








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (1): 247-257.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0217
Previous Articles Next Articles
CAO Xiu-kai1( ), WANG Shan2, GE Ling2, ZHANG Wei-bo2, SUN Wei1,2(
), WANG Shan2, GE Ling2, ZHANG Wei-bo2, SUN Wei1,2( )
)
Received:2021-02-25
Online:2022-01-26
Published:2022-02-22
Contact:
SUN Wei
E-mail:cxkai0909@163.com;dkxmsunwei@163.com
CAO Xiu-kai, WANG Shan, GE Ling, ZHANG Wei-bo, SUN Wei. Advances in Extrachromosomal Circular DNA and Their Application in Domestic Animal Breeding[J]. Biotechnology Bulletin, 2022, 38(1): 247-257.
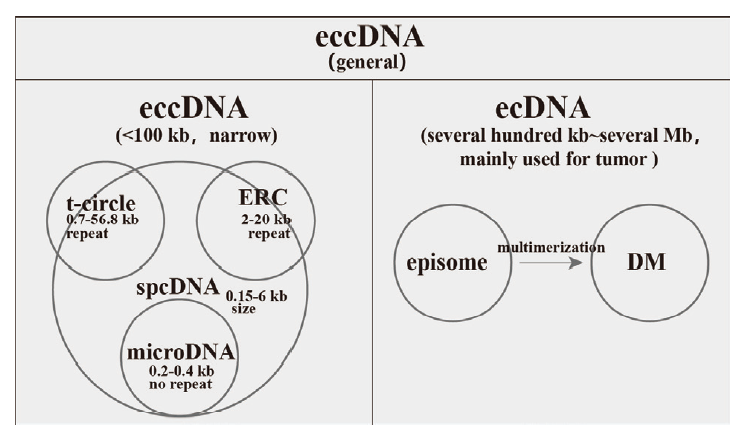
Fig.1 eccDNA classification eccDNA in narrow sense are < 100 kb,including t-circle,ERC and microDNA according to their origins. Notably,spcDNA is a concept in the early stage of studying eccDNA,it emphasizes their sizes rather than their sequence features. eccDNA with size ranging from several hundred kilobases to several megabases are common in tumor and termed as ecDNA
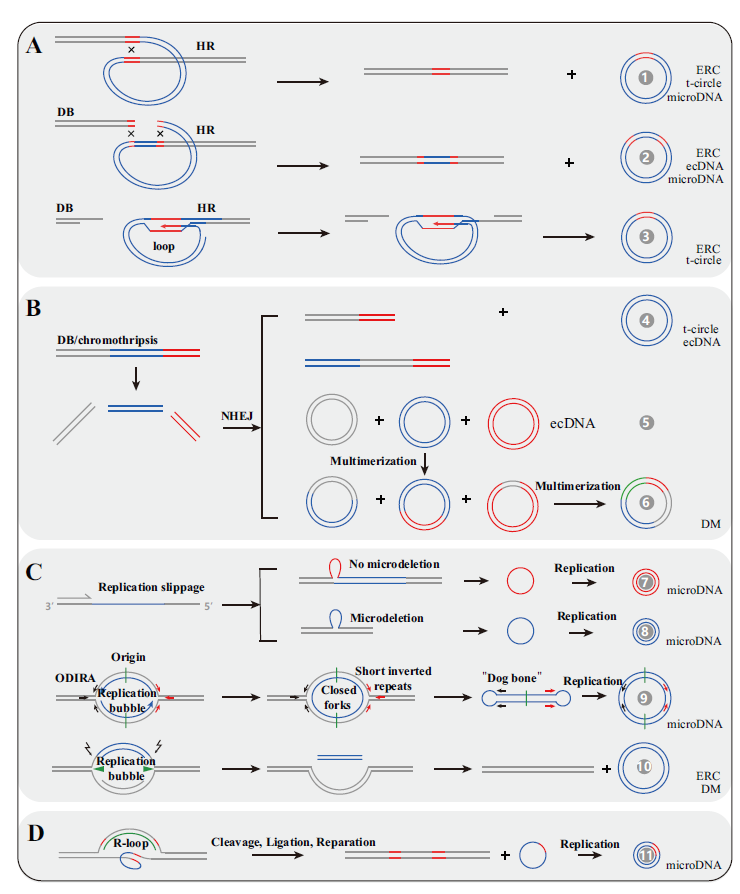
Fig.2 Possible mechanisms of forming eccDNA A:Formed by HR. B:Formed by NHEJ. C:Formed by DNA replication. D:Formed by transcription mediated by R-loop. There are 11 kinds of potential mechanisms for eccDNA formation and their detail information can be found in references listed in Table 1
| 机制编号 Mechanism No. | eccDNA类型eccDNA type | 时间/类型 Year/Type | 参考文献 Reference |
|---|---|---|---|
| 1 | ERC | 2019,Review | [ |
| 2019,Article | [ | ||
| t-circle | 2004,Review | [ | |
| 2019,Article | [ | ||
| microDNA | 2015,Article | [ | |
| 2019,Article | [ | ||
| 2 | ERC | 1999,Article | [ |
| ecDNA | 2010,Article | [ | |
| microDNA | 2018,Review | [ | |
| 3 | ERC | 2019,Article | [ |
| t-circle | 2004,Review | [ | |
| 2019,Article | [ | ||
| 4 | t-circle | 2004,Review | [ |
| ecDNA | 2020,Review | [ | |
| 2020,Review | [ | ||
| 5 | ecDNA | 2020,Review | [ |
| 2020,Review | [ | ||
| 2020,Review | [ | ||
| 6 | DM | 2020,Review | [ |
| 2020,Review | [ | ||
| 2020,Review | [ | ||
| 7 | microDNA | 2015,Article | [ |
| 2018,Review | [ | ||
| 8 | microDNA | 2015,Article | [ |
| 2018,Review | [ | ||
| 9 | microDNA | 2015,Article | [ |
| 2018,Review | [ | ||
| 10 | ERC | 2018,Article | [ |
| DM | 2004,Article | [ | |
| 2020,Review | [ | ||
| 11 | microDNA | 2018,Review | [ |
| 2020,Review | [ |
Table 1 Corresponding references of 11 kinds of potential mechanisms for eccDNA formation
| 机制编号 Mechanism No. | eccDNA类型eccDNA type | 时间/类型 Year/Type | 参考文献 Reference |
|---|---|---|---|
| 1 | ERC | 2019,Review | [ |
| 2019,Article | [ | ||
| t-circle | 2004,Review | [ | |
| 2019,Article | [ | ||
| microDNA | 2015,Article | [ | |
| 2019,Article | [ | ||
| 2 | ERC | 1999,Article | [ |
| ecDNA | 2010,Article | [ | |
| microDNA | 2018,Review | [ | |
| 3 | ERC | 2019,Article | [ |
| t-circle | 2004,Review | [ | |
| 2019,Article | [ | ||
| 4 | t-circle | 2004,Review | [ |
| ecDNA | 2020,Review | [ | |
| 2020,Review | [ | ||
| 5 | ecDNA | 2020,Review | [ |
| 2020,Review | [ | ||
| 2020,Review | [ | ||
| 6 | DM | 2020,Review | [ |
| 2020,Review | [ | ||
| 2020,Review | [ | ||
| 7 | microDNA | 2015,Article | [ |
| 2018,Review | [ | ||
| 8 | microDNA | 2015,Article | [ |
| 2018,Review | [ | ||
| 9 | microDNA | 2015,Article | [ |
| 2018,Review | [ | ||
| 10 | ERC | 2018,Article | [ |
| DM | 2004,Article | [ | |
| 2020,Review | [ | ||
| 11 | microDNA | 2018,Review | [ |
| 2020,Review | [ |
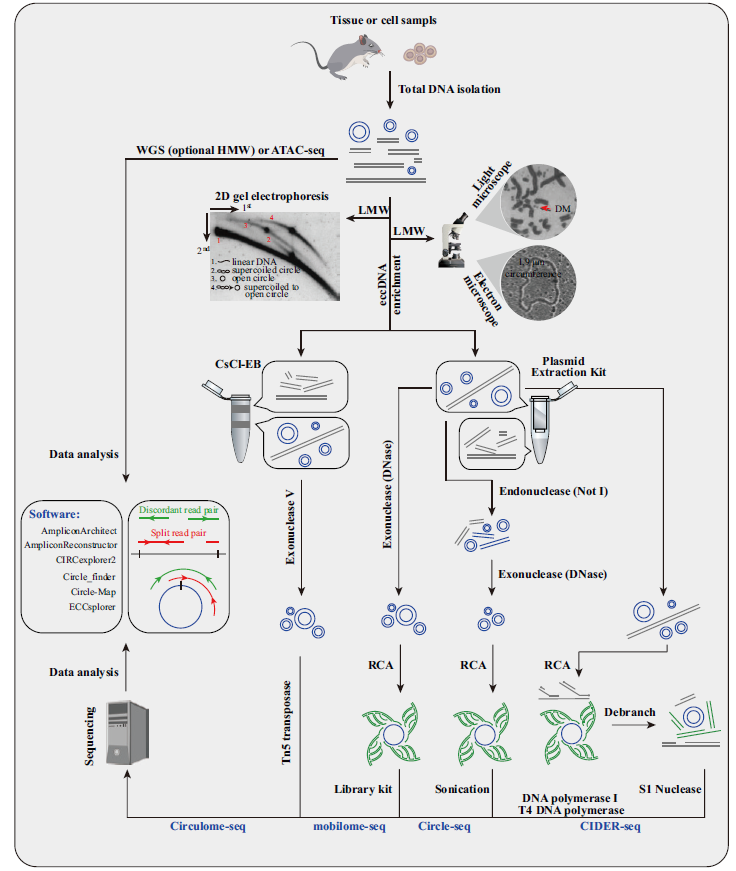
Fig.4 Methods for eccDNA identification Microscope and 2D electrophoresis are used for eccDNA identification in total DNA or after enrichment of low molecular weight(LMW)DNA[10,35,88]. High-throughput sequencing library is constructed by:eccDNA of expected sizes are enriched by CsCl-EB or plasmid extraction kit,and then high-throughput sequencing is conducted after rolling circle amplification(RCA)or Tn5 treatment. Split and discordant read pairs are used for eccDNA identification. Genome WGS and ATAC-seq data are also for eccDNA identification
| [1] |
Durkin K, Coppieters W, Drögemüller C, et al. Serial translocation by means of circular intermediates underlies colour sidedness in cattle[J]. Nature, 2012, 482(7383):81-84.
doi: 10.1038/nature10757 URL |
| [2] |
Møller HD, Ramos-Madrigal J, Prada-Luengo I, et al. Near-random distribution of chromosome-derived circular DNA in the condensed genome of pigeons and the larger, more repeat-rich human genome[J]. Genome Biol Evol, 2020, 12(1):3762-3777.
doi: 10.1093/gbe/evz281 URL |
| [3] |
Koo DH, Molin WT, Saski CA, et al. Extrachromosomal circular DNA-based amplification and transmission of herbicide resistance in crop weed Amaranthus palmeri[J]. PNAS, 2018, 115(13):3332-3337.
doi: 10.1073/pnas.1719354115 URL |
| [4] |
Molin WT, Yaguchi A, Blenner M, et al. The EccDNA replicon:a heritable, extranuclear vehicle that enables gene amplification and glyphosate resistance in Amaranthus palmeri[J]. Plant Cell, 2020, 32(7):2132-2140.
doi: 10.1105/tpc.20.00099 URL |
| [5] |
Dahm R. Friedrich Miescher and the discovery of DNA[J]. Dev Biol, 2005, 278(2):274-288.
doi: 10.1016/j.ydbio.2004.11.028 URL |
| [6] |
Watson JD, Crick FHC. The structure of dna[J]. Cold Spring Harb Symp Quant Biol, 1953, 18:123-131.
doi: 10.1101/SQB.1953.018.01.020 URL |
| [7] |
Hotta Y, Bassel A. Molecular size and circularity of DNA in cells of mammals and higher plants[J]. PNAS, 1965, 53:356-362.
doi: 10.1073/pnas.53.2.356 URL |
| [8] |
Yamagishi H, Kunisada T, Tsuda T. Small circular DNA complexes in eucaryotic cells[J]. Plasmid, 1982, 8(3):299-306.
pmid: 6294712 |
| [9] |
Cox D, Yuncken C, Spriggs AI. Minute chromatin bodies in malignant tumours of childhood[J]. Lancet, 1965, 1(7402):55-58.
doi: 10.1016/S0140-6736(02)95344-4 URL |
| [10] |
Radloff R, Bauer W, Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA:the closed circular DNA in HeLa cells[J]. PNAS, 1967, 57(5):1514-1521.
pmid: 5231757 |
| [11] |
Smith CA, Vinograd J. Small polydisperse circular DNA of HeLa cells[J]. J Mol Biol, 1972, 69(2):163-178.
pmid: 5070865 |
| [12] |
Stanfield S, Helinski DR. Small circular DNA in Drosophila melanogaster[J]. Cell, 1976, 9(2):333-345.
pmid: 824055 |
| [13] |
DeLap RJ, Rush MG, Zouzias D, et al. Isolation and preliminary characterization of the small circular DNA present in African green monkey kidney(BSC-1)cells[J]. Plasmid, 1978, 1(4):508-521.
pmid: 107539 |
| [14] |
DeLap RJ, Rush MG. Change in quantity and size distribution of small circular DNAs during development of chicken Bursa[J]. PNAS, 1978, 75(12):5855-5859.
pmid: 282606 |
| [15] |
Krolewski JJ, Bertelsen AH, Humayun MZ, et al. Members of the Alu family of interspersed, repetitive DNA sequences are in the small circular DNA population of monkey cells grown in culture[J]. J Mol Biol, 1982, 154(3):399-415.
pmid: 7077666 |
| [16] |
Bertelsen AH, Humayun MZ, Karfopoulos SG, et al. Molecular characterization of small polydisperse circular deoxyribonucleic acid from an African green monkey cell line[J]. Biochemistry, 1982, 21(9):2076-2085.
pmid: 7093232 |
| [17] |
Kazazian HH, Wong C, Youssoufian H, et al. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man[J]. Nature, 1988, 332(6160):164-166.
doi: 10.1038/332164a0 URL |
| [18] |
Schindler CW, Rush MG. The KpnI family of long interspersed nucleotide sequences is present on discrete sizes of circular DNA in monkey(BSC-1)cells[J]. J Mol Biol, 1985, 181(2):161-173.
pmid: 2984431 |
| [19] |
Kunisada T, Yamagishi H. Sequence organization of repetitive sequences enriched in small polydisperse circular DNAs from HeLa cells[J]. J Mol Biol, 1987, 198(4):557-565.
pmid: 3430621 |
| [20] |
Misra R, Matera AG, Schmid CW, et al. Recombination mediates production of an extrachromosomal circular DNA containing a transposon-like human element, THE-1[J]. Nucleic Acids Res, 1989, 17(20):8327-8341.
pmid: 2478961 |
| [21] |
Pont G, Degroote F, Picard G. Some extrachromosomal circular DNAs from Drosophila embryos are homologous to tandemly repeated genes[J]. J Mol Biol, 1987, 195(2):447-451.
pmid: 3116263 |
| [22] |
Regev A, Cohen S, Cohen E, et al. Telomeric repeats on small polydisperse circular DNA(spcDNA)and genomic instability[J]. Oncogene, 1998, 17(26):3455-3461.
pmid: 10030669 |
| [23] |
Cohen S, Agmon N, Sobol O, et al. Extrachromosomal circles of satellite repeats and 5S ribosomal DNA in human cells[J]. Mob DNA, 2010, 1(1):11.
doi: 10.1186/1759-8753-1-11 URL |
| [24] |
Cesare AJ, Griffith JD. Telomeric DNA in ALT cells is characterized by free telomeric circles and heterogeneous t-loops[J]. Mol Cell Biol, 2004, 24(22):9948-9957.
doi: 10.1128/MCB.24.22.9948-9957.2004 URL |
| [25] |
Tomaska L, Nosek J, Kramara J, et al. Telomeric circles:universal players in telomere maintenance?[J]. Nat Struct Mol Biol, 2009, 16(10):1010-1015.
doi: 10.1038/nsmb.1660 URL |
| [26] |
Kunisada T, Yamagishi H. Sequence repetition and genomic distribution of small polydisperse circular DNA purified from HeLa cells[J]. Gene, 1984, 31(1/2/3):213-223.
doi: 10.1016/0378-1119(84)90212-9 URL |
| [27] |
Stanfield SW, Helinski DR. Cloning and characterization of small circular DNA from Chinese hamster ovary cells[J]. Mol Cell Biol, 1984, 4(1):173-180.
doi: 10.1128/mcb.4.1.173-180.1984 pmid: 6700583 |
| [28] |
Nielsen JL, Walsh JT, Degen DR, et al. Evidence of gene amplification in the form of double minute chromosomes is frequently observed in lung cancer[J]. Cancer Genet Cytogenet, 1993, 65(2):120-124.
doi: 10.1016/0165-4608(93)90219-C URL |
| [29] |
Fletcher JA, Gebhardt MC, Kozakewich HP. Cytogenetic aberrations in osteosarcomas. Nonrandom deletions, rings, and double-minute chromosomes[J]. Cancer Genet Cytogenet, 1994, 77(1):81-88.
doi: 10.1016/0165-4608(94)90154-6 URL |
| [30] |
Vogt N, Lefèvre SH, Apiou F, et al. Molecular structure of double-minute chromosomes bearing amplified copies of the epidermal growth factor receptor gene in gliomas[J]. PNAS, 2004, 101(31):11368-11373.
doi: 10.1073/pnas.0402979101 URL |
| [31] |
Gibaud A, Vogt N, Hadj-Hamou NS, et al. Extrachromosomal amplification mechanisms in a glioma with amplified sequences from multiple chromosome loci[J]. Hum Mol Genet, 2010, 19(7):1276-1285.
doi: 10.1093/hmg/ddq004 pmid: 20056677 |
| [32] |
Reader JC, Zhao XF, Butler MS, et al. REL-positive double minute chromosomes in follicular lymphoma[J]. Leukemia, 2006, 20(9):1624-1626.
pmid: 16775615 |
| [33] |
Villa O, Salido M, Pérez-Vila ME, et al. Blast cells with nuclear extrusions in the form of micronuclei are associated with MYC amplification in acute myeloid leukemia[J]. Cancer Genet Cytogenet, 2008, 185(1):32-36.
doi: 10.1016/j.cancergencyto.2008.04.014 URL |
| [34] |
Benner SE, Wahl GM, von Hoff DD. Double minute chromosomes and homogeneously staining regions in tumors taken directly from patients versus in human tumor cell lines[J]. Anti Cancer Drugs, 1991, 2(1):11-25.
doi: 10.1097/00001813-199102000-00002 URL |
| [35] |
Hahn PJ. Molecular biology of double-minute chromosomes[J]. Bioessays, 1993, 15(7):477-484.
pmid: 7691058 |
| [36] |
Gebhart E. Double minutes, cytogenetic equivalents of gene amplification, in human neoplasia—a review[J]. Clin Transl Oncol, 2005, 7(11):477-485.
pmid: 16373058 |
| [37] |
Shibata Y, Kumar P, Layer R, et al. Extrachromosomal microDNAs and chromosomal microdeletions in normal tissues[J]. Science, 2012, 336(6077):82-86.
doi: 10.1126/science.1213307 URL |
| [38] |
Møller HD, Mohiyuddin M, Prada-Luengo I, et al. Circular DNA elements of chromosomal origin are common in healthy human somatic tissue[J]. Nat Commun, 2018, 9(1):1069.
doi: 10.1038/s41467-018-03369-8 URL |
| [39] |
Turner KM, Deshpande V, Beyter D, et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity[J]. Nature, 2017, 543(7643):122-125.
doi: 10.1038/nature21356 URL |
| [40] |
Verhaak RGW, Bafna V, Mischel PS. Extrachromosomal oncogene amplification in tumour pathogenesis and evolution[J]. Nat Rev Cancer, 2019, 19(5):283-288.
doi: 10.1038/s41568-019-0128-6 pmid: 30872802 |
| [41] |
Wu S, Turner KM, Nguyen N, et al. Circular ecDNA promotes accessible chromatin and high oncogene expression[J]. Nature, 2019, 575(7784):699-703.
doi: 10.1038/s41586-019-1763-5 URL |
| [42] |
Koche RP, Rodriguez-Fos E, Helmsauer K, et al. Extrachromosomal circular DNA drives oncogenic genome remodeling in neuroblastoma[J]. Nat Genet, 2020, 52(1):29-34.
doi: 10.1038/s41588-019-0547-z URL |
| [43] | Kinoshita Y, Ohnishi N, Yamada Y, et al. Extrachromosomal circular DNA from nuclear fraction of higher plants[J]. Plant and Cell Physiology, 1985, 26(7):1401-1409. |
| [44] | Kunisada T, Yamagishi H, Kinoshita I, et al. Amplification of extrachromosomal circular DNA in intact bean leaves treated with benzyladenine[J]. Plant Cell Physiol, 1986, 27(2):355-361. |
| [45] |
Carroll SM, Gaudray P, De Rose ML, et al. Characterization of an episome produced in hamster cells that amplify a transfected CAD gene at high frequency:functional evidence for a mammalian replication origin[J]. Mol Cell Biol, 1987, 7(5):1740-1750.
doi: 10.1128/mcb.7.5.1740-1750.1987 pmid: 2885742 |
| [46] |
Carroll SM, DeRose ML, Gaudray P, et al. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion[J]. Mol Cell Biol, 1988, 8(4):1525-1533.
doi: 10.1128/mcb.8.4.1525-1533.1988 pmid: 2898098 |
| [47] |
Ruiz JC, Choi KH, von Hoff DD, et al. Autonomously replicating episomes contain mdr1 genes in a multidrug-resistant human cell line[J]. Mol Cell Biol, 1989, 9(1):109-115.
doi: 10.1128/mcb.9.1.109-115.1989 pmid: 2648129 |
| [48] | Daniel D Von Hoff. New mechanisms of gene amplification in drug resistance[M]// Ozols RF. Molecular and clinical advances in anticancer drug resistance. New York:Springer Science & Business Media, 2012:1-8. |
| [49] |
Schoenlein PV, Barrett JT, Kulharya A, et al. Radiation therapy depletes extrachromosomally amplified drug resistance genes and oncogenes from tumor cells via micronuclear capture of episomes and double minute chromosomes[J]. Int J Radiat Oncol Biol Phys, 2003, 55(4):1051-1065.
doi: 10.1016/S0360-3016(02)04473-5 URL |
| [50] |
Storlazzi CT, Fioretos T, Surace C, et al. MYC-containing double minutes in hematologic malignancies:evidence in favor of the episome model and exclusion of MYC as the target gene[J]. Hum Mol Genet, 2006, 15(6):933-942.
pmid: 16452126 |
| [51] |
Shimizu N. Molecular mechanisms of the origin of micronuclei from extrachromosomal elements[J]. Mutagenesis, 2011, 26(1):119-123.
doi: 10.1093/mutage/geq053 URL |
| [52] |
Chiu RWK, Dutta A, Hensson AG, et al. What is extrachromosomal circular DNA and what does it do?[J]. Clin Chem, 2020, 66(6):754-759.
doi: 10.1093/clinchem/hvaa096 URL |
| [53] |
Hull RM, King M, Pizza G, et al. Transcription-induced formation of extrachromosomal DNA during yeast ageing[J]. PLoS Biol, 2019, 17(12):e3000471.
doi: 10.1371/journal.pbio.3000471 URL |
| [54] |
Dillon LW, Kumar P, Shibata Y, et al. Production of extrachromosomal MicroDNAs is linked to mismatch repair pathways and transcriptional activity[J]. Cell Rep, 2015, 11(11):1749-1759.
doi: 10.1016/j.celrep.2015.05.020 pmid: 26051933 |
| [55] |
Crossley MP, Bocek M, Cimprich KA. R-loops as cellular regulators and genomic threats[J]. Mol Cell, 2019, 73(3):398-411.
doi: S1097-2765(19)30044-9 pmid: 30735654 |
| [56] |
Brewer BJ, Payen C, Di Rienzi SC, et al. Origin-dependent inverted-repeat amplification:tests of a model for inverted DNA amplification[J]. PLoS Genet, 2015, 11(12):e1005699.
doi: 10.1371/journal.pgen.1005699 URL |
| [57] |
Meng X, Qi X, Guo H, et al. Novel role for non-homologous end joining in the formation of double minutes in methotrexate-resistant colon cancer cells[J]. J Med Genet, 2015, 52(2):135-144.
doi: 10.1136/jmedgenet-2014-102703 URL |
| [58] |
Cai M, Zhang H, Hou L, et al. Inhibiting homologous recombination decreases extrachromosomal amplification but has no effect on intrachromosomal amplification in methotrexate-resistant colon cancer cells[J]. Int J Cancer, 2019, 144(5):1037-1048.
doi: 10.1002/ijc.v144.5 URL |
| [59] |
Nelson JO, Watase GJ, Warsinger-Pepe N, et al. Mechanisms of rDNA copy number maintenance[J]. Trends Genet, 2019, 35(10):734-742.
doi: 10.1016/j.tig.2019.07.006 URL |
| [60] |
Yerlici VT, Lu MW, Hoge CR, et al. Programmed genome rearrangements in Oxytricha produce transcriptionally active extrachromosomal circular DNA[J]. Nucleic Acids Res, 2019, 47(18):9741-9760.
doi: 10.1093/nar/gkz725 pmid: 31504770 |
| [61] |
Tomaska L, McEachern MJ, Nosek J. Alternatives to telomerase:keeping linear chromosomes via telomeric circles[J]. FEBS Lett, 2004, 567(1):142-146.
doi: 10.1016/j.febslet.2004.04.058 URL |
| [62] |
Park PU, Defossez PA, Guarente L. Effects of mutations in DNA repair genes on formation of ribosomal DNA circles and life span in Saccharomyces cerevisiae[J]. Mol Cell Biol, 1999, 19(5):3848-3856.
doi: 10.1128/MCB.19.5.3848 pmid: 10207108 |
| [63] |
Gresham D, Usaite R, Germann SM, et al. Adaptation to diverse nitrogen-limited environments by deletion or extrachromosomal element formation of the GAP1 locus[J]. PNAS, 2010, 107(43):18551-18556.
doi: 10.1073/pnas.1014023107 pmid: 20937885 |
| [64] |
Paulsen T, Kumar P, Koseoglu MM, et al. Discoveries of extrachromosomal circles of DNA in normal and tumor cells[J]. Trends Genet, 2018, 34(4):270-278.
doi: 10.1016/j.tig.2017.12.010 URL |
| [65] |
Gu X, Yu J, Chai P, et al. Novel insights into extrachromosomal DNA:redefining the onco-drivers of tumor progression[J]. J Exp Clin Cancer Res, 2020, 39(1):215.
doi: 10.1186/s13046-020-01726-4 URL |
| [66] |
Yan YL, Guo GJ, Huang JZ, et al. Current understanding of extrachromosomal circular DNA in cancer pathogenesis and therapeutic resistance[J]. J Hematol Oncol, 2020, 13(1):124.
doi: 10.1186/s13045-020-00960-9 URL |
| [67] |
Liao Z, Jiang W, Ye L, et al. Classification of extrachromosomal circular DNA with a focus on the role of extrachromosomal DNA(ecDNA)in tumor heterogeneity and progression[J]. Biochim Biophys Acta Rev Cancer, 2020, 1874(1):188392.
doi: 10.1016/j.bbcan.2020.188392 URL |
| [68] | Mansisidor A, Molinar T Jr, Srivastava P Jr, et al. Genomic copy-number loss is rescued by self-limiting production of DNA circles[J]. Mol Cell, 2018, 72(3):583-593. e4. |
| [69] | Wei JX, Wu CC, Meng HB, et al. The biogenesis and roles of extrachromosomal oncogene involved in carcinogenesis and evolution[J]. Am J Cancer Res, 2020, 10(11):3532-3550. |
| [70] |
Ain Q, Schmeer C, Wengerodt D, et al. Extrachromosomal circular DNA:current knowledge and implications for CNS aging and neurodegeneration[J]. International Journal of Molecular Sciences, 2020, 21(7):2477.
doi: 10.3390/ijms21072477 URL |
| [71] | Qiu H, Shao ZY, Wen X, et al. New insights of extrachromosomal DNA in tumorigenesis and therapeutic resistance of cancer[J]. Am J Cancer Res, 2020, 10(12):4056-4065. |
| [72] |
Mazzucco G, Huda A, Galli M, et al. Telomere damage induces internal loops that generate telomeric circles[J]. Nat Commun, 2020, 11(1):5297.
doi: 10.1038/s41467-020-19139-4 URL |
| [73] |
Reddel RR. Alternative lengthening of telomeres, telomerase, and cancer[J]. Cancer Lett, 2003, 194(2):155-162.
pmid: 12757973 |
| [74] |
Sinclair DA, Guarente L. Extrachromosomal rDNA circles——a cause of aging in yeast[J]. Cell, 1997, 91(7):1033-1042.
pmid: 9428525 |
| [75] |
Hull RM, Houseley J. The adaptive potential of circular DNA accumulation in ageing cells[J]. Curr Genet, 2020, 66(5):889-894.
doi: 10.1007/s00294-020-01069-9 URL |
| [76] |
Møller HD, Parsons L, Jørgensen TS, et al. Extrachromosomal circular DNA is common in yeast[J]. PNAS, 2015, 112(24):E3114-E3122.
doi: 10.1073/pnas.1508825112 URL |
| [77] |
Payen C, Sunshine AB, Ong GT, et al. High-throughput identification of adaptive mutations in experimentally evolved yeast populations[J]. PLoS Genet, 2016, 12(10):e1006339.
doi: 10.1371/journal.pgen.1006339 URL |
| [78] |
Nathanson DA, Gini B, Mottahedeh J, et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA[J]. Science, 2014, 343(6166):72-76.
doi: 10.1126/science.1241328 pmid: 24310612 |
| [79] |
Nikolaev S, Santoni F, Garieri M, et al. Extrachromosomal driver mutations in glioblastoma and low-grade glioma[J]. Nat Commun, 2014, 5:5690.
doi: 10.1038/ncomms6690 pmid: 25471132 |
| [80] |
DeCarvalho AC, Kim H, Poisson LM, et al. Discordant inheritance of chromosomal and extrachromosomal DNA elements contributes to dynamic disease evolution in glioblastoma[J]. Nat Genet, 2018, 50(5):708-717.
doi: 10.1038/s41588-018-0105-0 URL |
| [81] |
Bailey C, Shoura MJ, Mischel PS, et al. Extrachromosomal DNA-relieving heredity constraints, accelerating tumour evolution[J]. Ann Oncol, 2020, 31(7):884-893.
doi: S0923-7534(20)36392-4 pmid: 32275948 |
| [82] | Morton AR, Dogan-Artun N, Faber ZJ, et al. Functional enhancers shape extrachromosomal oncogene amplifications[J]. Cell, 2019, 179(6):1330-1341. e13. |
| [83] |
Ott CJ. Circles with a point:new insights into oncogenic extrachromosomal DNA[J]. Cancer Cell, 2020, 37(2):145-146.
doi: 10.1016/j.ccell.2020.01.008 URL |
| [84] |
Sin STK, Jiang P, Deng J, et al. Identification and characterization of extrachromosomal circular DNA in maternal plasma[J]. PNAS, 2020, 117(3):1658-1665.
doi: 10.1073/pnas.1914949117 URL |
| [85] |
Kumar P, Dillon LW, Shibata Y, et al. Normal and cancerous tissues release extrachromosomal circular DNA(eccDNA)into the circulation[J]. Mol Cancer Res, 2017, 15(9):1197-1205.
doi: 10.1158/1541-7786.MCR-17-0095 URL |
| [86] |
Kim H, Nguyen NP, Turner K, et al. Extrachromosomal DNA is associated with oncogene amplification and poor outcome across multiple cancers[J]. Nat Genet, 2020, 52(9):891-897.
doi: 10.1038/s41588-020-0678-2 URL |
| [87] | Tandon I, Pal R, Pal JK, et al. Extrachromosomal circular DNAs:an extra piece of evidence to depict tumor heterogeneity[J]. Future Sci OA, 2019, 5(6):FSO390. |
| [88] |
Cohen S, Regev A, Lavi S. Small polydispersed circular DNA(spcDNA)in human cells:association with genomic instability[J]. Oncogene, 1997, 14(8):977-985.
pmid: 9050997 |
| [89] |
Cohen Z, Bacharach E, Lavi S. Mouse major satellite DNA is prone to eccDNA formation via DNA Ligase IV-dependent pathway[J]. Oncogene, 2006, 25(33):4515-4524.
pmid: 16547499 |
| [90] |
Cohen S, Segal D. Extrachromosomal circular DNA in eukaryotes:possible involvement in the plasticity of tandem repeats[J]. Cytogenet Genome Res, 2009, 124(3/4):327-338.
doi: 10.1159/000218136 URL |
| [91] |
Hollis M, Hindley J. Human Sau3A repeated DNA is enriched in small polydisperse circular DNA from normal lymphocytes[J]. Gene, 1986, 46(2/3):153-160.
doi: 10.1016/0378-1119(86)90399-9 URL |
| [92] |
Shoura MJ, Gabdank I, Hansen L, et al. Intricate and cell Type-Specific populations of endogenous circular dna(eccDNA)in Caenorhabditis elegans and Homo sapiens[J]. G3 Genes|Genomes|Genetics, 2017, 7(10):3295-3303.
doi: 10.1534/g3.117.300141 URL |
| [93] |
Lanciano S, Carpentier MC, Llauro C, et al. Sequencing the extrachromosomal circular mobilome reveals retrotransposon activity in plants[J]. PLoS Genet, 2017, 13(2):e1006630.
doi: 10.1371/journal.pgen.1006630 URL |
| [94] |
Mehta D, Cornet L, Hirsch-Hoffmann M, et al. Full-length sequencing of circular DNA viruses and extrachromosomal circular DNA using CIDER-Seq[J]. Nat Protoc, 2020, 15(5):1673-1689.
doi: 10.1038/s41596-020-0301-0 URL |
| [95] | Kumar P, Kiran S, Saha S, et al. ATAC-seq identifies thousands of extrachromosomal circular DNA in cancer and cell lines[J]. Sci Adv, 2020, 6(20):eaba2489. |
| [96] |
Deshpande V, Luebeck J, Nguyen ND, et al. Exploring the landscape of focal amplifications in cancer using AmpliconArchitect[J]. Nat Commun, 2019, 10(1):392.
doi: 10.1038/s41467-018-08200-y pmid: 30674876 |
| [97] |
Luebeck J, Coruh C, Dehkordi SR, et al. AmpliconReconstructor integrates NGS and optical mapping to resolve the complex structures of focal amplifications[J]. Nat Commun, 2020, 11(1):4374.
doi: 10.1038/s41467-020-18099-z pmid: 32873787 |
| [98] |
Zhang XO, Dong R, Zhang Y, et al. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs[J]. Genome Res, 2016, 26(9):1277-1287.
doi: 10.1101/gr.202895.115 URL |
| [99] |
Prada-Luengo I, Krogh A, Maretty L, et al. Sensitive detection of circular DNAs at single-nucleotide resolution using guided realignment of partially aligned reads[J]. BMC Bioinform, 2019, 20(1):1-9.
doi: 10.1186/s12859-018-2565-8 URL |
| [100] | Mann L, Seibt KM, Weber B, et al. Biopolis Dresden PhD Symposium 2020[C]. Dresden, 2020. |
| [101] |
Yu J, Xiang X, Huang J, et al. Haplotyping by CRISPR-mediated DNA circularization(CRISPR-hapC)broadens allele-specific gene editing[J]. Nucleic Acids Res, 2020, 48(5):e25.
doi: 10.1093/nar/gkz1233 URL |
| [102] |
Iparraguirre L, Prada-Luengo I, Regenberg B, et al. To be or not to be:circular RNAs or mRNAs from circular DNAs?[J]. Front Genet, 2019, 10:940.
doi: 10.3389/fgene.2019.00940 pmid: 31681407 |
| [103] |
Gaines TA, Patterson EL, Neve P. Molecular mechanisms of adaptive evolution revealed by global selection for glyphosate resistance[J]. New Phytol, 2019, 223(4):1770-1775.
doi: 10.1111/nph.15858 pmid: 31002387 |
| [1] | XIAO Liang, WU Zheng-dan, LU Liu-ying, SHI Ping-li, SHANG Xiao-hong, CAO Sheng, ZENG Wen-dan, YAN Hua-bing. Research Progress of Important Traits Genes in Cassava [J]. Biotechnology Bulletin, 2023, 39(6): 31-48. |
| [2] | SUN Hai-hang, GUAN Hui-lin, WANG Xu, WANG Tong, LI Hong-lin, PENG Wen-jie, LIU Bo-zhen, FAN Fang-ling. Effects of Biochar on the Soil Properties and Fungal Community Structure under Continuous Cropping of Panax notoginseng [J]. Biotechnology Bulletin, 2023, 39(2): 221-231. |
| [3] | CHEN Tian-ci, WU Shao-lan, YANG Guo-hui, JIANG Dan-xia, JIANG Yu-ji, CHEN Bing-zhi. Effects of Ganoderma resinaceum Alcohol Extract on Sleep and Intestinal Microbiota in Mice [J]. Biotechnology Bulletin, 2022, 38(8): 225-232. |
| [4] | ZHONG Hui, LIU Ya-jun, WANG Bin-hua, HE Meng-jie, WU Lan. Effects of Analysis Methods on the Analyzed Results of 16S rRNA Gene Amplicon Sequencing in Bacterial Communities [J]. Biotechnology Bulletin, 2022, 38(6): 81-92. |
| [5] | YANG Ya-jie, LI Yu-ying, SHEN Zhuang-zhuang, CHEN Tian, RONG Er-hua, WU Yu-xiang. Selection and Character Identification for Autopolyploid Progenies of Gossypium herbaceum [J]. Biotechnology Bulletin, 2022, 38(5): 64-73. |
| [6] | ZHAO Lin-yan, GUAN Hui-lin, XIANG Ping, LI Ze-cheng, BAI Yu-long, SONG Hong-chuan, SUN Shi-zhong, XU Wu-mei. Composition Features of Microbial Community in the Rhizospheric Soil of Bletilla striata with Root Rot [J]. Biotechnology Bulletin, 2022, 38(2): 67-74. |
| [7] | CHEN Yu-jie, ZHENG Hua-bao, ZHOU Xin-yan. Modified High-throughput Sequencing Reveals the Effects of Different Algicides towards Algal Community [J]. Biotechnology Bulletin, 2022, 38(11): 70-79. |
| [8] | KONG De-zhen, NIE Ying-bin, XU Hong-jun, CUI Feng-juan, MU Pei-yuan, TIAN Xiao-ming. Effects of Blend Seeding on the Yield,Purity and Yield Advantage of F1 in Three-line Hybrid Wheat [J]. Biotechnology Bulletin, 2022, 38(10): 132-139. |
| [9] | MAO Ting, NIU Yong-yan, ZHENG Qun, YANG Tao, MU Yong-song, ZHU Ying, JI Bin, WANG Zhi-ye. Effects of Microbial Inoculants on the Fermentation Quality and Microbial Community Diversity of Alfalfa Silage [J]. Biotechnology Bulletin, 2021, 37(9): 86-94. |
| [10] | TANG Die, ZHOU Qian. Research Advances in Plant Genome Assembly [J]. Biotechnology Bulletin, 2021, 37(6): 1-12. |
| [11] | ZHU Bin, GAN Chen-chen, WANG Hong-cheng. Characteristics of the Complete Chloroplast Genome of Dendrobium thyrsiflorum and Its Phylogenetic Relationship Analysis [J]. Biotechnology Bulletin, 2021, 37(5): 38-47. |
| [12] | SUN Ping-yong, ZHANG Wu-han, SHU Fu, HE Qiang, ZHANG Li, PENG Zhi-rong, DENG Hua-feng. Analysis of Mutation Sites of OsBADH2 Gene in Fragrant Rice and Development of Related Functional Marker [J]. Biotechnology Bulletin, 2021, 37(4): 1-7. |
| [13] | ZHANG Shu-hua, FANG Qian, JIA Hong-mei, HAN Gui-qi, YAN Zhu-yun, HE Dong-mei. Difference Analysis of Fungal Community Among Bulk Soil,Rhizosphere and Rhizomes of Ligusticum chuanxiong Hort. [J]. Biotechnology Bulletin, 2021, 37(4): 56-69. |
| [14] | GUO Yan-ping, ZHANG Hao, ZHAO Xin-gang, LUO Hai-ling, ZHANG Ying-jun. Applications of DNA Metabarcoding in Diet Identification of Herbivores [J]. Biotechnology Bulletin, 2021, 37(3): 252-260. |
| [15] | ZHENG Fang-fang, LIN Jun-sheng. Selection and Specificity of Nucleic Acid Aptamers for a Proliferation Inducing Ligand [J]. Biotechnology Bulletin, 2021, 37(10): 196-202. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||