








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (11): 22-27.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0941
Previous Articles Next Articles
XU Xin-xin1( ), LI Yan-jun1, ZHANG Wei1, HUANG Huo-qing2, LUO Hui-ying2, YAO Bin2(
), LI Yan-jun1, ZHANG Wei1, HUANG Huo-qing2, LUO Hui-ying2, YAO Bin2( )
)
Received:2025-09-02
Online:2025-11-26
Published:2025-12-09
Contact:
YAO Bin
E-mail:xuxinxin@caas.cn;yaobin@caas.cn
XU Xin-xin, LI Yan-jun, ZHANG Wei, HUANG Huo-qing, LUO Hui-ying, YAO Bin. Artificial Starch Biosynthesis Technology: Progress, Challenges, and Prospects[J]. Biotechnology Bulletin, 2025, 41(11): 22-27.
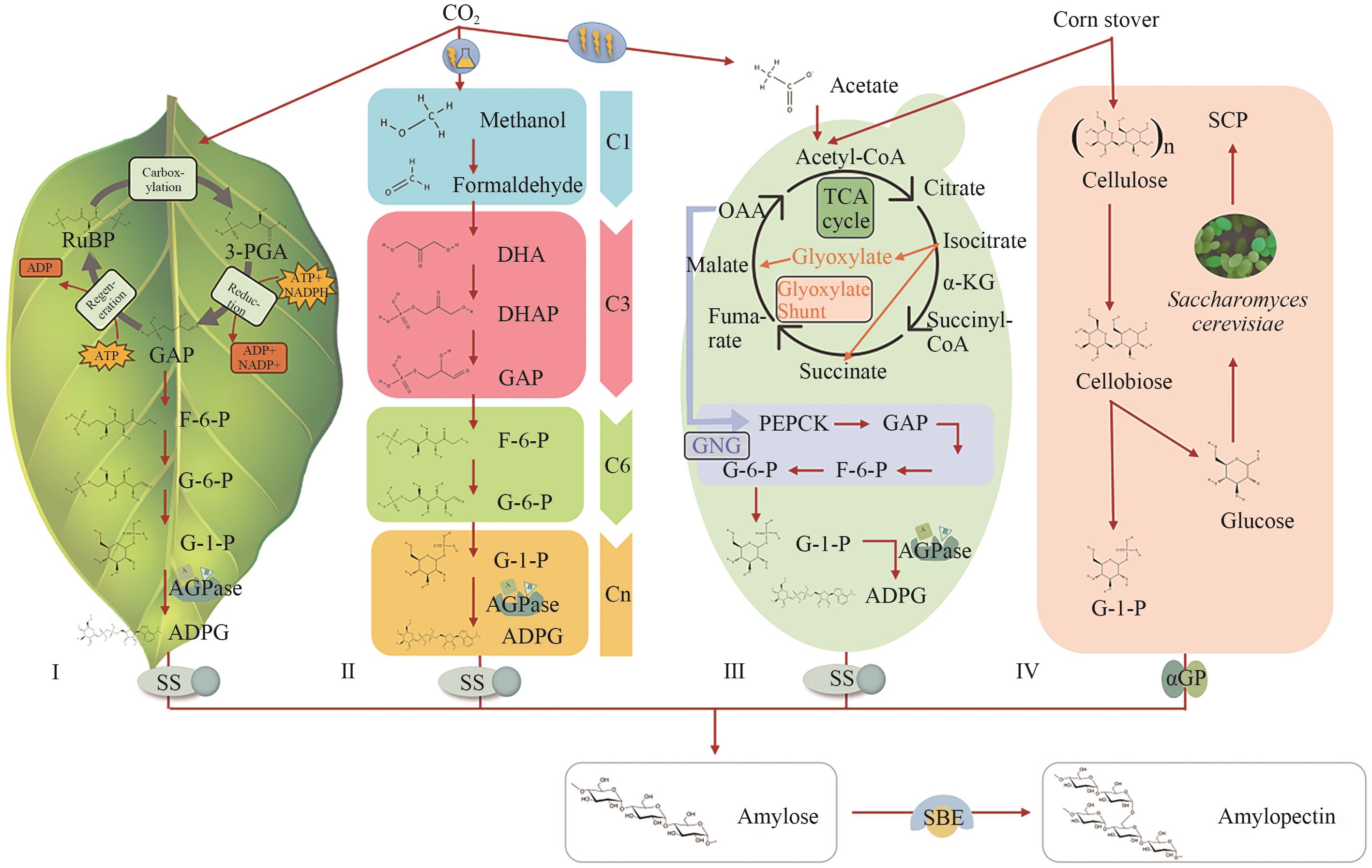
Fig. 1 Four representative pathways in starch biosynthesis(Ⅰ) Natural starch synthesis pathways in plants, where CO₂ is fixed through the Calvin-Benson cycle in chloroplasts, which are subsequently converted into ADP-glucose by AGPase and polymerized into amylose and amylopectin by SS and SBE. (Ⅱ) Artificial cell-free chemoenzymatic starch synthesis from CO₂, in which CO₂ is first reduced electrochemically to methanol, followed by stepwise enzymatic conversion to ADP-glucose, which serves as the direct precursor for amylose and amylopectin biosynthesis. (Ⅲ) Engineered yeast-mediated starch biosynthesis pathway, where CO₂-derived acetate is metabolized through the TCA cycle, glyoxylate shunt, and gluconeogenesis to generate glucose-6-phosphate, which is redirected via heterologously expressed AGPase, SS, and SBE for intracellular starch accumulation. (Ⅳ) Bio-refining system for co-production of artificial starch and SCP from corn stover. Pretreated corn stover is enzymatically hydrolyzed into cellobiose. Cellobiose is phosphorylated into G-1-P, which is then polymerized by αGP to generate synthetic amylose. Glucose is utilized by S. cerevisiae to produce microbial protein. RuBP: Ribulose-1,5-bisphosphate; 3-PGA: 3-phosphoglycerate; GAP: glyceraldehyde-3-phosphate; F-6-P: fructose-6-phosphate; G-6-P: glucose-6-phosphate; G-1-P: glucose-1-phosphate; ADPG: adenosine diphosphate glucose; DHA: dihydroxyacetone; DHAP: dihydroxyacetone phosphate; TCA cycle: tricarboxylic acid cycle; OAA: oxaloacetate; α-KG: α-ketoglutarate; PEPCK: phosphoenolpyruvate carboxykinase; GNG: gluconeogenesis; SCP: single-cell protein; AGPase: ADP-glucose pyrophosphorylase; SS: starch synthase; SBE: starch branching enzyme; αGP: α-glucan phosphorylase.
| [1] | Keeling PL, Myers AM. Biochemistry and genetics of starch synthesis [J]. Annu Rev Food Sci Technol, 2010, 1: 271-303. |
| [2] | Bahaji A, Li J, Sánchez-López ÁM, et al. Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields [J]. Biotechnol Adv, 2014, 32(1): 87-106. |
| [3] | Kong XB. China must protect high-quality arable land [J]. Nature, 2014, 506(7486): 7. |
| [4] | Lobell DB, Gourdji SM. The influence of climate change on global crop productivity [J]. Plant Physiol, 2012, 160(4): 1686-1697. |
| [5] | Elliott J, Glotter M, Ruane AC, et al. Characterizing agricultural impacts of recent large-scale US droughts and changing technology and management [J]. Agric Syst, 2018, 159: 275-281. |
| [6] | Laudien R, Schauberger B, Gleixner S, et al. Assessment of weather-yield relations of starchy maize at different scales in Peru to support the NDC implementation [J]. Agric For Meteor, 2020, 295: 108154. |
| [7] | Graham AE, Ledesma-Amaro R. The microbial food revolution [J]. Nat Commun, 2023, 14: 2231. |
| [8] | Zheng TT, Zhang ML, Wu LH, et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering [J]. Nat Catal, 2022, 5(5): 388-396. |
| [9] | Tang HT, Wu LH, Guo SY, et al. Metabolic engineering of yeast for the production of carbohydrate-derived foods and chemicals from C1-3 molecules [J]. Nat Catal, 2024, 7(1): 21-34. |
| [10] | Abt MR, Zeeman SC. Evolutionary innovations in starch metabolism [J]. Curr Opin Plant Biol, 2020, 55: 109-117. |
| [11] | Llorente B, Williams TC, Goold HD, et al. Harnessing bioengineered microbes as a versatile platform for space nutrition [J]. Nat Commun, 2022, 13: 6177. |
| [12] | Cai T, Sun HB, Qiao J, et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide [J]. Science, 2021, 373(6562): 1523-1527. |
| [13] | Bar-Even A, Noor E, Lewis NE, et al. Design and analysis of synthetic carbon fixation pathways [J]. Proc Natl Acad Sci USA, 2010, 107(19): 8889-8894. |
| [14] | Schwander T, Schada von Borzyskowski L, Burgener S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro [J]. Science, 2016, 354(6314): 900-904. |
| [15] | Miller TE, Beneyton T, Schwander T, et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts [J]. Science, 2020, 368(6491): 649-654. |
| [16] | Kirschbaum MUF. Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies [J]. Plant Physiol, 2011, 155(1): 117-124. |
| [17] | Hadadi N, Hafner J, Shajkofci A, et al. ATLAS of biochemistry: a repository of all possible biochemical reactions for synthetic biology and metabolic engineering studies [J]. ACS Synth Biol, 2016, 5(10): 1155-1166. |
| [18] | Erb TJ, Jones PR, Bar-Even A. Synthetic metabolism: metabolic engineering meets enzyme design [J]. Curr Opin Chem Biol, 2017, 37: 56-62. |
| [19] | Saithong T. A formal path inference of starch biosynthesis via mathematical modelling of metabolic changes in excess CO2 [J]. J Comput Sci Syst Biol, 2012, 5(2): 24-37. |
| [20] | Liu XT, Li LQ, Zhao G, et al. Optimization strategies for CO2 biological fixation [J]. Biotechnol Adv, 2024, 73: 108364. |
| [21] | Tan ZG, Li J, Hou J, et al. Designing artificial pathways for improving chemical production [J]. Biotechnol Adv, 2023, 64: 108119. |
| [22] | Yang X, Yuan QQ, Luo H, et al. Systematic design and in vitro validation of novel one-carbon assimilation pathways [J]. Metab Eng, 2019, 56: 142-153. |
| [23] | Shi ZH, Xu ZY, Rong WH, et al. Reprogramming yeast metabolism for customized starch-rich micro-grain through low-carbon microbial manufacturing [J]. Nat Commun, 2025, 16: 2784. |
| [24] | Hann EC, Overa S, Harland-Dunaway M, et al. A hybrid inorganic-biological artificial photosynthesis system for energy-efficient food production [J]. Nat Food, 2022, 3(6): 461-471. |
| [25] | Liu N, Qiao KJ, Stephanopoulos G. 13C metabolic flux analysis of acetate conversion to lipids by Yarrowia lipolytica [J]. Metab Eng, 2016, 38: 86-97. |
| [26] | Sáez-Sáez J, Wang GK, Marella ER, et al. Engineering the oleaginous yeast Yarrowia lipolytica for high-level resveratrol production [J]. Metab Eng, 2020, 62: 51-61. |
| [27] | Sharma J, Kumar V, Prasad R, et al. Engineering of Saccharomyces cerevisiae as a consolidated bioprocessing host to produce cellulosic ethanol: Recent advancements and current challenges [J]. Biotechnol Adv, 2022, 56: 107925. |
| [28] | FAO. Food outlook: biannual report on global food markets [M]. Rome: Food and Agriculture Organization of the United Nations, 2019. |
| [29] | Xu XX, Zhang W, You C, et al. Biosynthesis of artificial starch and microbial protein from agricultural residue [J]. Sci Bull, 2023, 68(2): 214-223. |
| [1] | LIU Yu-shi, LI Zhen, ZOU Yu-chen, TANG Wei-wei, LI Bin. Advances in Spatial Metabolomics in Medicinal Plants [J]. Biotechnology Bulletin, 2025, 41(9): 22-31. |
| [2] | WANG Bin, LIN Chong, YUAN Xiao, JIANG Yuan-yuan, WANG Yu-kun, XIAO Yan-hui. Cloning of bHLH Transcription Factor UNE10 and Its Regulatory Roles in the Biosynthesis of Volatile Compounds in Clove Basil [J]. Biotechnology Bulletin, 2025, 41(9): 207-218. |
| [3] | ZHENG Qian-ming, YAN Shuang, XIE Pu, WANG Hong-lin. Expression and Enzyme Activity Identification of Cell Wall Invertase Gene SmCWIN6 from Red Pitaya [J]. Biotechnology Bulletin, 2025, 41(8): 267-275. |
| [4] | CAI Ru-feng, YANG Yu-xuan, YU Ji-zheng, LI Jia-nan. Artificial Intelligence Transforms Protein Engineering: From Structural Analysis to Synthetic Biology through Algorithmic Advancements [J]. Biotechnology Bulletin, 2025, 41(8): 1-10. |
| [5] | LIU Jiao-jiao, MU De-mei, XIA Li-ming, FANG Yong, LIU Zuo-jun. Screening of Cellulose-degrading Bacteria in Pleurotus ostreatus Cultivation Substrate and Evaluation of Degradation Effect of Microbial Consortium [J]. Biotechnology Bulletin, 2025, 41(7): 326-335. |
| [6] | GAO Jing, CHENG Yi-cun, GAO Ming, ZHAO Yun-xiao, WANG Yang-dong. Regulation of Plant Tannin Synthesis and Mechanisms of Its Responses to Environment [J]. Biotechnology Bulletin, 2025, 41(7): 49-59. |
| [7] | HUANG Xu-sheng, ZHOU Ya-li, CHAI Xu-dong, WEN Jing, WANG Ji-ping, JIA Xiao-yun, LI Run-zhi. Cloning of Plastidial PfLPAT1B Gene from Perilla frutescens and Its Functional Analysis in Oil Biosynthesis [J]. Biotechnology Bulletin, 2025, 41(7): 226-236. |
| [8] | WU Ya, YAO Run, YANG Han-ting, LIU Wei, YANG Shuai, SONG Chi, CHEN Shi-lin. Genome-wide Identification and Expression Analysis of SDR Gene Family in Mentha suaveolens ‘Variegata’ [J]. Biotechnology Bulletin, 2025, 41(5): 175-185. |
| [9] | QU Shan, ZHAO Yue, LI Ya-hua, ZHENG Gui-ling, XIAN Hong-quan. A Study on the Interaction between Transcriptional Factor and Protein of Tachi2 Chitinase Gene in Trichoderma asperellum [J]. Biotechnology Bulletin, 2025, 41(5): 310-319. |
| [10] | LIU Li, WANG Hui, GUAN Tian-shu, LI Bai-hong, YU Shu-yi. Screening the Interacting Protein of Abscisic Acid Receptor VvPYL4 and the Gene Expression of the Interacting Protein in Grape [J]. Biotechnology Bulletin, 2025, 41(4): 188-197. |
| [11] | LU Tian-yi, LI Ai-peng, FEI Qiang. Research Progress in the Biosynthesis of Polylactic Acid [J]. Biotechnology Bulletin, 2025, 41(4): 47-60. |
| [12] | LI Xiao-ming, SHANG Xiu-hua, WANG You-shuang, WU Zhi-hua. Research Progress in Benzoxazinoids in Plants [J]. Biotechnology Bulletin, 2025, 41(4): 9-20. |
| [13] | YU Ting, HUANG Dan-dan, ZHU Yan-hui, YANG Mei-hong, AI Ju, GAO Dong-li. Screening and Interaction Verification of Transcription Factors Stpatatin 05 Gene in Potato [J]. Biotechnology Bulletin, 2025, 41(3): 137-145. |
| [14] | LU Feng, HUANG Yu-hong, LIN Yan-na, MA Fu-qiang. Advances on Molecular Modifications of Formate Dehydrogenase for CO₂ Reduction [J]. Biotechnology Bulletin, 2025, 41(3): 14-24. |
| [15] | CHE Jian-mei, ZHENG Xue-fang, WANG Jie-ping, CHEN Yan-ping, CHEN Bing-xing, LIU Bo. Screening, Identification and Whole Genome Analysis of a Cellulase Producing Strain [J]. Biotechnology Bulletin, 2025, 41(3): 294-307. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||