








生物技术通报 ›› 2020, Vol. 36 ›› Issue (11): 94-102.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0220
收稿日期:2020-03-05
出版日期:2020-11-26
发布日期:2020-11-20
作者简介:朱彩林,女,硕士研究生,研究方向:脂肪酶改造;E-mail: 基金资助:
ZHU Cai-lin( ), LÜ Xiang, XIA Xiao-le(
), LÜ Xiang, XIA Xiao-le( )
)
Received:2020-03-05
Published:2020-11-26
Online:2020-11-20
摘要:
T1脂肪酶来源于 Geobacillus zalihae 菌株,常作为生物催化剂广泛应用于各领域。为了综合改善T1脂肪酶的酶学性质,前期通过对该酶盖子结构进行理性设计得到3个效果较好的突变体L188M、A190L、A190Y,在此基础上,将单点突变体进行组合得到L188M/A190L和L188M/A190Y两个复合突变体。在该实验中对突变体脂肪酶酶学性质展开研究,同时将单点与组合突变效果进行比较。结果显示野生型及突变体最适pH均为9.0,复合突变体的最适温度提高5℃。单点突变及组合突变均使T1脂肪酶的酶学性质得到不同程度的改善,且复合突变体效果更显著。组合突变体使脂肪酶的温度、pH稳定性显著提高,L188M/A190L对于有机溶剂二甲基亚砜及正十六烷的耐受性为野生型的1.88倍和2.73倍,L188M/A190Y为野生型的1.96倍和2.58倍。此外,对C16和C18底物的选择性也明显增强,L188M/A190L为野生型的1.65和2.7倍,L188M/A190Y为野生型的1.5倍和2.25倍。该研究成果在一定程度上拓宽了T1脂肪酶的应用前景,同时为其他脂肪酶的改造提供了一定理论依据。
朱彩林, 吕祥, 夏小乐. 盖子区域氨基酸的定点突变对T1脂肪酶酶学性质的影响[J]. 生物技术通报, 2020, 36(11): 94-102.
ZHU Cai-lin, LÜ Xiang, XIA Xiao-le. Effect of Site-directed Mutagenesis of Amino Acids in Lid Region on the Enzymatic Properties of T1 Lipase[J]. Biotechnology Bulletin, 2020, 36(11): 94-102.
| 突变体 | 引物(5'-3') |
|---|---|
| A190L | 上游:5'- AAAGCAGTGTTAGAACTGGCCGCCGTGGC- AAGCAACGTG -3' |
| 下游:5'- GCTTGCCACGGCGGCCAGTTCTAACACTGC- TTTCTGCAG -3' | |
| L188M | 上游:5'- CTGCAGAAAGCAGTGATGGAAGCAGCCGCC- GTGGCA -3' |
| 下游:5'- TGCCACGGCGGCTGCTTCCATCACTGCTTTC- TGCAG -3' | |
| A190Y | 上游: 5'-CAGAAAGCAGTGTTAGAATACGCCGCCGTGG- CAAGC-3' |
| 下游: 5'-GCTTGCCACGGCGGCGTATTCTAACACTGCTTT- CTG-3' |
表1 突变体引物
| 突变体 | 引物(5'-3') |
|---|---|
| A190L | 上游:5'- AAAGCAGTGTTAGAACTGGCCGCCGTGGC- AAGCAACGTG -3' |
| 下游:5'- GCTTGCCACGGCGGCCAGTTCTAACACTGC- TTTCTGCAG -3' | |
| L188M | 上游:5'- CTGCAGAAAGCAGTGATGGAAGCAGCCGCC- GTGGCA -3' |
| 下游:5'- TGCCACGGCGGCTGCTTCCATCACTGCTTTC- TGCAG -3' | |
| A190Y | 上游: 5'-CAGAAAGCAGTGTTAGAATACGCCGCCGTGG- CAAGC-3' |
| 下游: 5'-GCTTGCCACGGCGGCGTATTCTAACACTGCTTT- CTG-3' |
| 试剂 | 体积/μL |
|---|---|
| ddH2O | 18 |
| 2×Max Buffer | 25 |
| dNTP Mix(10 mmol/L) | 1 |
| pET28a-T1 | 1 |
| 上游引物(10 μmol/L) | 2 |
| 下游引物(10 μmol/L) | 2 |
| Phanta Max Super-Fidelity DNA Ploymerase | 1 |
表2 突变体扩增反应体系
| 试剂 | 体积/μL |
|---|---|
| ddH2O | 18 |
| 2×Max Buffer | 25 |
| dNTP Mix(10 mmol/L) | 1 |
| pET28a-T1 | 1 |
| 上游引物(10 μmol/L) | 2 |
| 下游引物(10 μmol/L) | 2 |
| Phanta Max Super-Fidelity DNA Ploymerase | 1 |
| 温度/℃ | 时间 | 循环数 |
|---|---|---|
| 95 | 30 s | 1 |
| 95 | 15 s | |
| 68 | 15 s | 30 |
| 72 | 6 min | |
| 72 | 5 min | 1 |
表3 突变体扩增条件
| 温度/℃ | 时间 | 循环数 |
|---|---|---|
| 95 | 30 s | 1 |
| 95 | 15 s | |
| 68 | 15 s | 30 |
| 72 | 6 min | |
| 72 | 5 min | 1 |
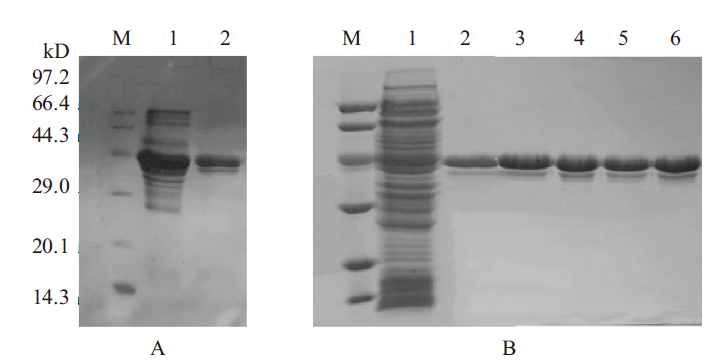
图1 纯化蛋白SDS-PAGE电泳验证结果图 A. M:Marker;1:野生型T1粗酶液;2:野生型T1纯化酶液。B. M:Marker;1:突变体粗酶液;2:L188M纯化酶液;3:A190L纯化酶液;4:A190Y纯化酶液;5:L188M/A190L纯化酶液;6:L188M/A190Y纯化酶液
| 突变体 | 总酶活/U | 总蛋白含 量/mg | 比酶活/ (U/mg) | 蛋白收 率/% |
|---|---|---|---|---|
| 野生型 | 844 | 2.5 | 337 | 45 |
| A190L | 1 657 | 4.2 | 395 | 49 |
| L188M | 1 434 | 3.8 | 377 | 57 |
| A190Y | 1 479 | 3.6 | 411 | 55 |
| L188M/A190L | 1 760 | 4.4 | 400 | 43 |
| L188M/A190Y | 1 337 | 3.3 | 405 | 48 |
表4 突变体纯化小结
| 突变体 | 总酶活/U | 总蛋白含 量/mg | 比酶活/ (U/mg) | 蛋白收 率/% |
|---|---|---|---|---|
| 野生型 | 844 | 2.5 | 337 | 45 |
| A190L | 1 657 | 4.2 | 395 | 49 |
| L188M | 1 434 | 3.8 | 377 | 57 |
| A190Y | 1 479 | 3.6 | 411 | 55 |
| L188M/A190L | 1 760 | 4.4 | 400 | 43 |
| L188M/A190Y | 1 337 | 3.3 | 405 | 48 |
| [1] | Wang T, Qin G. Lipases and its application in food industry[J]. Meat Research, 2010,9:82-84. |
| [2] | Abdul Wahab R, Basri M, Raja Abdul Rahman RNZ, et al. Development of a catalytically stable and efficient lipase through an increase in hydrophobicity of the oxyanion residue[J]. Journal of Molecular Catalysis B:Enzymatic, 2015,122:282-288. |
| [3] |
Reis P, Holmberg K, Watzke H, et al. Lipases at interfaces:A review[J]. Advances in Colloid and Interface Science, 2009, 147-148:237-250.
URL pmid: 18691682 |
| [4] | 薛龙吟, 林瑞凤, 舒正玉, 等. 黑曲霉脂肪酶盖子结构域突变对其活性的影响[J]. 生物技术通报, 2010(2):173-177. |
| Xue LY, Lin RF, Shu ZY, et al. Effect of Mutation at the lid domain of Aspergillus niger lipase on activity[J]. Biotechnology Bulletin, 2010(2):173-177. | |
| [5] | 易华伟, 唐晓峰. 基于氨基酸序列和模拟结构预测蛋白质稳定性的研究进展[J]. 生物技术通报, 2017,33(4):83-89. |
| Yi HW, Tang XF. Research progress on the prediction of protein stability based on amino acid sequence and simulated structure[J]. Biotechnology Bulletin, 2017,33(4):83-89. | |
| [6] |
Shih TW, Pan TM. Substitution of Asp189 residue alters the activity and thermostability of Geobacillus sp. NTU 03 lipase[J]. Biotechnology Letters, 2011,33(9):1841-1846.
doi: 10.1007/s10529-011-0635-3 URL pmid: 21544610 |
| [7] |
Santarossa G, Lafranconi PG, Alquati C, et al. Mutations in the “lid” region affect chain length specificity and thermostability of a Pseudomonas fragi lipase[J]. FEBS Lett, 2005,579(11):2383-2386.
doi: 10.1016/j.febslet.2005.03.037 URL pmid: 15848176 |
| [8] |
Panizza P, Cesarini S, Diaz P, et al. Saturation mutagenesis in selected amino acids to shift Pseudomonas sp. acidic lipase Lip I. 3 substrate specificity and activity[J]. Chem Commun, 2015,51(7):1330-1333.
doi: 10.1039/C4CC08477B URL |
| [9] |
Karkhane AA, Yakhchali B, Jazii FR, et al. The effect of substitution of Phe181 and Phe182 with Ala on activity, substrate specificity and stabilization of substrate at the active site of Bacillus thermocatenulatus lipase[J]. Journal of Molecular Catalysis B:Enzymatic, 2009,61(3-4):162-167.
doi: 10.1016/j.molcatb.2009.06.006 URL |
| [10] | 林瑞凤. 扩展青霉脂肪酶 “盖子” 结构域突变对其活性影响的研究[D]. 福州:福建师范大学, 2010. |
| Lin RF. Effect of Mutation at the lid subdomain of Penicillium expansum lipase on its activity[D]. Fuzhou:Fujian Normal University, 2010. | |
| [11] |
Wong H, Schotz MC. The lipase gene family[J]. Journal of Lipid Research, 2002,43(7):993-999.
doi: 10.1194/jlr.r200007-jlr200 URL pmid: 12091482 |
| [12] |
Bloom JD, Labthavikul ST, Otey CR, et al. Protein stability promotes evolvability[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006,103(15):5869-5874.
doi: 10.1073/pnas.0510098103 URL pmid: 16581913 |
| [13] |
Ruslan R, Abd Rahman RN, Leow TC, et al. Improvement of thermal stability via outer-loop ion pair interaction of mutated T1 lipase from Geobacillus zalihae strain T1[J]. International Journal of Molecular Sciences, 2012,13(1):943-960.
doi: 10.3390/ijms13010943 URL pmid: 22312296 |
| [14] | Wang Y, Wei DQ, Wang JF. Molecular dynamics studies on T1 lipase:insight into a double-flap mechanism[J]. Journal of Chemical Information, 2010,50(5):875-878. |
| [15] |
Tang QY, Lan DM, Yang B, et al. Site-directed mutagenesis studies of hydrophobic residues in the lid region of T1 lipase[J]. European Journal of Lipid Science and Technology, 2017,119(3):1600107.
doi: 10.1002/ejlt.201600107 URL |
| [16] |
Wahab RA, Basri M, Rahman RN, et al. Manipulation of the conformation and enzymatic properties of T1 lipase by site-directed mutagenesis of the protein core[J]. Applied Biochemistry and Biotechnology, 2012,167(3):612-620.
doi: 10.1007/s12010-012-9728-2 URL |
| [17] |
Meersman F, Dobson CM., Heremans K. Protein unfolding, amyloid fibril formation and configurational energy landscapes under high pressure conditions[J]. Chemical Society Reviews, 2006,35(10):908-917.
doi: 10.1039/b517761h URL pmid: 17003897 |
| [18] | Eisenmenger MJ, Reyes-De-Corcuera JI. High pressure enhance-ment of enzymes:A review[J]. Enzyme and Microbial Technol-ogy, 2009,45(5):331-347. |
| [19] |
Li H, Zhang X. Characterization of thermostable lipase from thermophilic Geobacillus sp. TW1[J]. Protein Expression and Purification, 2005,42(1):153-159.
doi: 10.1016/j.pep.2005.03.011 URL pmid: 15939301 |
| [20] | 汪璞, 王方华, 唐庆芸, 等. 固定化嗜热嗜碱土芽孢杆菌T1脂肪酶的制备及其催化特性研究[J]. 现代食品科技, 2015,31(5):175-180. |
| Wang P, Wang FH, Tang QY, et al. Preparation and catalytic properties of immobilized thermoalkaliphilic T1 lipase from Geobacillus sp. strain T1[J]. Modern Food Science and Technology, 2015,31(5):175-180. | |
| [21] | Chen R, Guo L, Dang H. Gene cloning, expression and characterization of a cold-adapted lipase from a psychrophilic deep-sea bacterium Psychrobacter sp. C18[J]. World Journal of Microbiology and Biotechnology, 2010,27(2):431-441. |
| [22] |
Zhang Y, Zhao J, Zeng R. Expression and characterization of a novel mesophilic protease from metagenomic library derived from Antarctic coastal sediment[J]. Extremophiles, 2010,15(1):23-29.
doi: 10.1007/s00792-010-0332-5 URL pmid: 21069403 |
| [23] |
Leow TC, Rahman RN, Basri M, et al. A thermoalkaliphilic lipase of Geobacillus sp. T1[J]. Extremophiles, 2007,11(3):527-535.
doi: 10.1007/s00792-007-0069-y URL pmid: 17426920 |
| [24] | 孙清扬, 张静静, 李冰清, 等. 金属离子对重组深渊滕黄单胞菌低温淀粉酶LamA的活性和稳定性影响[J]. 微生物学通报, 2019,46(11):2848-2856. |
| Sun QY, Zhang JJ, Li BQ, et al. Effect of metal ions on the activity and stability of recombinant cold-adapted α-amylase LamA from Luteimonas abyssi[J]. Microbiology China, 2019,46(11):2848-2856. | |
| [25] |
Zhou H, Zhou Y. Quantifying the effect of burial of amino acid residues on protein stability[J]. Proteins, 2004,54(2):315-322.
doi: 10.1002/prot.10584 URL pmid: 14696193 |
| [26] |
Mabrouk SB, Aghajari N, Ali MB, et al. Enhancement of the thermostability of the maltogenic amylase MAUS149 by Gly312Ala and Lys436Arg substitutions[J]. Bioresource Technology, 2011,102(2):1740-1746.
URL pmid: 20855205 |
| [27] |
Behera RK, Mazumdar S. Thermodynamic basis of the thermostability of CYP175A1 from Thermus thermophilus[J]. International Journal of Biological Macromolecules, 2010,46(4):412-418.
URL pmid: 20138909 |
| [28] |
Kannan N, Vishveshwara S. Aromatic clusters:a determinant of thermal stability of thermophilic proteins[J]. Protein Engineering, 2000,13(11):753-761.
doi: 10.1093/protein/13.11.753 URL pmid: 11161106 |
| [29] | 黄奎. 疏棉状嗜热丝孢菌脂肪酶在毕赤酵母表面展示[D]. 广州:华南理工大学, 2017. |
| Huang K. Cell surface displaying of Thermomyces lanuginosus lipase on Pichia pastoris[D]. Guangzhou:South China University of Technology, 2017. | |
| [30] |
Rodrigues DS, Mendes AA, Filice M, et al. Different derivatives of a lipase display different regioselectivity in the monohydrolysis of per-O-acetylated 1-O-substituted-β-galactopyranosides[J]. Journal of Molecular Catalysis B:Enzymatic, 2009,58(1-4):36-40.
doi: 10.1016/j.molcatb.2008.11.001 URL |
| [31] |
Buettner K, Hertel TC, Pietzsch M. Increased thermostability of microbial transglutaminase by combination of several hot spots evolved by random and saturation mutagenesis[J]. Amino Acids, 2012,42(2-3):987-996.
doi: 10.1007/s00726-011-1015-y URL pmid: 21863232 |
| [32] |
Reetz MT, Carballeira JD, Vogel A. Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability[J]. Angewandte Chemie International Edition, 2006,45(46):7745-7751.
doi: 10.1002/anie.200602795 URL pmid: 17075931 |
| [33] |
Bhardwaj A, Leelavathi S, Mazumdar-Leighton S, et al. The critical role of N-and C-terminal contact in protein stability and folding of a family 10 xylanase under extreme conditions[J]. PLoS One, 2010,5(6):e11347.
doi: 10.1371/journal.pone.0011347 URL pmid: 20596542 |
| [1] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [2] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [3] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [4] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [5] | 常晴, 束月蓉, 王文韬, 蒋昊, 延泉德, 钱政, 高雪纯, 吴金鸿, 张勇. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131. |
| [6] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [7] | 岑潇龙, 雷曦, 马诗云, 陈倩茹, 黄遵锡, 周峻沛, 张蕊. 金黄色葡萄球菌透明质酸裂解酶HylS的异源表达与特性研究[J]. 生物技术通报, 2022, 38(1): 157-167. |
| [8] | 田嘉慧, 封佳丽, 卢俊桦, 毛林静, 胡著然, 王莹, 楚杰. 一色齿毛菌漆酶LacT-1的分离纯化与性质研究[J]. 生物技术通报, 2021, 37(8): 186-194. |
| [9] | 张瑶心, 王亮节, 郑文, 徐汉琴, 郑恋, 钟静. 产几丁质酶的无色杆菌ZWW8的发酵产酶及酶学性质研究[J]. 生物技术通报, 2021, 37(4): 96-106. |
| [10] | 刘珊, 叶伟, 朱牧孜, 李赛妮, 邓张双, 章卫民. 一种新型酰基转移酶GPAT的克隆、表达与酶学性质研究[J]. 生物技术通报, 2021, 37(11): 257-266. |
| [11] | 赵海燕, 宋晨斌, 刘正亚, 马兴荣, 尚会会, 李安华, 关现军, 王建设. 来源于Laceyella sp.的α-淀粉酶基因克隆、重组表达及酶学性质研究[J]. 生物技术通报, 2020, 36(8): 23-33. |
| [12] | 王惠兰, 吴金勇, 陈祥松, 袁丽霞, 朱薇薇, 姚建铭. N-乙酰神经氨酸醛缩酶的固定化及固定化酶性质研究[J]. 生物技术通报, 2020, 36(6): 165-173. |
| [13] | 张庆芳, 逄飞, 于爽, 肖景惠, 窦少华, 迟乃玉. 海洋高产尿酸氧化酶菌株筛选鉴定及酶学性质研究[J]. 生物技术通报, 2019, 35(7): 61-69. |
| [14] | 董聪, 高庆华, 王玥, 罗同阳. 基于密码子优化的FAD依赖葡萄糖脱氢酶在毕赤酵母中的高效表达及酶学性质[J]. 生物技术通报, 2019, 35(7): 114-120. |
| [15] | 徐珊 ,李任强 ,郑振华 ,张云 ,孙爱君 ,胡云峰. 红树林微生物DH-2胞外蛋白酶的性质及产酶条件优化[J]. 生物技术通报, 2018, 34(6): 120-127. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||