








生物技术通报 ›› 2025, Vol. 41 ›› Issue (5): 310-319.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0992
• 研究报告 • 上一篇
曲珊1( ), 赵月1, 李雅华1, 郑桂玲2, 咸洪泉1(
), 赵月1, 李雅华1, 郑桂玲2, 咸洪泉1( )
)
收稿日期:2024-10-12
出版日期:2025-05-26
发布日期:2025-06-05
通讯作者:
咸洪泉,男,博士,教授,研究方向 :分子生物学和植物病害生物防治;E-mail: hqxian00517@163.com作者简介:曲珊,女,硕士,研究方向 :微生物及分子生物学;E-mail: raymond0416@163.com基金资助:
QU Shan1( ), ZHAO Yue1, LI Ya-hua1, ZHENG Gui-ling2, XIAN Hong-quan1(
), ZHAO Yue1, LI Ya-hua1, ZHENG Gui-ling2, XIAN Hong-quan1( )
)
Received:2024-10-12
Published:2025-05-26
Online:2025-06-05
摘要:
目的 生防菌棘孢木霉(Trichoderma asperellum)TD3104产生的几丁质酶Tachi2在植物病害防治过程中发挥重要作用,47号转录因子作用于特异响应几丁质诱导的Tachi2基因启动子顺式作用元件。探究47号转录因子与一种新调控蛋白H63的互作关系,为解析几丁质诱导调控基因转录表达机制提供科学依据。 方法 利用酵母双杂交系统对棘孢木霉几丁质酶基因Tachi2中47号转录因子的候选互作蛋白H63进行体内点对点互作鉴定;采用大肠杆菌表达系统分别对H63蛋白与47号转录因子基因进行原核诱导表达,利用亲和层析技术纯化融合蛋白,通过GST pull-down实验进行体外蛋白互作检测;利用农杆菌介导的洋葱表皮细胞亚细胞定位技术和BiFC实验进一步检测细胞内蛋白的相互作用。 结果 H63蛋白与47号转录因子在酵母细胞内存在互作关系;原核表达的重组H63蛋白、47号转录因子大小分别为36 kD和18 kD,二者在体外存在互作关系;成功构建了双分子荧光互补载体,证实H63蛋白与47号转录因子在洋葱内表皮细胞中存在相互作用,并且互作发生在细胞核内。 结论 证实H63蛋白与47号转录因子在细胞内外均存在相互作用,为解析真菌几丁质酶基因的表达调控、几丁质酶在农业和生物医药领域的开发利用奠定理论基础。
曲珊, 赵月, 李雅华, 郑桂玲, 咸洪泉. 调控棘孢木霉几丁质酶Tachi2基因的转录因子和蛋白的互作研究[J]. 生物技术通报, 2025, 41(5): 310-319.
QU Shan, ZHAO Yue, LI Ya-hua, ZHENG Gui-ling, XIAN Hong-quan. A Study on the Interaction between Transcriptional Factor and Protein of Tachi2 Chitinase Gene in Trichoderma asperellum[J]. Biotechnology Bulletin, 2025, 41(5): 310-319.
| 引物名称 Primer name | 序列 Sequence (5′-3′) | 限制性内切酶 Restriction enzyme | 用途 Usage |
|---|---|---|---|
| 47S | CCG | EcoR Ⅰ | 点对点验证 |
| 47A | CG | BamH Ⅰ | 点对点验证 |
| H63S | TCCC | Xma I | 点对点验证 |
| H63A | CG | BamH Ⅰ | 点对点验证 |
| 5′AD | CTATTCGATGATGAAGATACCCCACCAAACCC | - | 点对点验证 |
| 3′AD | AGTGAACTTGGGGGGTTTTTCAGTATCTACGAT | - | 点对点验证 |
| H63S-B/N | CG | BamH Ⅰ | GST pull-down |
| H63A-B/N | ATTT | Not I | GST pull-down |
| CE63S | CG | BamH I | BiFC |
| CE63A | GG | Kpn I | BiFC |
| NE47S | CG | BamH I | BiFC |
| NE47A | GG | Kpn I | BiFC |
表1 实验所用引物
Table 1 Primers used in the experiment
| 引物名称 Primer name | 序列 Sequence (5′-3′) | 限制性内切酶 Restriction enzyme | 用途 Usage |
|---|---|---|---|
| 47S | CCG | EcoR Ⅰ | 点对点验证 |
| 47A | CG | BamH Ⅰ | 点对点验证 |
| H63S | TCCC | Xma I | 点对点验证 |
| H63A | CG | BamH Ⅰ | 点对点验证 |
| 5′AD | CTATTCGATGATGAAGATACCCCACCAAACCC | - | 点对点验证 |
| 3′AD | AGTGAACTTGGGGGGTTTTTCAGTATCTACGAT | - | 点对点验证 |
| H63S-B/N | CG | BamH Ⅰ | GST pull-down |
| H63A-B/N | ATTT | Not I | GST pull-down |
| CE63S | CG | BamH I | BiFC |
| CE63A | GG | Kpn I | BiFC |
| NE47S | CG | BamH I | BiFC |
| NE47A | GG | Kpn I | BiFC |
| 组别 Group | 蛋白 Protein |
|---|---|
| 实验组1 | GST-H63蛋白+His-47蛋白 |
| 实验组2 | GST标签蛋白+His-47蛋白 |
| 实验组3 | GST标签蛋白 |
| 对照组1 | 纯化的His-47蛋白 |
| 对照组2 | 纯化的GST-H63蛋白 |
表2 GST pull-down实验设计
Table 2 GST pull-down experimental design
| 组别 Group | 蛋白 Protein |
|---|---|
| 实验组1 | GST-H63蛋白+His-47蛋白 |
| 实验组2 | GST标签蛋白+His-47蛋白 |
| 实验组3 | GST标签蛋白 |
| 对照组1 | 纯化的His-47蛋白 |
| 对照组2 | 纯化的GST-H63蛋白 |
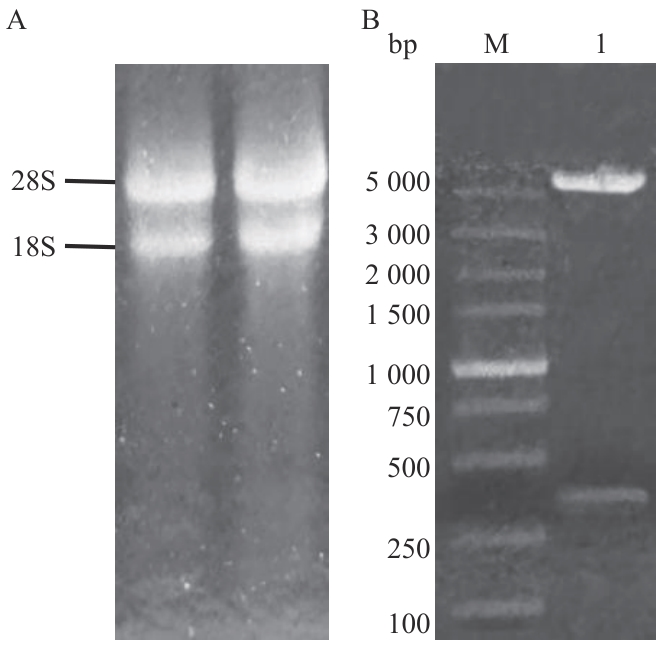
图1 pGBKT7-47载体构建A:棘孢木霉RNA;B:PGBKT7-47双酶切验证(1:pGBKT7-47双酶切产物;M:DL5000 bp DNA marker)
Fig. 1 pGBKT7-47 vector constructionA: RNA of Trichoderma asperellum; B: PGBKT7-47 double enzyme digestion verification (pGBKT7-47 double enzyme-digested product; M: 5000 bp DNA marker)
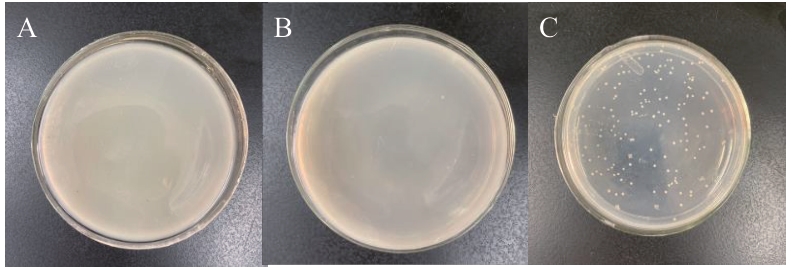
图2 pGADT7-47 载体自激活和毒性检测A:pGBKT7-47/pGADT7的Y2H Gold酵母转化子;B:pGADT7-T/pGBKT7-lam的Y2H Gold酵母转化子;C:pGADT7-T/pGBKT7-53的Y2H Gold酵母转化子
Fig. 2 pGADT7-47 vector self-activation and toxicity testA: Y2H Gold yeast transformant of pGBKT7-47 and pGADT7; B: Y2H Gold yeast transformant of pGADT7-T and pGBKT7-lam; C: Y2H Gold yeast transformant of pGADT7-T and pGBKT7-53
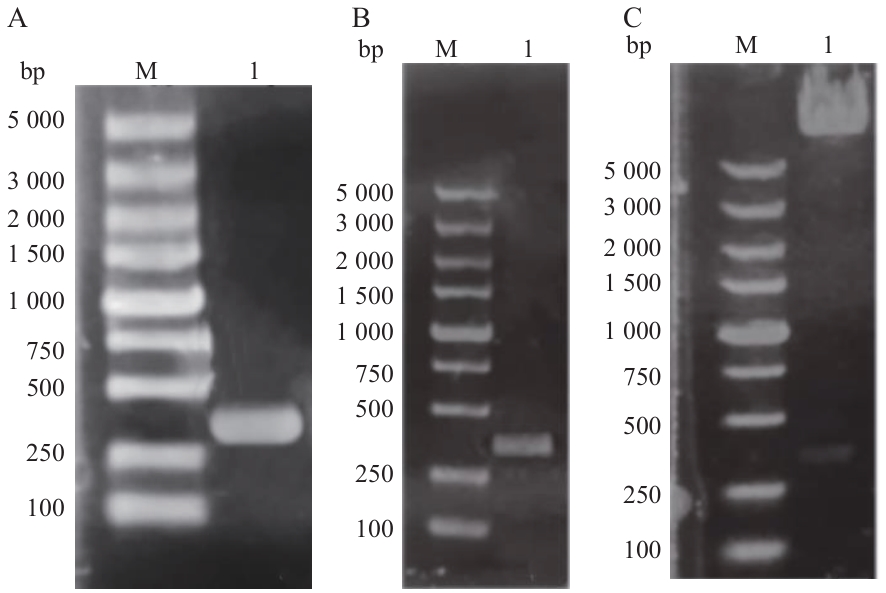
图3 pGADT7-63载体构建A:H63基因PCR产物;B:H63菌液PCR产物验证;C:pGADT7-63双酶切产物;M:DL5000 bp DNA marker
Fig. 3 Construction of interacting pGADT7-H63 vectorA: H63 gene PCR product; B: H63 bacterial liquid PCR product validation; C: PGADT7-63 double enzyme digestion product; M: DL5000 bp DNA marker
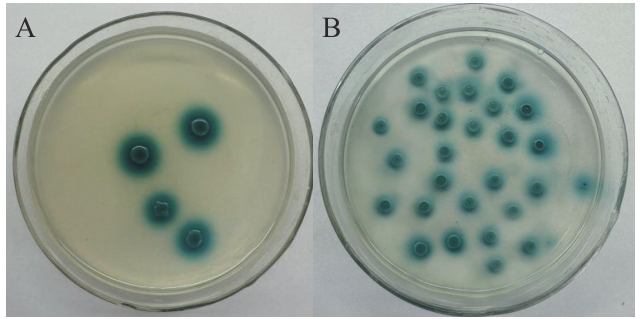
图4 酵母双杂交验证pGBKT7-47与 pGADT7-H63互作A:pGBKT7-47/pGADT7-H63的Y2H Gold酵母转化子;B:pGADT7-T/pGBKT7-53的Y2H Gold酵母转化子
Fig. 4 Verification of the interaction of pGBKT7-47 with pGADT7-H63 through yeast two-hybridA: Y2H Gold yeast transformant of pGBKT7-47 and pGADT7-H63; B: Y2H Gold yeast transformant of pGADT7-T and pGBKT7-53
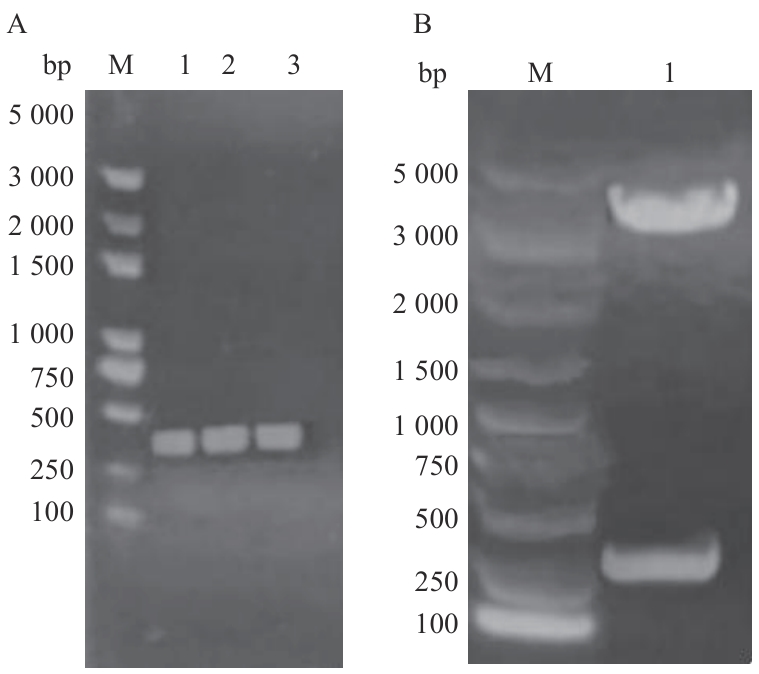
图5 pGEX4T-H63载体构建A:H63基因PCR产物;B:pGEX4T-H63双酶切产物;M:DL5000 bp DNA marker
Fig. 5 Construction of pGEX4T-H63 vectorA: H63 gene PCR product; B: pGEX4T-H63 double enzyme digestion product; M: DL5000 bp DNA marker
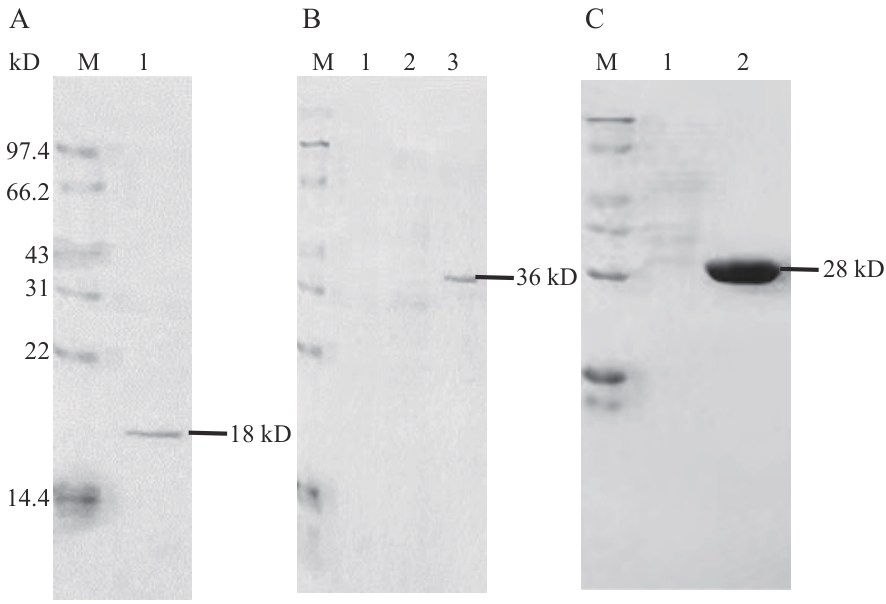
图6 重组蛋白SDS-PAGE电泳A:纯化后的47蛋白;B:纯化后的GST-H63蛋白;C:纯化后的GST蛋白;M :蛋白分子量标准
Fig. 6 SDS-PAGE electrophoresis map of recombinant proteinA: Purified 47 protein; B: purified pGEX4T-1-H63 protein; C: purified pGEX4T-1 protein; M: standard protein marker
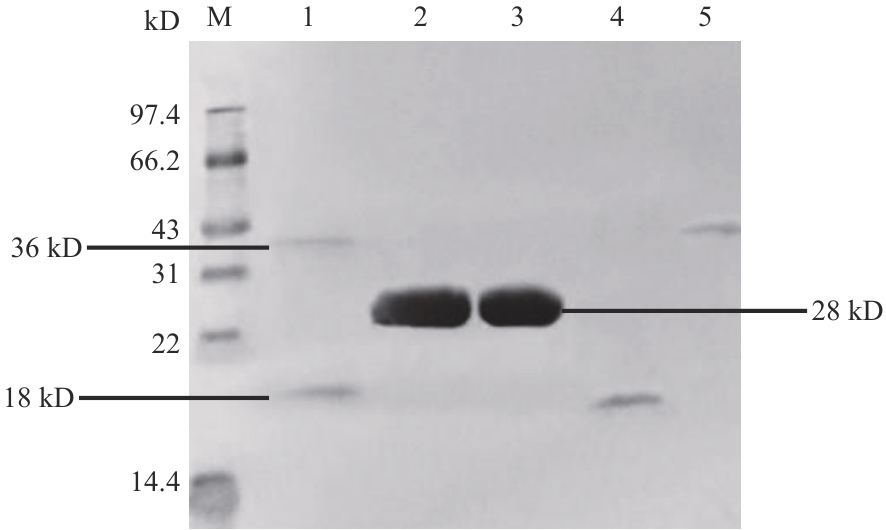
图7 GST-H63和His-47 蛋白体外互作验证1:GST-H63和His-47蛋白;2:His-47 和GST蛋白;3:GST蛋白;4:His-47蛋白;5:GST-H63蛋白;M:蛋白分子量标准
Fig. 7 Protein interaction validation of GST-H63 and His-47 in vitro1: GST-H63 protein and His-47 protein; 2: His-47 protein and GST protein; 3: GST; 4: His-47 protein; 5: GST-H63 protein; M: standard protein marker
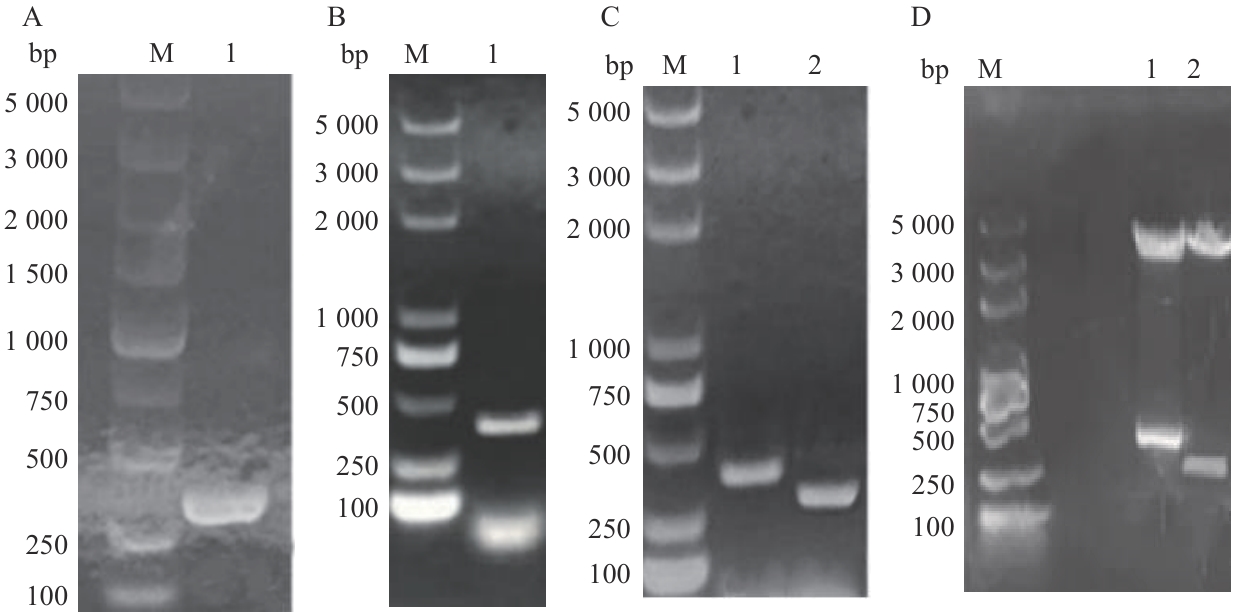
图8 BiFC实验载体构建A:H63基因PCR产物;B:47号转录因子基因PCR产物;C:菌液PCR验证(1:DH5α/B-T1-47菌液PCR产物;2:DH5α/B-T1-H63菌液PCR产物);D:双酶切验证(1:NE-47双酶切产物;2:CE-63双酶切产物);M :DL5000 bp DNA marker
Fig. 8 Construction of BiFC experimental carrierA: H63 gene PCR product; B: PCR product of transcription factor gene 47; C: PCR validation of bacterial liquid (1: PCR product of DH5 α/B-T1-47 bacterial liquid; 2: PCR product of DH5 α/B-T1-H63 bacterial liquid); D: double enzyme digestion validation (1: NE-47 double enzyme cleavage product; 2: CE-63 double enzyme cleavage product); M: DL5000bp DNA marker
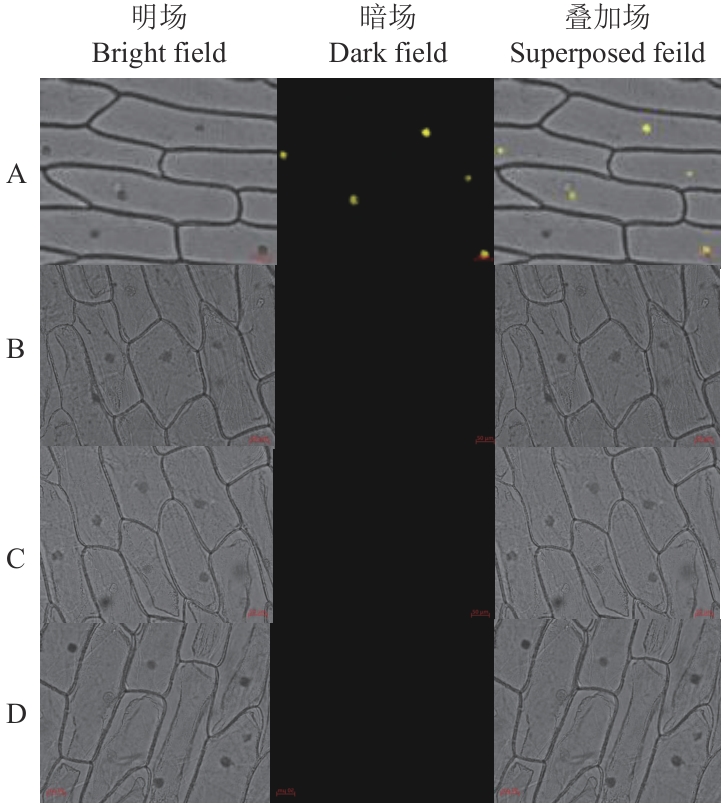
图9 BiFC实验验证蛋白互作A: GV3101/NE-47 and GV3101/CE-H63; B: GV3101/NE and GV3101/CE; C: GV3101/NE and GV3101/CE-H63; D: GV3101/CE and GV3101/NE-47
Fig. 9 BiFC experiment validating protein interaction
| 1 | Sharon E, Chet I, Spiegel Y. Trichoderma as a biological control agent [M]//Davies K, Spiegel Y, eds. Biological Control of Plant-Parasitic Nematodes. Dordrecht: Springer Netherlands, 2011: 183-201. |
| 2 | Mukherjee PK, Buensanteai N, Moran-Diez ME, et al. Functional analysis of non-ribosomal peptide synthetases (NRPSs) in Trichoderma virens reveals a polyketide synthase (PKS)/NRPS hybrid enzyme involved in the induced systemic resistance response in maize [J]. Microbiology, 2012, 158(Pt 1): 155-165. |
| 3 | Woo SL, Hermosa R, Lorito M, et al. Trichoderma: a multipurpose, plant-beneficial microorganism for eco-sustainable agriculture [J]. Nat Rev Microbiol, 2023, 21(5): 312-326. |
| 4 | Kappel L, Münsterkötter M, Sipos G, et al. Chitin and chitosan remodeling defines vegetative development and Trichoderma biocontrol [J]. PLoS Pathog, 2020, 16(2): e1008320. |
| 5 | Bae SJ, Park YH, Bae HJ, et al. Molecular identification, enzyme assay, and metabolic profiling of Trichoderma spp [J]. J Microbiol Biotechnol, 2017, 27(6): 1157-1162. |
| 6 | Al-Ani LKT. A patent survey of Trichoderma spp. (from 2007 to 2017) [M]//Intellectual Property Issues in Microbiology. Singapore: Springer Singapore, 2019: 163-192. |
| 7 | Manzar N, Kashyap AS, Goutam RS, et al. Trichoderma: advent of versatile biocontrol agent, its secrets and insights into mechanism of biocontrol potential [J]. Sustainability, 2022, 14(19): 12786. |
| 8 | 薛德星, 李美, 高兴祥, 等. 生防菌棘孢木霉的分离鉴定及生物学特性研究 [J]. 山东农业科学, 2023, 55(10): 118-123. |
| Xue DX, Li M, Gao XX, et al. Isolation, identification and biological characteristics of Trichoderma asperellum GT30 [J]. Shandong Agric Sci, 2023, 55(10): 118-123. | |
| 9 | 廉华, 马光恕, 李梅, 等. 棘孢木霉菌剂对黄瓜生理特性及产质量的影响 [J]. 中国农业大学学报, 2021, 26(6): 42-52. |
| Lian H, Ma GS, Li M, et al. Effects of Trichoderma asperellum agents on physiological characteristics, yield and quality of cucumber [J]. J China Agric Univ, 2021, 26(6): 42-52. | |
| 10 | Di Rosa M, Distefano G, Zorena K, et al. Chitinases and immunity: Ancestral molecules with new functions [J]. Immunobiology, 2016, 221(3): 399-411. |
| 11 | Dukare AS, Paul S, Asha AD, et al. Role of bacterial and fungal chitinases in integrated management of pest and diseases of agro-horticultural crops [M]//Microbes for Sustainable lnsect Pest Management. Cham: Springer International Publishing, 2021: 33-57. |
| 12 | 卢传琦. 海洋产几丁质酶菌株的筛选鉴定、基因组分析及几丁质酶酶学性质评价 [D]. 武汉: 武汉轻工大学, 2023. |
| Lu CQ. Screening, identification, genome analysis and enzymatic properties evaluation of chitinase-producing strain from the sea [D]. Wuhan: Wuhan Polytechnic University, 2023. | |
| 13 | 周玉玲, 蒋思婧, 贺妮莎, 等. 微生物几丁质酶研究进展及其在N-乙酰氨基葡萄糖制备中的应用 [J]. 微生物学报, 2021, 61(8): 2192-2204. |
| Zhou YL, Jiang SJ, He NS, et al. Research progress of microbial chitinase and its application in the preparation of N-acetylglucosamine [J]. Acta Microbiol Sin, 2021, 61(8): 2192-2204. | |
| 14 | Poveda J. Glucosinolates profile of Arabidopsis thaliana modified root colonization of Trichoderma species [J]. Biol Contr, 2021, 155: 104522. |
| 15 | Tue NH, Cat Tuong TG, Trang PTH, et al. Cloning the root-specific Asy promoter and genes encoding chitinase 42 kDa of Trichoderma asperellum into the plant expression vector [J]. J App Biol Biotech, 2022: 7-11. |
| 16 | Liu XG, Yu Y, Liu Q, et al. A Na2CO3-responsive chitinase gene from Leymus chinensis improve pathogen resistance and saline-alkali stress tolerance in transgenic tobacco and maize [J]. Front Plant Sci, 2020, 11: 504. |
| 17 | Kabir SR, Rahman MM, Tasnim S, et al. Purification and characterization of a novel chitinase from Trichosanthes dioica seed with antifungal activity [J]. Int J Biol Macromol, 2016, 84: 62-68. |
| 18 | 王琳, 陈雅如, 程湄婕, 等. 微生物几丁质酶研究进展及应用 [J]. 中国生物工程杂志, 2022, 42(12): 101-110. |
| Wang L, Chen YR, Cheng MJ, et al. Research advances in microbial chitinase and its applications [J]. China Biotechnol, 2022, 42(12): 101-110. | |
| 19 | Limón MC, Lora JM, García I, et al. Primary structure and expression pattern of the 33-kDa chitinase gene from the mycoparasitic fungus Trichoderma harzianum [J]. Curr Genet, 1995, 28(5): 478-483. |
| 20 | Wang C, Zeng ZQ, Zhuang WY. Comparative molecular evolution of chitinases in ascomycota with emphasis on mycoparasitism lifestyle [J]. Microb Genom, 2021, 7(9): 000646. |
| 21 | 咸洪泉, 张磊, 李雅华, 等. 特异性顺式作用元件及含该顺式作用元件的启动子和核酸构建体及其应用: CN106754915B [P]. 2019-07-26. |
| Xian HQ, Zhang L, Li YH, et al. Specific cis-acting elements, promoters and nucleic acid constructs containing such cis-acting elements, and their applications: CN106754915B [P]. 2019-07-26. | |
| 22 | Poria V, Rana A, Kumari A, et al. Current perspectives on chitinolytic enzymes and their agro-industrial applications [J]. Biology, 2021, 10(12): 1319. |
| 23 | Jeong GJ, Khan F, Tabassum N, et al. Chitinases as key virulence factors in microbial pathogens: Understanding their role and potential as therapeutic targets [J]. Int J Biol Macromol, 2023, 249: 126021. |
| 24 | Badrhadad A, Nazarian-Firouzabadi F, Ismaili A. Fusion of a chitin-binding domain to an antibacterial peptide to enhance resistance to Fusarium solani in tobacco (Nicotiana tabacum) [J]. 3 Biotech, 2018, 8(9): 391. |
| 25 | Naher L, Yusuf UK, Habib SH, et al. Mycoparasitism activity of Trichoderm harzianum associated with chitinase expression against Ganoderma boninense [J]. Pak J Bot, 2018, 50(3): 1241-1245. |
| 26 | 韩静, 安一博, 季世达, 等. 棘孢木霉Myb27转录因子基因特性分析、原核表达及产物纯化 [J]. 吉林农业大学学报, 2024, 46(1): 49-57. |
| Han J, An YB, Ji SD, et al. Characteristics analysis, prokaryotic expression and product purification of Trichoderma asperellum Myb27 transcription factor [J]. J Jilin Agric Univ, 2024, 46(1): 49-57. | |
| 27 | Ptashne M. How eukaryotic transcriptional activators work [J]. Nature, 1988, 335(6192): 683-689. |
| 28 | Maués DB, Maraschin JC, Duarte DÂ, et al. Overexpression of the transcription factor Azf1 reveals novel regulatory functions and impacts β-glucosidase production in Trichoderma reesei [J]. J Fungi, 2023, 9(12): 1173. |
| 29 | Mu YT, Dong YH, Li XC, et al. JrPHL8-JrWRKY4-JrSTH2L module regulates resistance to Colletotrichum gloeosporioides in walnut [J]. Hortic Res, 2024, 11(7): uhae148. |
| 30 | 王秋月, 段鹏亮, 李海笑, 等. 玉米大斑病菌cDNA文库的构建及转录因子StMR1互作蛋白的筛选 [J]. 生物技术通报, 2024, 40(6): 281-289. |
| Wang QY, Duan PL, Li HX, et al. Construction of cDNA library of Setosphaeria Turcica and screening of transcription factor StMR1 interacting proteins [J]. Biotechnol Bull, 2024, 40(6): 281-289. |
| [1] | 杨春, 王晓倩, 王红军, 晁跃辉. 蒺藜苜蓿MtZHD4基因克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2025, 41(5): 244-254. |
| [2] | 胡若群, 曾菁菁, 梁婉凤, 曹佳玉, 黄小苇, 梁晓英, 仇明月, 陈莹. 转录组和代谢组联合分析探究不同遮光条件下金线莲类胡萝卜素合成代谢机制[J]. 生物技术通报, 2025, 41(5): 231-243. |
| [3] | 周志国, 樊双虎, 邓晨, 冯雪. 2,4-表油菜素内酯对镉胁迫下胡萝卜幼苗生理特性的影响[J]. 生物技术通报, 2025, 41(5): 165-174. |
| [4] | 彭绍智, 王登科, 张祥, 戴雄泽, 徐昊, 邹学校. 辣椒CaFD1基因克隆、表达特征及功能验证[J]. 生物技术通报, 2025, 41(5): 153-164. |
| [5] | 罗嗣芳, 张祖铭, 谢丽芳, 郭紫晶, 陈兆星, 杨月华, 严翔, 张洪铭. 山金柑GATA基因家族全基因组鉴定及在果实发育中的表达分析[J]. 生物技术通报, 2025, 41(5): 218-230. |
| [6] | 杨朝结, 张兰, 陈红, 黄娟, 石桃雄, 朱丽伟, 陈庆富, 李洪有, 邓娇. 苦荞转录因子基因FtbHLH3调控类黄酮生物合成的功能鉴定[J]. 生物技术通报, 2025, 41(4): 134-144. |
| [7] | 王天禧, 杨炳松, 潘荣君, 盖文贤, 梁美霞. 苹果PLATZ基因家族鉴定及MdPLATZ9基因功能研究[J]. 生物技术通报, 2025, 41(4): 176-187. |
| [8] | 刘丽, 王辉, 关天舒, 李柏宏, 于舒怡. 葡萄脱落酸受体VvPYL4互作蛋白的筛选及互作蛋白基因表达[J]. 生物技术通报, 2025, 41(4): 188-197. |
| [9] | 马利花, 侯梦娟, 朱新霞. 陆地棉GhNFD4在棉花干旱响应中的功能[J]. 生物技术通报, 2025, 41(3): 104-111. |
| [10] | 宋姝熠, 蒋开秀, 刘欢艳, 黄亚成, 刘林娅. ‘红阳’猕猴桃TCP基因家族鉴定及其在果实中的表达分析[J]. 生物技术通报, 2025, 41(3): 190-201. |
| [11] | 王斌, 王玉昆, 肖艳辉. 丁香罗勒(Ocimum gratissimum)叶片响应镉胁迫的比较转录组学分析[J]. 生物技术通报, 2025, 41(3): 255-270. |
| [12] | 刘洁, 王飞, 陶婷, 张玉静, 陈浩婷, 张瑞星, 石玉, 张毅. 过表达SlWRKY41提高番茄幼苗抗旱性[J]. 生物技术通报, 2025, 41(2): 107-118. |
| [13] | 李艳伟, 杨妍妍, 孙亚玲, 霍雨猛, 王振宝, 刘冰江. 基于转录组分析植物激素对洋葱鳞茎膨大发育的调控机制[J]. 生物技术通报, 2025, 41(2): 187-201. |
| [14] | 钱政毅, 吴绍芳, 曹舒怡, 宋雅欣, 潘鑫峰, 李兆伟, 范凯. 睡莲NAC转录因子的鉴定及其表达分析[J]. 生物技术通报, 2025, 41(2): 234-247. |
| [15] | 黄颖, 遇文婧, 刘雪峰, 刁桂萍. 山新杨谷胱甘肽转移酶基因的生物信息学与表达模式分析[J]. 生物技术通报, 2025, 41(2): 248-256. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||