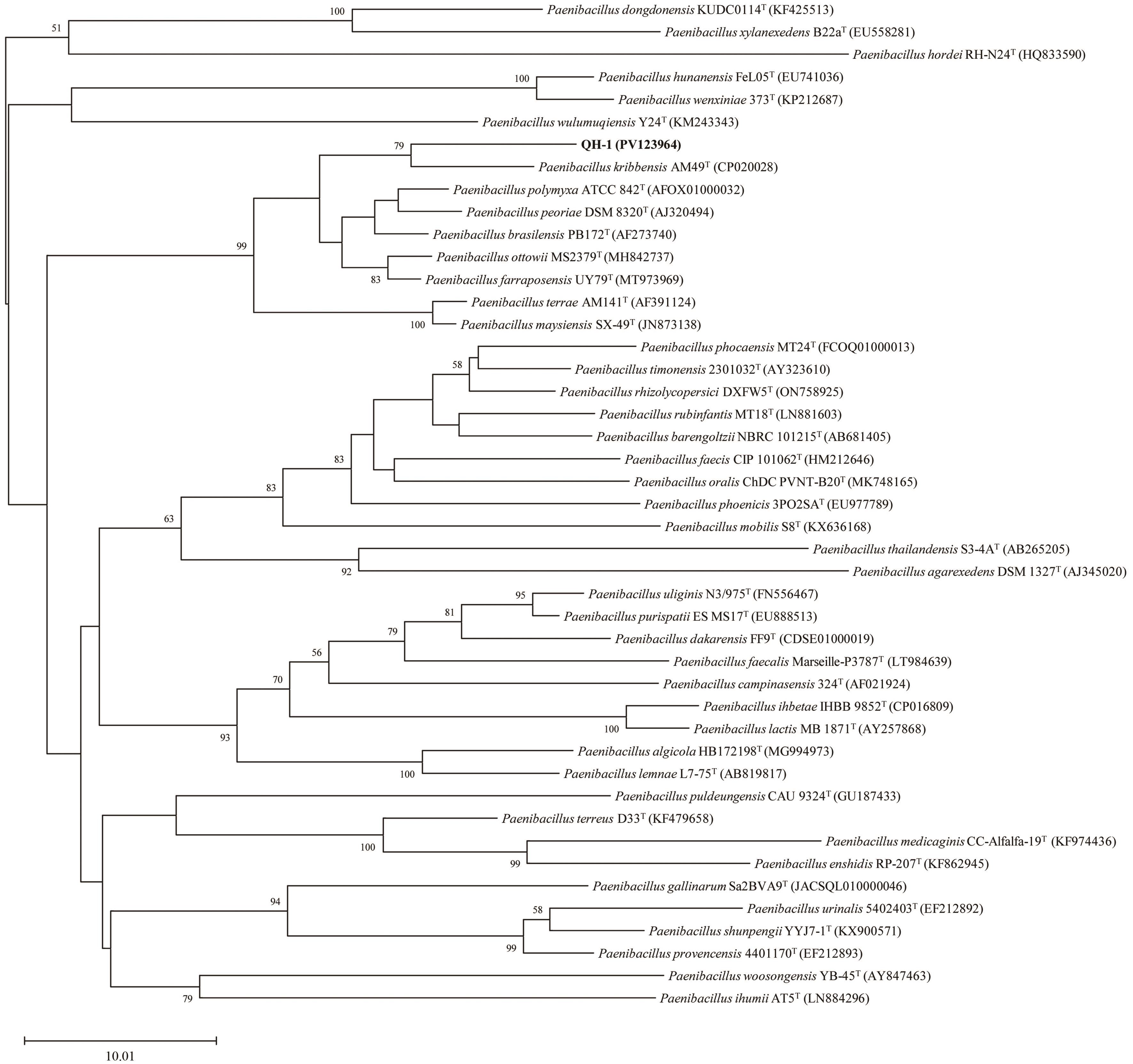
Fig. 3 Neighbour-joining tree showing the phylogenetic position of strain QH-1 based on 16S rRNA gene sequencesBootstrap values >50% (based on 1 000 replications) are shown at branch points. Bar: 10.01 substitutions per nucleotide position
| 特征Characteristic | QH-1 | P. polymyxa ATCC 842T | P. ottowii MS2379T | P. kribbensis AM49T |
|---|---|---|---|---|
| pH | 6-8 | ND | 6-10 | 5-8 |
| 温度 Temperature (℃) | 10-55 | ND | 10-45 | 10-44 |
| NaCl耐受 NaCl tolerance (%) | 5 | 2 | 3 | 4 |
| 硝酸盐还原 Nitrate reduction | + | + | + | + |
| 过氧化氢酶 Catalase | + | ND | + | - |
| 淀粉酶 Amylase | + | + | + | + |
| 吐温80 Tween 80 | + | - | ND | + |
| 牛奶胨化 Milk peptonization | + | + | + | + |
| 明胶液化 Gelatin liquefaction | + | + | + | + |
| 脲酶 Urease | - | - | - | - |
Table 3 Comparison of physiological and biochemical characteristics between QH-1 and its most similar strains
| 特征Characteristic | QH-1 | P. polymyxa ATCC 842T | P. ottowii MS2379T | P. kribbensis AM49T |
|---|---|---|---|---|
| pH | 6-8 | ND | 6-10 | 5-8 |
| 温度 Temperature (℃) | 10-55 | ND | 10-45 | 10-44 |
| NaCl耐受 NaCl tolerance (%) | 5 | 2 | 3 | 4 |
| 硝酸盐还原 Nitrate reduction | + | + | + | + |
| 过氧化氢酶 Catalase | + | ND | + | - |
| 淀粉酶 Amylase | + | + | + | + |
| 吐温80 Tween 80 | + | - | ND | + |
| 牛奶胨化 Milk peptonization | + | + | + | + |
| 明胶液化 Gelatin liquefaction | + | + | + | + |
| 脲酶 Urease | - | - | - | - |
| Item | QH-1-P. polymyxa ATCC 842T | QH-1-P. ottowii MS2379T | QH-1-P. kribbensis AM49T |
|---|---|---|---|
| dDDH (%) | 84.5 | 72.1 | 50.1 |
| ANI (%) | 97.98 | 91.73 | 85.26 |
Table 4 dDDH and ANI of QH-1 and its similar strains
| Item | QH-1-P. polymyxa ATCC 842T | QH-1-P. ottowii MS2379T | QH-1-P. kribbensis AM49T |
|---|---|---|---|
| dDDH (%) | 84.5 | 72.1 | 50.1 |
| ANI (%) | 97.98 | 91.73 | 85.26 |
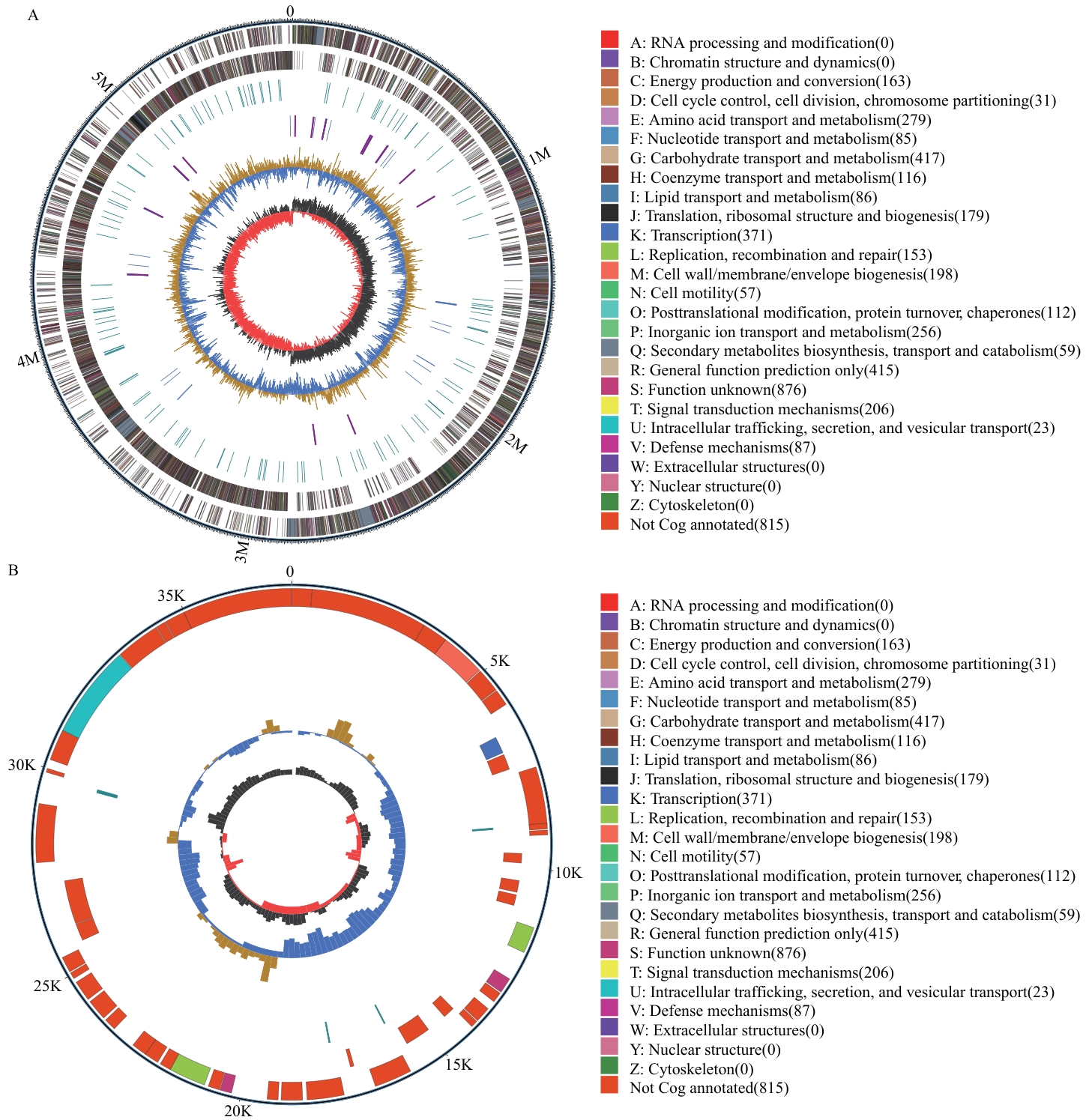
Fig. 5 Genomic circle diagram of QH-1A: Chromosome circular map. B: Plasmid circular map. The outermost circle is the genome size. The second and third circles are the CDS on the positive and negative strands. The fourth circle is repeat sequences. The fifth circle is rRNA and tRNA. The sixth circle is the GC content. The innermost circle is GC-skew value
编号 Number | 基因编号 Gene number | 酶定义 Enzyme definition | 家族 Family |
|---|---|---|---|
| 1 | GE000403 | Chitinase EC 3.2.1.14 | GH18 |
| 2 | GE001510 | Chitinase EC 3.2.1.14 | GH18 |
| 3 | GE004051 | Chitinase EC 3.2.1.14 | GH18 |
| 4 | GE000622 | Cellulase EC 3.2.1.4 | GH5 |
| 5 | GE000624 | Cellulase EC 3.2.1.4 | GH5 |
| 6 | GE002468 | Endo-β-1,4-mannosidase EC 3.2.1.78 | GH5 |
| 7 | GE003415 | β-galactosidase EC 3.2.1.23 | GH5 |
| 8 | GE003585 | Endoglucanase EC 3.2.1.4 | GH5 |
| 9 | GE004895 | Cellulase EC 3.2.1.4 | GH5 |
| 10 | GE001031 | Endoglucanase EC 3.2.1.4 | GH6 |
| 11 | GE000656 | Glucosylceramidase EC 3.2.1.45 | GH30 |
| 12 | GE000930 | Glucosylceramidase EC 3.2.1.45 | GH30 |
| 13 | GE004379 | Endo-β-1,4-mannosidase EC 3.2.1.78 | GH44GH26 |
| 14 | GE002307 | Cellulase EC 3.2.1.4 | GH48 |
| 15 | GE002395 | Endo-β-(1,3)(1,4)-glucanase EC 3.2.1.6 | GH16 |
| 16 | GE004210 | Endo-β-1,3-glucanase EC 3.2.1.39 | GH81 |
Table 5 Enzymes related to cell structural component degradation in the genome of strain QH-1
编号 Number | 基因编号 Gene number | 酶定义 Enzyme definition | 家族 Family |
|---|---|---|---|
| 1 | GE000403 | Chitinase EC 3.2.1.14 | GH18 |
| 2 | GE001510 | Chitinase EC 3.2.1.14 | GH18 |
| 3 | GE004051 | Chitinase EC 3.2.1.14 | GH18 |
| 4 | GE000622 | Cellulase EC 3.2.1.4 | GH5 |
| 5 | GE000624 | Cellulase EC 3.2.1.4 | GH5 |
| 6 | GE002468 | Endo-β-1,4-mannosidase EC 3.2.1.78 | GH5 |
| 7 | GE003415 | β-galactosidase EC 3.2.1.23 | GH5 |
| 8 | GE003585 | Endoglucanase EC 3.2.1.4 | GH5 |
| 9 | GE004895 | Cellulase EC 3.2.1.4 | GH5 |
| 10 | GE001031 | Endoglucanase EC 3.2.1.4 | GH6 |
| 11 | GE000656 | Glucosylceramidase EC 3.2.1.45 | GH30 |
| 12 | GE000930 | Glucosylceramidase EC 3.2.1.45 | GH30 |
| 13 | GE004379 | Endo-β-1,4-mannosidase EC 3.2.1.78 | GH44GH26 |
| 14 | GE002307 | Cellulase EC 3.2.1.4 | GH48 |
| 15 | GE002395 | Endo-β-(1,3)(1,4)-glucanase EC 3.2.1.6 | GH16 |
| 16 | GE004210 | Endo-β-1,3-glucanase EC 3.2.1.39 | GH81 |
基因簇编号 Cluster ID | 基因簇类型 Type | 起始位置 Start | 终止位置 End | 已知基因簇 Similar cluster | 相似度 Similarity (%) | 基因数量 Gene number |
|---|---|---|---|---|---|---|
| C1 | NRPS | 67511 | 131240 | Fusaricidin B | 100 | 34 |
| C2 | Ranthipeptide | 388821 | 410242 | - | - | 12 |
| C3 | Lanthipeptide-class-i, Cyclic-lactone-autoinducer | 1050199 | 1077551 | Paenibacillin | 100 | 22 |
| C4 | Transat-pks NRPS | 1092823 | 1170216 | - | - | 42 |
| C5 | Proteusin | 1283266 | 1303502 | - | - | 19 |
| C6 | NRPS Transat-pks | 1333876 | 1433854 | - | - | 50 |
| C7 | Lassopeptide | 1470252 | 1494319 | - | - | 23 |
| C8 | Lanthipeptide-class-i | 1777757 | 1804766 | Paenilan | 100 | 22 |
| C9 | NRPS-like, Cyclic-lactone-autoinducer | 2091488 | 2153613 | - | - | 54 |
| C10 | NRPS | 2528543 | 2622242 | Tridecaptin | 100 | 38 |
| C11 | NRPS, T1PKS | 2809874 | 2886013 | Laterocidine | 5 | 38 |
| C12 | Betalactone | 2965408 | 2996287 | Varlaxin 1046A/Varlaxin 1022A | 9 | 29 |
| C13 | Cyclic-lactone-autoinducer | 3037002 | 3057550 | - | - | 20 |
| C14 | Transat-pks NRPS, T3PKS, PKS-like | 3563899 | 3665823 | Aurantinin B/aurantinin C/aurantinin D | 35 | 55 |
| C15 | Lanthipeptide | 4368109 | 4393496 | - | - | 16 |
| C16 | NRPS | 4886738 | 4966292 | Polymyxin | 100 | 33 |
| C17 | Lanthipeptide-class-i | 5378895 | 5405329 | Paenicidin A | 100 | 19 |
Table 6 Predicted results of gene clusters for synthesis of secondary metabolites of strain QH-1
基因簇编号 Cluster ID | 基因簇类型 Type | 起始位置 Start | 终止位置 End | 已知基因簇 Similar cluster | 相似度 Similarity (%) | 基因数量 Gene number |
|---|---|---|---|---|---|---|
| C1 | NRPS | 67511 | 131240 | Fusaricidin B | 100 | 34 |
| C2 | Ranthipeptide | 388821 | 410242 | - | - | 12 |
| C3 | Lanthipeptide-class-i, Cyclic-lactone-autoinducer | 1050199 | 1077551 | Paenibacillin | 100 | 22 |
| C4 | Transat-pks NRPS | 1092823 | 1170216 | - | - | 42 |
| C5 | Proteusin | 1283266 | 1303502 | - | - | 19 |
| C6 | NRPS Transat-pks | 1333876 | 1433854 | - | - | 50 |
| C7 | Lassopeptide | 1470252 | 1494319 | - | - | 23 |
| C8 | Lanthipeptide-class-i | 1777757 | 1804766 | Paenilan | 100 | 22 |
| C9 | NRPS-like, Cyclic-lactone-autoinducer | 2091488 | 2153613 | - | - | 54 |
| C10 | NRPS | 2528543 | 2622242 | Tridecaptin | 100 | 38 |
| C11 | NRPS, T1PKS | 2809874 | 2886013 | Laterocidine | 5 | 38 |
| C12 | Betalactone | 2965408 | 2996287 | Varlaxin 1046A/Varlaxin 1022A | 9 | 29 |
| C13 | Cyclic-lactone-autoinducer | 3037002 | 3057550 | - | - | 20 |
| C14 | Transat-pks NRPS, T3PKS, PKS-like | 3563899 | 3665823 | Aurantinin B/aurantinin C/aurantinin D | 35 | 55 |
| C15 | Lanthipeptide | 4368109 | 4393496 | - | - | 16 |
| C16 | NRPS | 4886738 | 4966292 | Polymyxin | 100 | 33 |
| C17 | Lanthipeptide-class-i | 5378895 | 5405329 | Paenicidin A | 100 | 19 |
| [25] | Liu HW, Li JY, Carvalhais LC, et al. Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens [J]. New Phytol, 2021, 229(5): 2873-2885. |
| [26] | Gao M, Xiong C, Gao C, et al. Disease-induced changes in plant microbiome assembly and functional adaptation [J]. Microbiome, 2021, 9(1): 187. |
| [27] | Yuan HB, Yuan MJ, Shi BK, et al. Biocontrol activity and action mechanism of Paenibacillus polymyxa strain Nl4 against pear Valsa canker caused by Valsa pyri [J]. Front Microbiol, 2022, 13: 950742. |
| [28] | Taheri E, Tarighi S, Taheri P. Characterization of root endophytic Paenibacillus polymyxa isolates with biocontrol activity against Xanthomonas translucens and Fusarium graminearum [J]. Biol Control, 2022, 174: 105031. |
| [29] | Weselowski B, Nathoo N, Eastman AW, et al. Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production [J]. BMC Microbiol, 2016, 16(1): 244. |
| [30] | Jung WJ, An KN, Jin YL, et al. Biological control of damping-off caused by Rhizoctonia solani using chitinase-producing Paenibacillus illinoisensis KJA-424 [J]. Soil Biol Biochem, 2003, 35(9): 1261-1264. |
| [31] | Zhai Y, Zhu JX, Tan TM, et al. Isolation and characterization of antagonistic Paenibacillus polymyxa HX-140 and its biocontrol potential against Fusarium wilt of cucumber seedlings [J]. BMC Microbiol, 2021, 21(1): 75. |
| [32] | Liu Y, Wang RH, Cao YH, et al. Identification and antagonistic activity of endophytic bacterial strain Paenibacillus sp. 5L8 isolated from the seeds of maize (Zea mays L., Jingke 968) [J]. Ann Microbiol, 2016, 66(2): 653-660. |
| [33] | Xu SJ, Kim BS. Evaluation of Paenibacillus polymyxa strain SC09-21 for biocontrol of Phytophthora blight and growth stimulation in pepper plants [J]. Trop Plant Pathol, 2016, 41(3): 162-168. |
| [1] | 国家药典委员会. 中华人民共和国药典-一部2015年版 [M]. 北京: 中国医药科技出版社, 2015. |
| National Pharmacopoeia Committee. People’s republic of China (PRC) pharmacopoeia-No.1 department [M]. Beijing: China Medical Science Press, 2015. | |
| [2] | Bian ZY, Zhang R, Zhang X, et al. Extraction, structure and bioactivities of polysaccharides from Rehmannia glutinosa: a review [J]. J Ethnopharmacol, 2023, 305: 116132. |
| [3] | 吴廷娟, 杜权, 王玉星, 等. 地黄根腐病病原菌的分离鉴定及其防治 [J]. 江苏农业科学, 2021, 49(22): 132-136. |
| Wu TJ, Du Q, Wang YX, et al. Isolation, identification and control of root rot pathogen of Rehmannia glutinosa [J]. Jiangsu Agric Sci, 2021, 49(22): 132-136. | |
| [4] | Lim JD, Yun SJ, Chung IM, et al. Resveratrol synthase transgene expression and accumulation of resveratrol glycoside in Rehmannia glutinosa [J]. Mol Breed, 2005, 16(3): 219-233. |
| [5] | Wu LK, Wang JY, Huang WM, et al. Plant-microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture [J]. Sci Rep, 2015, 5: 15871. |
| [6] | Cao LL, Kang QY, Tian Y. Pesticide residues: Bridging the gap between environmental exposure and chronic disease through omics [J]. Ecotoxicol Environ Saf, 2024, 287: 117335. |
| [7] | Mei LC, Chen HM, Dong AY, et al. Pesticide informatics platform (PIP): an international platform for pesticide discovery, residue, and risk evaluation [J]. J Agric Food Chem, 2022, 70(22): 6617-6623. |
| [8] | Pan XY, Yue Y, Zhao FJ, et al. Rhizosphere microbes facilitate the break of chlamydospore dormancy and root colonization of rice false smut fungi [J]. Cell Host Microbe, 2025, 33(5): 731-744.e5. |
| [9] | Wang X, Xu Q, Hu K, et al. A coculture of Enterobacter and Comamonas species reduces cadmium accumulation in rice [J]. Mol Plant Microbe Interactions® , 2023, 36(2): 95-108. |
| [10] | Wang LW, Zhang YB, Wang Y, et al. Inoculation with Penicillium citrinum aids ginseng in resisting Fusarium oxysporum by regulating the root and rhizosphere microbial communities [J]. Rhizosphere, 2022, 22: 100535. |
| [11] | Kamoun S, Furzer O, Jones JDG, et al. The Top 10 oomycete pathogens in molecular plant pathology [J]. Mol Plant Pathol, 2015, 16(4): 413-434. |
| [12] | 李文均, 刘兰, 焦建宇, 等. 细菌古菌系统分类学原理及鉴定技术 [M]. 北京: 高等教育出版社, 2024. |
| Li WJ, Liu L, Jiao JY, et al. Systematic taxonomy principle and identification technology of bacteria and archaea [M]. Beijing: Higher Education Press, 2024. | |
| [13] | He HR, Huang JR, Zhao ZZ, et al. Whole genome analysis of Streptomyces sp. RerS4, a Rehmannia glutinosa rhizosphere microbe producing a new lipopeptide [J]. Heliyon, 2023, 9(9): e19543. |
| [14] | 郭芳芳, 谢镇, 卢鹏, 等. 一株多粘类芽胞杆菌的鉴定及其生防促生效果初步测定 [J]. 中国生物防治学报, 2014, 30(4): 489-496. |
| Guo FF, Xie Z, Lu P, et al. Identification of a novel Paenibacillus polymyxa strain and its biocontrol and plant growth-promoting effects [J]. Chin J Biol Control, 2014, 30(4): 489-496. | |
| [15] | 杨剑锋, 张园园, 王娜, 等. 苜蓿根腐病病原菌的分离鉴定及苜蓿品种的抗性评价 [J]. 中国草地学报, 2020, 42(3): 52-60. |
| Yang JF, Zhang YY, Wang N, et al. Isolation and identification of pathogens causing root rot disease in alfalfa and the evaluation of resistant ability of alfalfa varieties to Fusarium equiseti and F. tricinctum [J]. Chin J Grassland, 2020, 42(3): 52-60. | |
| [16] | 陈逢玲, 孙卓, 林红梅, 等. 关防风根腐病拮抗细菌筛选与鉴定 [J]. 微生物学通报, 2022, 49(8): 3192-3204. |
| Chen FL, Sun Z, Lin HM, et al. Screening and identification of the antagonistic bacteria against root rot of Saposhnikovia divaricata [J]. Microbiol China, 2022, 49(8): 3192-3204. | |
| [17] | 黄家锐, 杜鹏强, 李文均, 等. 地黄内生放线菌的分离及地黄轮纹病拮抗菌Streptomyces folium leaf-16的新种鉴定 [J]. 微生物学报, 2022, 62(12): 4953-4963. |
| Huang JR, Du PQ, Li WJ, et al. Isolation of endophytic actinomycetes from Rehmannia glutinosa and identification of a new species Streptomyces folium leaf-16 resistant to Phoma herbarum [J]. Acta Microbiol Sin, 2022, 62(12): 4953-4963. | |
| [18] | Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees [J]. Mol Biol Evol, 1987, 4(4): 406-425. |
| [19] | Blin K, Shaw S, Kloosterman AM, et al. antiSMASH 6.0: improving cluster detection and comparison capabilities [J]. Nucleic Acids Res, 2021, 49(W1): W29-W35. |
| [20] | Liu K, Newman M, McInroy JA, et al. Selection and assessment of plant growth-promoting rhizobacteria for biological control of multiple plant diseases [J]. Phytopathology® , 2017, 107(8): 928-936. |
| [21] | Dobrzyński J, Naziębło A. Paenibacillus as a biocontrol agent for fungal phytopathogens: is P. polymyxa the only one worth attention? [J]. Microb Ecol, 2024, 87(1): 134. |
| [22] | Luo CH, He YJ, Chen YP. Rhizosphere microbiome regulation: Unlocking the potential for plant growth [J]. Curr Res Microb Sci, 2025, 8: 100322. |
| [23] | Liu HW, Brettell LE, Qiu ZG, et al. Microbiome-mediated stress resistance in plants [J]. Trends Plant Sci, 2020, 25(8): 733-743. |
| [24] | Wen T, Zhao ML, Yuan J, et al. Root exudates mediate plant defense against foliar pathogens by recruiting beneficial microbes [J]. Soil Ecol Lett, 2021, 3(1): 42-51. |
| [1] | LI Ya-tao, ZHANG Zhi-peng, ZHAO Meng-yao, LYU Zhen, GAN Tian, WEI Hao, WU Shu-feng, MA Yu-chao. Whole Genome Analysis of Bradyrhizobium sp. Bd1 and the Negative Regulating Function of TetR3 during Cell Growth and Nodulation [J]. Biotechnology Bulletin, 2025, 41(9): 289-301. |
| [2] | LI Kai-yue, DENG Xiao-xia, YIN Yuan, DU Ya-tong, XU Yuan-jing, WANG Jing-hong, YU Song, LIN Ji-xiang. Identification of LEA Gene Family and Analysis on Its Response to Aluminum Stress in Ricinus communis L. [J]. Biotechnology Bulletin, 2025, 41(7): 128-138. |
| [3] | WU Ze-yin, HUANG Chen-yang, ZHAO Meng-ran, ZHANG Li-jiao, YAO Fang-jie. Genome-specific Analysis of Pleurotus cornucopiae CCMSSC 04611 with Short Stipe [J]. Biotechnology Bulletin, 2025, 41(5): 320-332. |
| [4] | ZHANG Hui, LU Wen-cai, WANG Dong, LIU Qian, MA Lian-jie. Identification of Bacillus cereus YT2-1C with High Indoleacetic Acid Yield and Its Growth-promoting Effect [J]. Biotechnology Bulletin, 2025, 41(5): 300-309. |
| [5] | SONG Ying-pei, WANG Can, ZHOU Hui-wen, KONG Ke-ke, XU Meng-ge, WANG Rui-kai. Analysis of Soybean Pod Dehiscence Habit Based on Whole Genome Association Analysis and Genetic Diversity [J]. Biotechnology Bulletin, 2025, 41(2): 97-106. |
| [6] | LIU Wen-zhi, HE Dan, LI Peng, FU Ying-lin, ZHANG Yi-xin, WEN Hua-jie, YU Wen-qing. Paenibacillus polymyxa New Strain X-11 and Its Growth-promoting Effects on Tomato and Rice [J]. Biotechnology Bulletin, 2024, 40(9): 249-259. |
| [7] | ZHOU Jiang-hong, XIA Fei, ZHONG Li, QIU Lan-fen, LI Guang, LIU Qian, ZHANG Guo-feng, SHAO Jin-li, LI Na, CHE Shao-chen. Whole Genome Sequencing and Comparative Genomic Analysis of Antagonistic Bacterium CCBC3-3-1 against Verticillium dahlia [J]. Biotechnology Bulletin, 2024, 40(7): 235-246. |
| [8] | TIAN Tong-tong, GE Jia-zhen, GAO Peng-cheng, LI Xue-rui, SONG Guo-dong, ZHENG Fu-ying, CHU Yue-feng. Whole Genome Sequencing and Bioinformatics Analysis of Mycoplasma ovipneumoniae GH3-3 Strain [J]. Biotechnology Bulletin, 2024, 40(7): 323-334. |
| [9] | XU Wei-fang, LI He-yu, ZHANG Hui, HE Zi-ang, GAO Wen-heng, XIE Zi-yang, WANG Chuan-wen, YIN Deng-ke. Efficacy and Its Mechanism of Bacterial Strain HX0037 on the Control of Anthracnose Disease of Trichosanthes kirilowii Maxim [J]. Biotechnology Bulletin, 2024, 40(4): 228-241. |
| [10] | LU Yu-dan, LIU Xiao-chi, FENG Xin, CHEN Gui-xin, CHEN Yi-ting. Identification of the Kiwifruit BBX Gene Family and Analysis of Their Transcriptional Characteristics [J]. Biotechnology Bulletin, 2024, 40(2): 172-182. |
| [11] | YIN Zi-wei, HONG Yu. Study on the Effect of Rhodococcus rhodochrous NB1 on the Tolerance to Salt and Growth-promoting of Maize and Its Whole Genome [J]. Biotechnology Bulletin, 2024, 40(12): 193-207. |
| [12] | WANG Zi, SHI Jin-chuan, WANG Yong-qiang, SUN Miao, MENG Ling-hao, GENG Chao, LIU Kai. Whole Genome Sequencing and Genome Evolution Analysis of Capsular Serotype A and D Pasteurella multocida of Bovine [J]. Biotechnology Bulletin, 2024, 40(12): 282-290. |
| [13] | WANG Teng-hui, GE Wen-dong, LUO Ya-fang, FAN Zhen-yu, WANG Yu-shu. Gene Mapping of Kale White Leaves Based on Whole Genome Re-sequencing of Extreme Mixed Pool(BSA) [J]. Biotechnology Bulletin, 2023, 39(9): 176-182. |
| [14] | FANG Lan, LI Yan-yan, JIANG Jian-wei, CHENG Sheng, SUN Zheng-xiang, ZHOU Yi. Isolation, Identification and Growth-promoting Characteristics of Endohyphal Bacterium 7-2H from Endophytic Fungi of Spiranthes sinensis [J]. Biotechnology Bulletin, 2023, 39(8): 272-282. |
| [15] | GUO Shao-hua, MAO Hui-li, LIU Zheng-quan, FU Mei-yuan, ZHAO Ping-yuan, MA Wen-bo, LI Xu-dong, GUAN Jian-yi. Whole Genome Sequencing and Comparative Genome Analysis of a Fish-derived Pathogenic Aeromonas Hydrophila Strain XDMG [J]. Biotechnology Bulletin, 2023, 39(8): 291-306. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||