








生物技术通报 ›› 2024, Vol. 40 ›› Issue (3): 242-250.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0884
刘佳宁1( ), 李梦1, 杨新森1, 吴伟1, 裴新梧2, 袁潜华1(
), 李梦1, 杨新森1, 吴伟1, 裴新梧2, 袁潜华1( )
)
收稿日期:2023-09-13
出版日期:2024-03-26
发布日期:2024-04-08
通讯作者:
袁潜华,男,硕士,研究员,研究方向:作物遗传育种与栽培;E-mail: qhyuan@163.com作者简介:刘佳宁,男,硕士,研究方向:作物栽培生态;E-mail: 15873169509@163.com
基金资助:
LIU Jia-ning1( ), LI Meng1, YANG Xin-sen1, WU Wei1, PEI Xin-wu2, YUAN Qian-hua1(
), LI Meng1, YANG Xin-sen1, WU Wei1, PEI Xin-wu2, YUAN Qian-hua1( )
)
Received:2023-09-13
Published:2024-03-26
Online:2024-04-08
摘要:
【目的】 山栏稻的栽培方式单一,传统刀耕火种的种植手段破坏环境,水作是山栏稻栽培的新模式。研究山栏稻在大田水作环境下根际微生物的变化情况。【方法】 设置正常灌溉和干旱管理的山栏稻栽培处理,分别对山栏稻根际土壤细菌群落进行16S rRNA基因扩增序列测定,测序数据结合土壤理化性质进行综合分析。【结果】 栽培方式的改变明显影响了根际细菌群落的组成。正常灌溉处理的Nitrosprota和Proteobacteria相对丰度低于干旱管理,Firmicutes的相对丰度高于干旱管理。相对丰度前10的细菌门群落与土壤理化性质具有相关性。相关性网络分析显示,正常灌溉处理中更多的细菌群落具有相互作用,正常灌溉处理拥有更复杂的细菌网络。【结论】 不同水分管理栽培方式的山栏稻根际细菌群落组成发生显著变化,Nitrospirota、Proteobacteria细菌类群可能通过促进水稻根部吸收氮素来增强山栏稻抗逆性。正常灌溉处理中更多细菌群落相互作用,增强了生态系统的稳定性。
刘佳宁, 李梦, 杨新森, 吴伟, 裴新梧, 袁潜华. 不同水分管理栽培方式对山栏稻根际土壤细菌群落的影响[J]. 生物技术通报, 2024, 40(3): 242-250.
LIU Jia-ning, LI Meng, YANG Xin-sen, WU Wei, PEI Xin-wu, YUAN Qian-hua. Impact of Different Water Management Cultivation Methods on the Rhizosphere Bacteria Community of Shanlan Upland Rice[J]. Biotechnology Bulletin, 2024, 40(3): 242-250.

图1 不同水分管理处理对根际土壤细菌群落门水平相对丰度的影响 横坐标为处理,H为干旱管理处理,S为正常灌溉处理;纵坐标为相对丰度百分比。不同颜色表示不同细菌门群落;堆叠柱为门水平相对丰度Top10的分类群
Fig. 1 Effect of irrigated and drying treatment on the relative abundance of bacterial community in rhizosphere soil at phylum level The abscissa refers to treatment, H to drying management treatment, and S to normal irrigation treatment. The ordinate is the relative abundance percentage. Different colors indicate different bacterial phyla. The stacked column is a taxon with a relative abundance of top 10 at the phylum level

图2 不同水分管理处理的Alpha多样性指数差异箱线图 横坐标为分组名称,H为干旱管理处理,S为正常灌溉处理,纵坐标为相应的指数值;箱的上下端线分别表示样本上下四分位数(IQR);中位线表示样本中位数;上下边缘表示样本最大最小内围值(1.5倍的IQR);位于上下边缘的外侧的点代表异常值;柱子间的连线上的数字为t检验的P值
Fig. 2 Box graph of differences in alpha diversity index between irrigated and drying treatment groups The abscissa refers to the grouping name, H to drying management treatment, S to normal irrigation treatment, and the ordinate refers to the value of alpha diversity index. The upper and lower sides of the box: the upper quartile and the lower quartile(IQR); middle line: the median of the sample; upper and lower margins: maximum and minimum inner circumference(IQR×1.5). The outer points of the edges indicate abnormal values. The number on the line between the two columns is the P-value of the t-test represent outliers
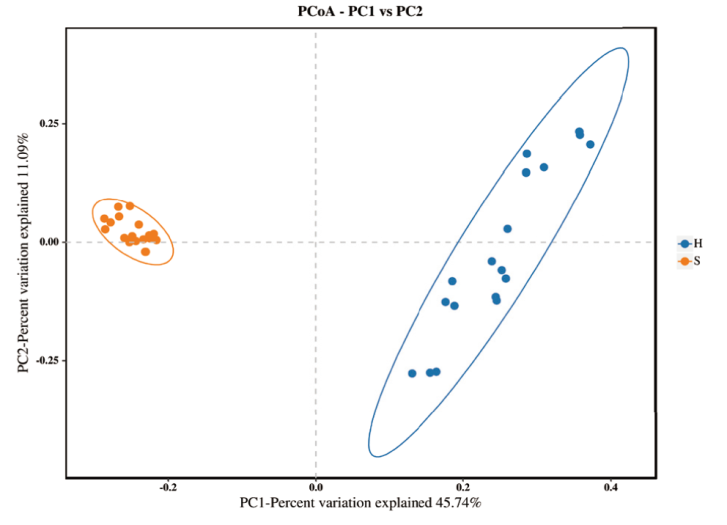
图3 PcoA分析 图中每个点代表一个样品;蓝色点代表干旱管理处理、橙色点代表正常灌溉处理;椭圆形圈表示其为95%置信区间;横坐标PC1代表第一主成分,百分比代表第一主成分对样品差异的贡献度;纵坐标PC2代表第二主成分;百分比代表第二主成分对样品差异的贡献度
Fig. 3 PcoA analysis Each point in the figure indicates a sample. The blue dot represents drying management treatment, and the orange dot represents normal irrigation treatment. An elliptical circle indicates that it is a 95% confidence interval. The abscissa PC1 is the first principal component, and the percentage is the contribution rate of the first principal component to the sample difference. The ordinate PC2 is the second principal component. Percentage is the contribution rate of the second principal component to the sample difference
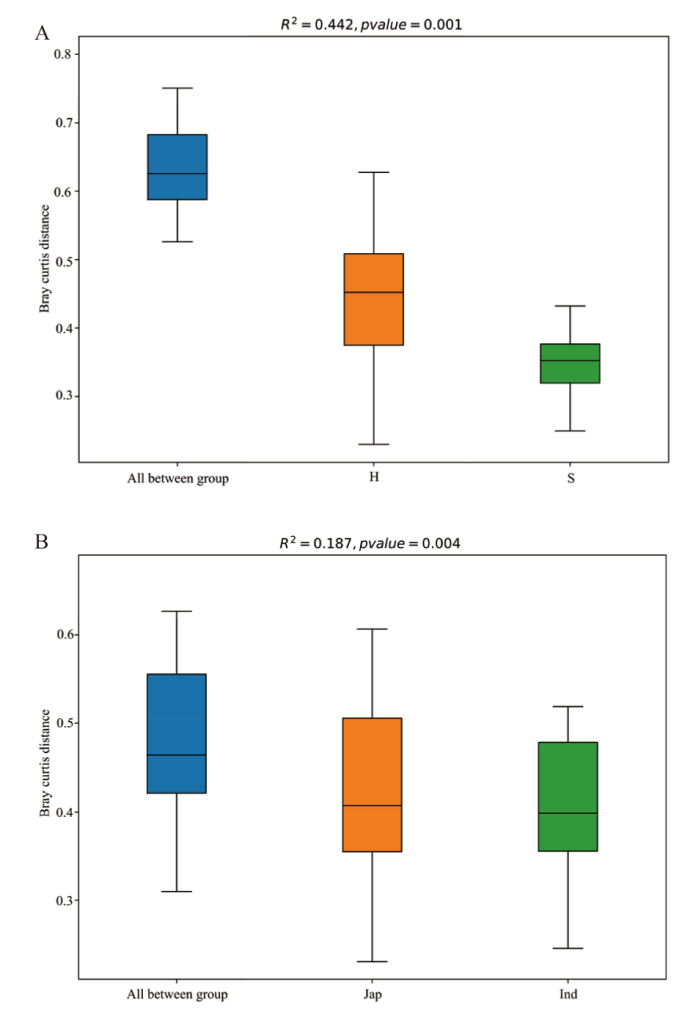
图4 PERMANOVA分析箱型图 A:处理;B:品种。纵坐标代表Bray-Curtis距离;“All between *”上方的箱图代表全部组间样品Beta距离数据;后面的箱型图分别是干旱管理处理和正常灌溉处理的组内样品间的Beta距离数据;H代表干旱管理处理,S代表正常灌溉处理;R2表示对样品差异的解释度
Fig. 4 PERMANOVA analysis box diagram A: Treatment; B: variety. The ordinate indicates the Bray-Curtis distance. The box diagram above “All between *” indicates the beta distance data of all inter group samples. The box chart below shows the beta distance data between samples in the group treated with drying management and normal irrigation, respectively. H refers to drying management treatment, and S to normal irrigation treatment. R2 is the degree of explanation for sample differences

图5 物种进化树的样本群落分布图 右上角图例为门水平物种名称,内圈为物种进化树,内圈物种中同一个门显示同一个颜色;外圈表示该物种在不同处理中的相对丰度占比,H:干旱管理处理,S:正常灌溉处理
Fig. 5 Sample community distribution map of species evolution tree The legend in the upper right corner shows the names of species at the phylum level, the inner circle shows the evolutionary tree of species, and the same phylum in the inner circle is in the same color. The outer circle indicates the relative abundance proportion of this species in different treatments. H: Drying management. S: Normal irrigation.
| 细菌门 Bacterial phylum | 相对丰度Relative abundance | P | |
|---|---|---|---|
| 正常灌溉 Normal irrigation | 干旱管理 Drought management | ||
| Acidobacteriota | 0.091 4 | 0.075 5 | <0.01 |
| Armatimonadota | 0.003 4 | 0.001 6 | <0.01 |
| Bdellovibrionota | 0.006 3 | 0.011 3 | <0.01 |
| Sumerlaeota | 0.001 3 | 0.002 2 | <0.01 |
| Planctomycetota | 0.016 8 | 0.032 5 | <0.01 |
| Dadabacteria | 0.003 3 | 0.009 1 | <0.01 |
| Dependentiae | 0.004 0 | 0.007 9 | <0.01 |
| Entotheonellaeota | 0.000 1 | 0.000 4 | <0.01 |
| Fibrobacterota | 0.002 7 | 0.001 4 | <0.01 |
| Patescibacteria | 0.014 2 | 0.030 2 | <0.01 |
| Nitrospirota | 0.069 1 | 0.098 9 | <0.01 |
| Myxococcota | 0.063 3 | 0.045 1 | <0.01 |
| Methylomirabilota | 0.005 8 | 0.008 8 | <0.01 |
| Gemmatimonadota | 0.012 2 | 0.007 9 | <0.01 |
| Verrucomicrobiota | 0.046 8 | 0.037 5 | <0.01 |
| Calditrichota | 0.000 6 | 0.001 5 | <0.01 |
| Firmicutes | 0.140 5 | 0.107 7 | <0.01 |
| Proteobacteria | 0.157 5 | 0.178 6 | <0.01 |
| Hydrogenedentes | 0.000 6 | 0.000 9 | 0.02 |
表1 不同水分管理栽培处理显著差异的细菌门群落
Table 1 Bacterial phylum communities with significant differences in different water management cultivation treatments
| 细菌门 Bacterial phylum | 相对丰度Relative abundance | P | |
|---|---|---|---|
| 正常灌溉 Normal irrigation | 干旱管理 Drought management | ||
| Acidobacteriota | 0.091 4 | 0.075 5 | <0.01 |
| Armatimonadota | 0.003 4 | 0.001 6 | <0.01 |
| Bdellovibrionota | 0.006 3 | 0.011 3 | <0.01 |
| Sumerlaeota | 0.001 3 | 0.002 2 | <0.01 |
| Planctomycetota | 0.016 8 | 0.032 5 | <0.01 |
| Dadabacteria | 0.003 3 | 0.009 1 | <0.01 |
| Dependentiae | 0.004 0 | 0.007 9 | <0.01 |
| Entotheonellaeota | 0.000 1 | 0.000 4 | <0.01 |
| Fibrobacterota | 0.002 7 | 0.001 4 | <0.01 |
| Patescibacteria | 0.014 2 | 0.030 2 | <0.01 |
| Nitrospirota | 0.069 1 | 0.098 9 | <0.01 |
| Myxococcota | 0.063 3 | 0.045 1 | <0.01 |
| Methylomirabilota | 0.005 8 | 0.008 8 | <0.01 |
| Gemmatimonadota | 0.012 2 | 0.007 9 | <0.01 |
| Verrucomicrobiota | 0.046 8 | 0.037 5 | <0.01 |
| Calditrichota | 0.000 6 | 0.001 5 | <0.01 |
| Firmicutes | 0.140 5 | 0.107 7 | <0.01 |
| Proteobacteria | 0.157 5 | 0.178 6 | <0.01 |
| Hydrogenedentes | 0.000 6 | 0.000 9 | 0.02 |
| 环境因子 Environmental factor | 正常灌溉 Normal irrigation | 干旱管理 Drought management | P |
|---|---|---|---|
| 有效钾/(mg·kg-1) | 69.99 ± 0.81 | 68.12±0.54 | 0.030 |
| 有效磷/(mg·kg-1) | 3.26 ± 0.08 | 4.81±0.11 | <0.001 |
| 有机质/(g·kg-1) | 11.85±0.19 | 23.30±0.03 | <0.001 |
| 碱解氮/(mg·kg-1) | 63.23±1.69 | 117.75±1.68 | <0.001 |
| 全氮/(g·kg-1) | 0.80±0.02 | 1.46±0.04 | <0.001 |
| pH | 8.61±0.10 | 8.20±.030 | 0.003 |
表2 不同水分管理栽培处理土壤环境因子的差异
Table 2 Differences in soil environmental factors under different water management cultivation treatments
| 环境因子 Environmental factor | 正常灌溉 Normal irrigation | 干旱管理 Drought management | P |
|---|---|---|---|
| 有效钾/(mg·kg-1) | 69.99 ± 0.81 | 68.12±0.54 | 0.030 |
| 有效磷/(mg·kg-1) | 3.26 ± 0.08 | 4.81±0.11 | <0.001 |
| 有机质/(g·kg-1) | 11.85±0.19 | 23.30±0.03 | <0.001 |
| 碱解氮/(mg·kg-1) | 63.23±1.69 | 117.75±1.68 | <0.001 |
| 全氮/(g·kg-1) | 0.80±0.02 | 1.46±0.04 | <0.001 |
| pH | 8.61±0.10 | 8.20±.030 | 0.003 |
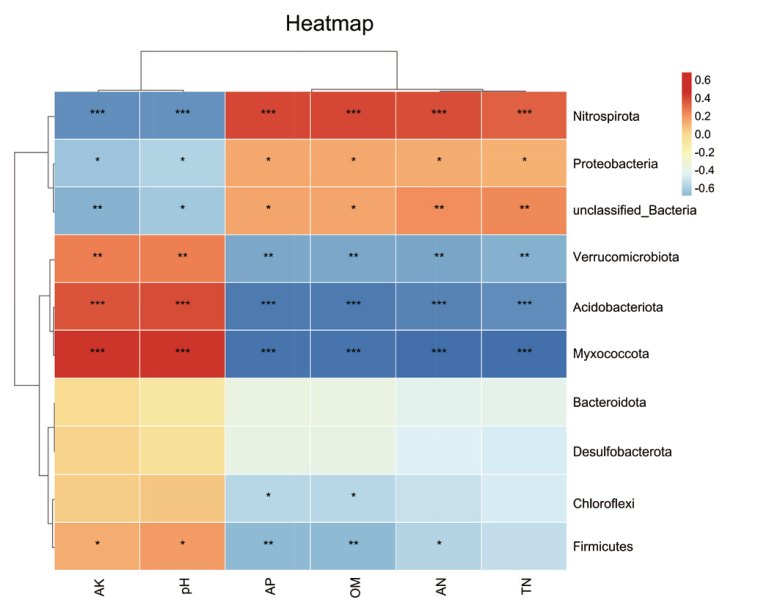
图6 环境因子与相对丰度前十的细菌门群落的相关性热图 横坐标为各环境因子;AK(有效钾)、pH、AP(有效磷)、OM(有机质)、AN(碱解氮)、TN(全氮);纵坐标为相对丰度前十的细菌门群落;红色表示正相关,蓝色表示负相关;颜色深浅代表相关性大小;显著性以*表示,*表示P值小于0.05,**表示P值小于0.01,***表示P值小于0.001
Fig. 6 Heat map of correlation between environmental factors and the top ten bacterial communities with relative abundance The abscissa refers to various environmental factors; AK(available potassium), pH, AP(available phosphorus), OM(organic matter), AN(alkali hydrolyzed nitrogen), TN(total nitrogen).The vertical coordinate is the top ten bacterial communities with relative abundance. Red indicates positive correlation. Blue indicates negative correlation. The color depth indicates the correlation size. The significance is indicated by *,* indicates that the P-value < 0.05, ** indicates that the P-value <0.01, and *** indicates that the P-value <0.001
| 处理 Treatment | 节点数 Number of nodes | 边数 Number of edges | 正相关边数Number of positive correlation edges | 负相关边数Number of negative correlation edges | 聚类系数Clustering coefficient | 模块性 Modularity |
|---|---|---|---|---|---|---|
| 干旱管理 | 36 | 100 | 47 | 53 | 0.498 | 0.292 |
| 正常灌溉 | 50 | 100 | 49 | 51 | 0.403 | 0.444 |
表3 不同水分管理栽培处理相关性网络属性
Table 3 Network attributes of different water management cultivation treatment
| 处理 Treatment | 节点数 Number of nodes | 边数 Number of edges | 正相关边数Number of positive correlation edges | 负相关边数Number of negative correlation edges | 聚类系数Clustering coefficient | 模块性 Modularity |
|---|---|---|---|---|---|---|
| 干旱管理 | 36 | 100 | 47 | 53 | 0.498 | 0.292 |
| 正常灌溉 | 50 | 100 | 49 | 51 | 0.403 | 0.444 |
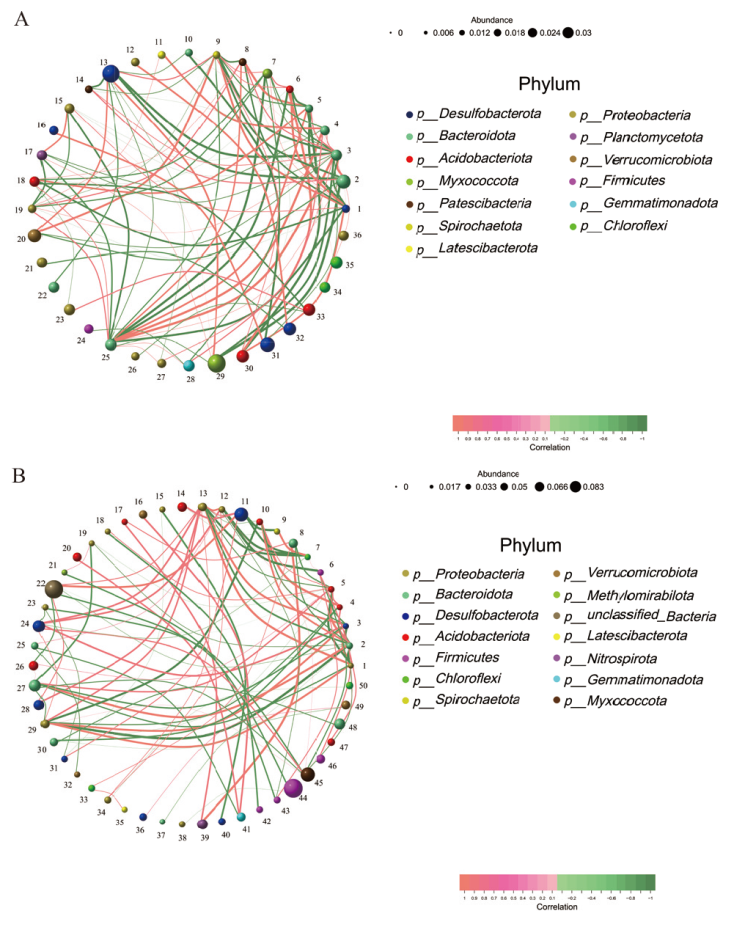
图7 属水平各物种网络图 A:干旱管理;B:正常灌溉。圆球代表细菌属;圆球的颜色代表细菌门;圆球的大小代表细菌属平均丰度的大小;连线表示2个细菌属相关;连线的粗细表示相关性的强弱;连线的颜色中,红色表示正相关,绿色表示负相关
Fig. 7 Network Diagram of various species at the genus level A: Drought management; B: normal irrigation. The sphere indicates the bacterial genus. The color of the sphere indicates the bacterial phylum. The size of the sphere indicates the average abundance of the bacterial genus. The line indicates that two bacterial genera are related. The thickness of the line indicates the strength of the correlation. Color of the connecting line: Red indicates positive correlation; green indicates negative correlation
| [1] | 黄孟雨, 刘志超, 谢新鑫, 等. 水旱栽培方式对山栏稻源库流特性的影响[J]. 南方农业学报, 2020, 51(4): 806-813. |
| Huang MY, Liu ZC, Xie XX, et al. Effects of different flooding and drying cultivation methods on characteristics of source, sink and flow of Shanlan upland rice[J]. J South Agric, 2020, 51(4): 806-813. | |
| [2] | 刘华招, 季春德. 海南山栏稻种质资源的保护与利用[J]. 热带农业科学, 2016, 36(12): 49-51. |
| Liu HZ, Ji CD. Conservation and utilization of Shanlan upland rice germplasm resources in Hainan Province[J]. Chin J Trop Agric, 2016, 36(12): 49-51. | |
| [3] | 黄昭奋, 黎瑞波, 麦全法, 等. 海南农业生物多样性与社会经济发展水平关系研究[J]. 热带农业科学, 2005, 25(2): 25-28. |
| Huang ZF, Li RB, Mai QF, et al. The relationship between agrobidiversity and socially economic development in Hainan and its protective strategy[J]. Chin J Trop Agric, 2005, 25(2): 25-28. | |
| [4] | 吴丹, 吴川德, 何美丹, 等. 水作和旱作对山栏稻生长的影响[J]. 热带生物学报, 2017, 8(3): 318-323. |
| Wu D, Wu CD, He MD, et al. The effects of paddy and upland cultivation on physiological parameters, agronomic traits and yield of shanlan upland rice[J]. J Trop Biol, 2017, 8(3): 318-323. | |
| [5] | 刘志超, 黄孟雨, 翟楠鑫, 等. 水旱两种栽培模式下海南山栏稻对白叶枯病抗性鉴定与评价[J]. 植物保护, 2021, 47(3): 191-199, 216. |
| Liu ZC, Huang MY, Zhai NX, et al. Resistance evaluation and molecular identification of Hainan Shanlan upland rice to bacterial blight under flooding and drying cultivation conditions[J]. Plant Prot, 2021, 47(3): 191-199, 216. | |
| [6] | 柯智, 黄孟雨, 刘志超, 等. 栽培方式对山栏稻光合作用和产量的影响[J]. 热带生物学报, 2019, 10(4): 331-337, 438. |
| Ke Z, Huang MY, Liu ZC, et al. Effects of cultivation patterns on photosynthesis, and yield and its components of shanlan upland rice[J]. J Trop Biol, 2019, 10(4): 331-337, 438. | |
| [7] | 何光亮. 不同栽培方式对山栏稻农艺性状和产量的影响[D]. 海口: 海南大学, 2018. |
| He GL. Effects of different cultivation methods on agronomic traits and yield of shanlan upland rice[D]. Haikou: Hainan University, 2018. | |
| [8] |
Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health[J]. Trends Plant Sci, 2012, 17(8): 478-486.
doi: 10.1016/j.tplants.2012.04.001 pmid: 22564542 |
| [9] | Edwards J, Johnson C, Santos-Medellín C, et al. Structure, variation, and assembly of the root-associated microbiomes of rice[J]. Proc Natl Acad Sci USA, 2015, 112(8): E911-E920. |
| [10] |
Pang ZQ, Xu P, Yu DQ. Environmental adaptation of the root microbiome in two rice ecotypes[J]. Microbiol Res, 2020, 241: 126588.
doi: 10.1016/j.micres.2020.126588 URL |
| [11] |
Schmidt JE, Poret-Peterson A, Lowry CJ, et al. Has agricultural intensification impacted maize root traits and rhizosphere interactions related to organic N acquisition?[J]. AoB Plants, 2020, 12(4): plaa026.
doi: 10.1093/aobpla/plaa026 URL |
| [12] |
Qu Q, Zhang ZY, Peijnenburg WJGM, et al. Rhizosphere microbiome assembly and its impact on plant growth[J]. J Agric Food Chem, 2020, 68(18): 5024-5038.
doi: 10.1021/acs.jafc.0c00073 URL |
| [13] |
Shrestha PM, Kube M, Reinhardt R, et al. Transcriptional activity of paddy soil bacterial communities[J]. Environ Microbiol, 2009, 11(4): 960-970.
doi: 10.1111/j.1462-2920.2008.01821.x pmid: 19170728 |
| [14] |
Gu YF, Zhang XP, Tu SH, et al. Soil microbial biomass, crop yields, and bacterial community structure as affected by long-term fertilizer treatments under wheat-rice cropping[J]. Eur J Soil Biol, 2009, 45(3): 239-246.
doi: 10.1016/j.ejsobi.2009.02.005 URL |
| [15] |
Bao XZ, Zou JX, Zhang B, et al. Arbuscular mycorrhizal fungi and microbes interaction in rice mycorrhizosphere[J]. Agronomy, 2022, 12(6): 1277.
doi: 10.3390/agronomy12061277 URL |
| [16] |
Doni F, Suhaimi NSM, Mispan MS, et al. Microbial contributions for rice production: from conventional crop management to the use of ‘omics’ technologies[J]. Int J Mol Sci, 2022, 23(2): 737.
doi: 10.3390/ijms23020737 URL |
| [17] |
Leng GY, Hall J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future[J]. Sci Total Environ, 2019, 654: 811-821.
doi: 10.1016/j.scitotenv.2018.10.434 URL |
| [18] | Khan A, Ding ZT, Ishaq M, et al. Applications of beneficial plant growth promoting rhizobacteria and mycorrhizae in rhizosphere and plant growth: a review[J]. Int J Agric Biol Eng, 2020, 13(5): 199-208. |
| [19] |
Xiong JB, Lu JQ, Li XH, et al. Effect of rice(Oryza sativa L.)genotype on yield: evidence from recruiting spatially consistent rhizosphere microbiome[J]. Soil Biol Biochem, 2021, 161: 108395.
doi: 10.1016/j.soilbio.2021.108395 URL |
| [20] | 张静, 可文静, 刘娟, 等. 不同深度土壤控水对稻田土壤微生物区系及细菌群落多样性的影响[J]. 中国生态农业学报: 中英文, 2019, 27(2): 277-285. |
| Zhang J, Ke WJ, Liu J, et al. Influence of water controlling depth on soil microflora and bacterial community diversity in paddy soil[J]. Chin J Eco Agric, 2019, 27(2): 277-285. | |
| [21] |
劳承英, 申章佑, 李艳英, 等. 基于高通量测序技术分析不同耕作方式下水稻根际土壤真菌多样性[J]. 热带作物学报, 2021, 42(9): 2717-2726.
doi: 10.3969/j.issn.1000-2561.2021.09.038 |
| Lao CY, Shenzhang Y, Li YY, et al. Diversity analysis of fungal in rhizosphere soils of rice under different tillage methods based on high-throughput sequencing technique[J]. Chin J Trop Crops, 2021, 42(9): 2717-2726. | |
| [22] |
Xie ZC, Chu YK, Zhang WJ, et al. Bacillus pumilus alleviates drought stress and increases metabolite accumulation in Glycyrrhiza uralensis Fisch[J]. Environ Exp Bot, 2019, 158: 99-106.
doi: 10.1016/j.envexpbot.2018.11.021 URL |
| [23] |
Yin Y, Wang YF, Cui HL, et al. Distinctive structure and assembly of phyllosphere microbial communities between wild and cultivated rice[J]. Microbiol Spectr, 2023, 11(1): e0437122.
doi: 10.1128/spectrum.04371-22 URL |
| [24] |
Cheng HY, Yuan MS, Tang L, et al. Integrated microbiology and metabolomics analysis reveal responses of soil microorganisms and metabolic functions to phosphorus fertilizer on semiarid farm[J]. Sci Total Environ, 2022, 817: 152878.
doi: 10.1016/j.scitotenv.2021.152878 URL |
| [25] |
Zhu JY, Li A, Zhang J, et al. Effects of nitrogen application after abrupt drought-flood alternation on rice root nitrogen uptake and rhizosphere soil microbial diversity[J]. Environ Exp Bot, 2022, 201: 105007.
doi: 10.1016/j.envexpbot.2022.105007 URL |
| [26] |
Jang SW, Yoou MH, Hong WJ, et al. Re-analysis of 16S amplicon sequencing data reveals soil microbial population shifts in rice fields under drought condition[J]. Rice, 2020, 13(1): 44.
doi: 10.1186/s12284-020-00403-6 |
| [27] |
Zhou GX, Xu XF, Qiu XW, et al. Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure[J]. Bioresour Technol, 2019, 272: 10-18.
doi: 10.1016/j.biortech.2018.09.135 URL |
| [28] |
Kanasugi M, Sarkodee-Addo E, Ansong Omari R, et al. Exploring rice root microbiome; the variation, specialization and interaction of bacteria and fungi in six tropic savanna regions in Ghana[J]. Sustainability, 2020, 12(14): 5835.
doi: 10.3390/su12145835 URL |
| [29] |
Zhang JY, Liu YX, Zhang N, et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice[J]. Nat Biotechnol, 2019, 37(6): 676-684.
doi: 10.1038/s41587-019-0104-4 pmid: 31036930 |
| [30] | 刘文静, 张建伟, 邱崇文, 等. 水旱轮作对土壤微生物群落构建过程的影响机制[J]. 土壤, 2020, 52(4): 710-717. |
| Liu WJ, Zhang JW, Qiu CW, et al. Study on community assembly processes under paddy-upland rotation[J]. Soils, 2020, 52(4): 710-717. |
| [1] | 王雨晴, 马子奇, 侯嘉欣, 宗钰琪, 郝晗睿, 刘国元, 魏辉, 连博琳, 陈艳红, 张健. 盐胁迫下植物根系分泌物的成分分析与生态功能研究进展[J]. 生物技术通报, 2024, 40(1): 12-23. |
| [2] | 赵林艳, 徐武美, 王豪吉, 王昆艳, 魏富刚, 杨绍周, 官会林. 施用生物炭对连作三七根际真菌群落与存活率的影响[J]. 生物技术通报, 2023, 39(7): 219-227. |
| [3] | 孙卓, 王妍, 韩忠明, 王云贺, 赵淑杰, 杨利民. 防风根际真菌的分离鉴定及其生防潜力评价[J]. 生物技术通报, 2023, 39(1): 264-273. |
| [4] | 赵忠娟, 杨凯, 扈进冬, 魏艳丽, 李玲, 徐维生, 李纪顺. 盐胁迫条件下哈茨木霉ST02对椒样薄荷生长及根区土壤理化性质的影响[J]. 生物技术通报, 2022, 38(7): 224-235. |
| [5] | 王宁, 李蕙秀, 李季, 丁国春. 堆肥调控作物根际微生物组抑制植物病害的研究进展[J]. 生物技术通报, 2022, 38(5): 4-12. |
| [6] | 杨露, 辛建攀, 田如男. 根际微生物对植物重金属胁迫的缓解作用及其机理研究进展[J]. 生物技术通报, 2022, 38(3): 213-225. |
| [7] | 陆玉芳, 施卫明. 根际化学信号物质与土壤养分转化[J]. 生物技术通报, 2020, 36(9): 14-24. |
| [8] | 孙雨, 常晶晶, 田春杰. 作物根际微生物组重组构建技术体系探讨[J]. 生物技术通报, 2020, 36(9): 25-30. |
| [9] | 许来鹏, 万鲜花, 孙向丽, 曹艳芳, 李慧, 田亚东, 刘小军, 康相涛, 王彦彬. 畜禽粪肥和秸秆还田对玉米根际微生物群落结构的影响[J]. 生物技术通报, 2020, 36(9): 137-146. |
| [10] | 翟楠鑫, 迟会, 夏玥琳, 刘彩月, 裴新梧, 袁潜华. 海南山栏稻抗旱基因转录组分析[J]. 生物技术通报, 2020, 36(12): 12-20. |
| [11] | 张卓, 刘茂炎, 王培, 黄文坤, 刘二明, 彭焕, 彭德良. 抗草甘膦转基因大豆AG5601对根际微生物群落功能多样性的影响[J]. 生物技术通报, 2019, 35(7): 17-24. |
| [12] | 王端, 姚香梅, 叶健. 根际微生物-植物-病毒-介体昆虫多元互作研究进展[J]. 生物技术通报, 2018, 34(2): 54-65. |
| [13] | 赵佳, 黄静, 陈哲, 聂园军, 梁宏. 西瓜枯萎病拮抗菌Lh-1的鉴定及生物防治效果研究[J]. 生物技术通报, 2017, 33(4): 130-136. |
| [14] | 姚宇飞,王英,庄南生,高和琼. 利用SRAP标记分析海南旱稻地方品种的遗传多样性及其分子身份证的构建[J]. 生物技术通报, 2014, 0(11): 97-106. |
| [15] | 赵佳, 孙毅, 梁宏, 黄静, 杜建中. 现代生物技术在根际微生物群落研究中的应用[J]. 生物技术通报, 2012, 0(12): 65-70. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||