








生物技术通报 ›› 2023, Vol. 39 ›› Issue (4): 259-267.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1083
陈楠楠1,3( ), 王春来1,3, 蒋振忠1,3, 焦鹏1,3, 关淑艳2,3(
), 王春来1,3, 蒋振忠1,3, 焦鹏1,3, 关淑艳2,3( ), 马义勇2,3(
), 马义勇2,3( )
)
收稿日期:2022-09-01
出版日期:2023-04-26
发布日期:2023-05-16
通讯作者:
关淑艳,女,博士,教授,研究方向:作物遗传育种与基因工程;E-mail: guanshuyan@jlau.edu.cn;作者简介:陈楠楠,女,硕士研究生,研究方向:作物重要性状形成的分子机制;E-mail: 2356014208@qq.com
基金资助:
CHEN Nan-nan1,3( ), WANG Chun-lai1,3, JIANG Zhen-zhong1,3, JIAO Peng1,3, GUAN Shu-yan2,3(
), WANG Chun-lai1,3, JIANG Zhen-zhong1,3, JIAO Peng1,3, GUAN Shu-yan2,3( ), MA Yi-yong2,3(
), MA Yi-yong2,3( )
)
Received:2022-09-01
Published:2023-04-26
Online:2023-05-16
摘要:
玉米起源于亚热带,是喜温作物,易受低温胁迫的影响,脱水素(dehydrin,DHN)作为胚胎发育晚期丰富蛋白(late embryogenesis abundant protein, LEA)Ⅱ家族成员,是一类在植物非生物胁迫中发挥重要功能的蛋白。本研究克隆获得ZmDHN15基因,使用生物信息学手段,实时荧光定量PCR等技术对该基因的基本特性、组织表达特性进行分析,并进行植物过表达载体的构建及烟草的遗传转化,对T2代阳性植株进行抗冷性功能验证。结果表明,ZmDHN15基因全长1 442 bp,共编码290个氨基酸,分子量为31.44 kD,理论等电点为6.05,是亲水性非跨膜蛋白,具有脱水素家族特有保守结构域。RT-qPCR分析表明ZmDHN15基因的在玉米叶片中表达量较高且在冷胁迫条件下表达量增加;获得T2代转基因烟草植株9 株;在冷胁迫下转基因烟草与野生型相比,萌发率提高1.40 倍,根长提高1.58 倍,其叶片萎蔫程度更低,脯氨酸含量、丙二醛含量和过氧化物酶活性分别降低41.17%、28.47%和23.33%,可溶性糖含量、氧化物酶活性和超氧化物歧化酶活性分别升高58.97%、47.85%和47.53%,H2O2 和O2-积累量分别减少34.78%和47.00%。综上所述,过表达ZmDHN15 基因可以有效提高烟草植株对冷胁迫的耐受性,为进一步研究ZmDHN15基因在玉米中的功能奠定基础。
陈楠楠, 王春来, 蒋振忠, 焦鹏, 关淑艳, 马义勇. 玉米ZmDHN15基因在烟草中的遗传转化及抗冷性分析[J]. 生物技术通报, 2023, 39(4): 259-267.
CHEN Nan-nan, WANG Chun-lai, JIANG Zhen-zhong, JIAO Peng, GUAN Shu-yan, MA Yi-yong. Genetic Transformation and Chilling Resistance Analysis of Maize ZmDHN15 Gene in Tobacco[J]. Biotechnology Bulletin, 2023, 39(4): 259-267.
| 名称 Name | 引物序列 Primer sequence(5'-3') |
|---|---|
| ZmDHN15 for RT-qPCR-F | AAGCCAAAAGGCACTGAAGAAG |
| ZmDHN15 for RT-qPCR-R | ACAGAACAGATCAGCAGGCTAGCTA |
| Act-F | TTGAGGTAGGATGAGACT |
| Act-R | GGAGTGAAGCAGATGATT |
| ZmDHN15-F | AAGGCACTGAAGAAGCCAGTCA |
| ZmDHN15-R | GAAACCAAAGCAATTATTAACGCAT |
| ZmDHN15-3301-F | actcttgaccatggtagatctAAGGCACTGAAGAAG- CCAGTCA |
| ZmDHN15-3301-R | ggggaaattcgagctggtcaccGAAACCAAAGCAA- TTATTAACGCAT |
| Bar-F | TGACGCACAATCCCACTATCCT |
| Bar-R | GAAACCCACGTCATGCCAGT |
表1 引物序列
Table 1 Primer sequences
| 名称 Name | 引物序列 Primer sequence(5'-3') |
|---|---|
| ZmDHN15 for RT-qPCR-F | AAGCCAAAAGGCACTGAAGAAG |
| ZmDHN15 for RT-qPCR-R | ACAGAACAGATCAGCAGGCTAGCTA |
| Act-F | TTGAGGTAGGATGAGACT |
| Act-R | GGAGTGAAGCAGATGATT |
| ZmDHN15-F | AAGGCACTGAAGAAGCCAGTCA |
| ZmDHN15-R | GAAACCAAAGCAATTATTAACGCAT |
| ZmDHN15-3301-F | actcttgaccatggtagatctAAGGCACTGAAGAAG- CCAGTCA |
| ZmDHN15-3301-R | ggggaaattcgagctggtcaccGAAACCAAAGCAA- TTATTAACGCAT |
| Bar-F | TGACGCACAATCCCACTATCCT |
| Bar-R | GAAACCCACGTCATGCCAGT |
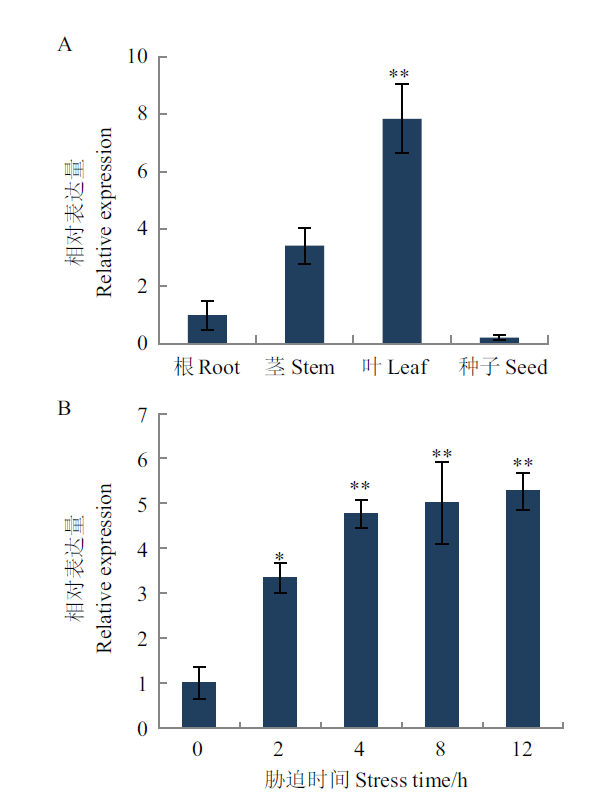
图2 ZmDHN15 基因表达模式分析 A:ZmDHN15基因在不同部位表达模式分析;B:ZmDHN15基因在叶片中冷处理条件下表达模式分析;星号(*)表示各组织差异显著,P < 0.05(*), P < 0.01(**)
Fig. 2 ZmDHN15 gene expression pattern analysis A: ZmDHN15 gene analysis of expression patterns in different parts. B: Analysis of the expression pattern of ZmDHN15 gene in leaves under intercooling treatment. * indicate significant differences among organizations, P < 0.05(*), P < 0.01(**)

图3 烟草的遗传转化 A:萌发;B:共培养;C:筛选;D分化;E:生根;F:移栽;G:开花;H:收种
Fig. 3 Genetic transformation of tobacco A: Germination; B: co-culture; C: screening; D: differentiation; E: rooting; F: transplant; G: flowering; H: seed collection
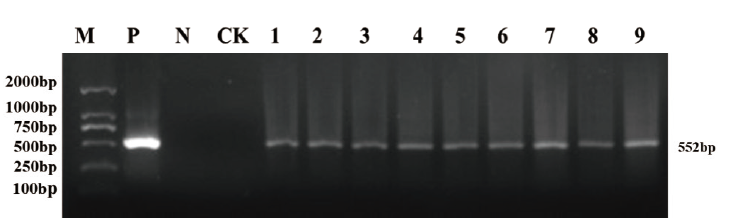
图4 T2代烟草的PCR验证 M:DNA marker DL 2000 ;P:质粒对照;N:阴性对照;CK:对照植株;1-9:阳性植株
Fig. 4 PCR validation of T2 generation tobacco M: DNA marker DL 2000; P: plasmid groups; N: negative control; CK: controlled plant ;1-9: positive plants
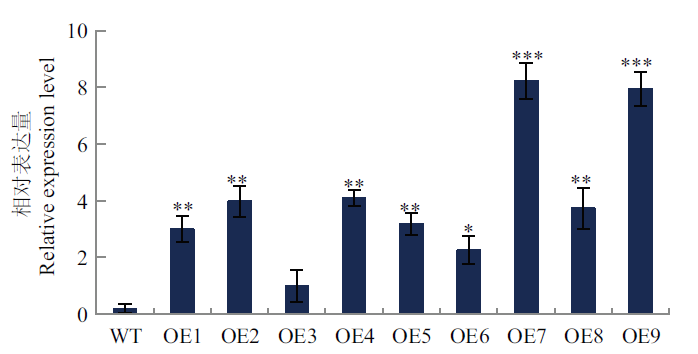
图5 T2代转基因烟草的RT-qPCR验证 WT:野生型,OE1-OE9:转基因植株;星号(*)表示与野生型差异显著,P < 0.05(*), P < 0.01(**), P < 0.001(***)。下同
Fig. 5 RT-qPCR validation of transgenic tobacco of T2 ge-neration WT: Wild type, OE1-OE9: transgenic plants. * indicate significant differences from wild-type, P < 0.05(*), P < 0.01(**), and P < 0.001(***). The same below
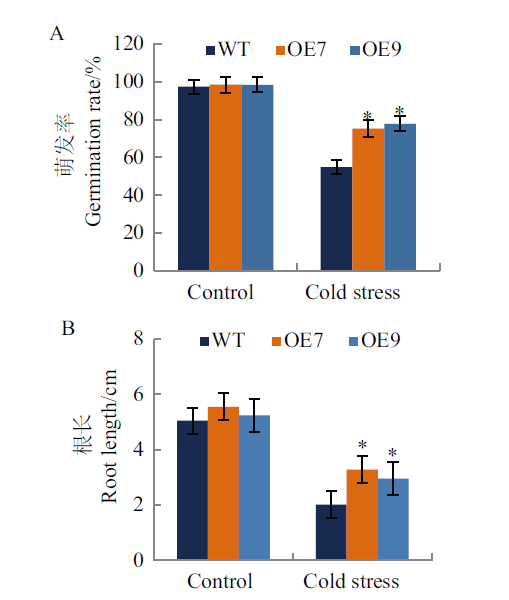
图6 冷胁迫处理下转基因烟草萌发率和根长的测定 A:萌发率统计;B:根长统计。Control:对照组;Cold stress:4℃低温胁迫。下同
Fig. 6 Determination of germination rate and root length of transgenic tobacco plants under cold stress tre-atment A: Germination rate statistics; B: root length statistics. Control: Control group. Cold stress: Chilling stress at 4℃.The same below
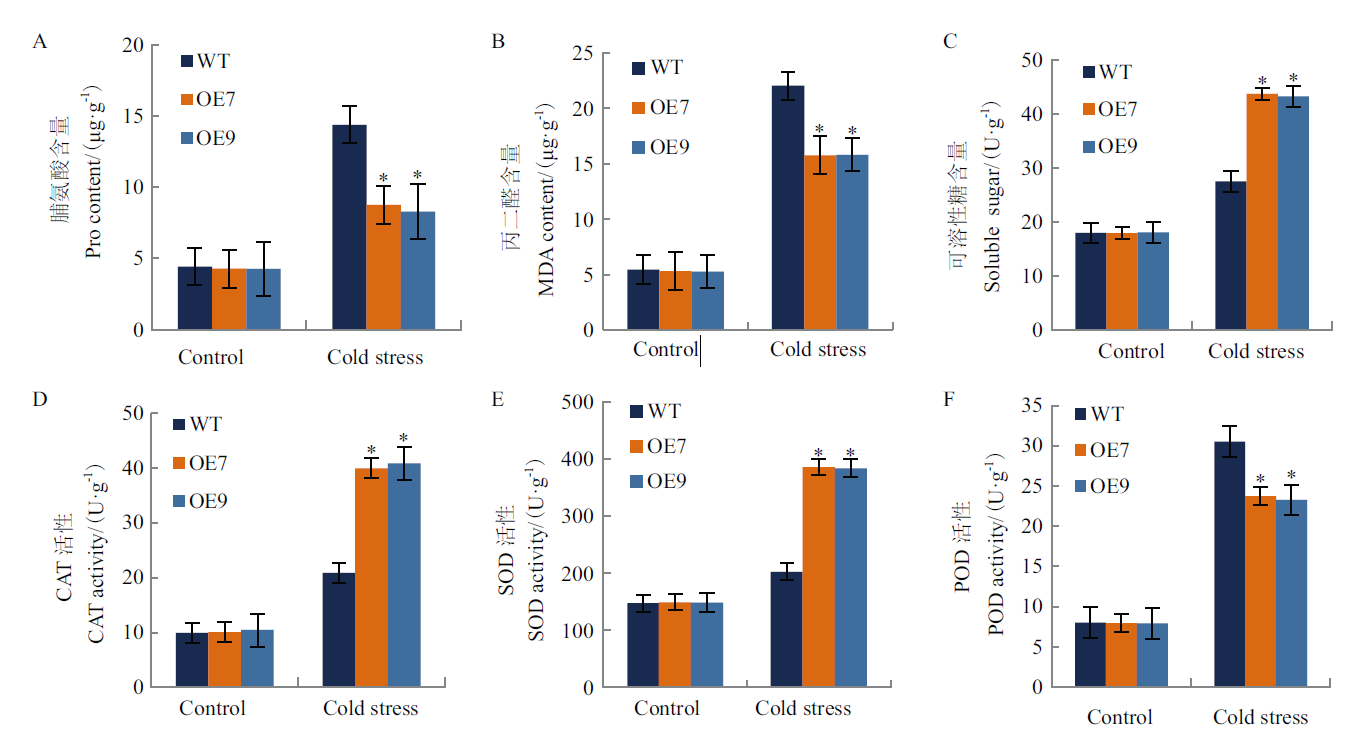
图8 生理生化指标测定 A-C:Pro、MDA、可溶性糖含量; D-F:CAT、SOD、POD酶活性
Fig. 8 Determination of physiological and biochemical indexes A-C: Pro, MDA and soluble sugar contents. D-F:CAT, SOD and POD enzyme activities
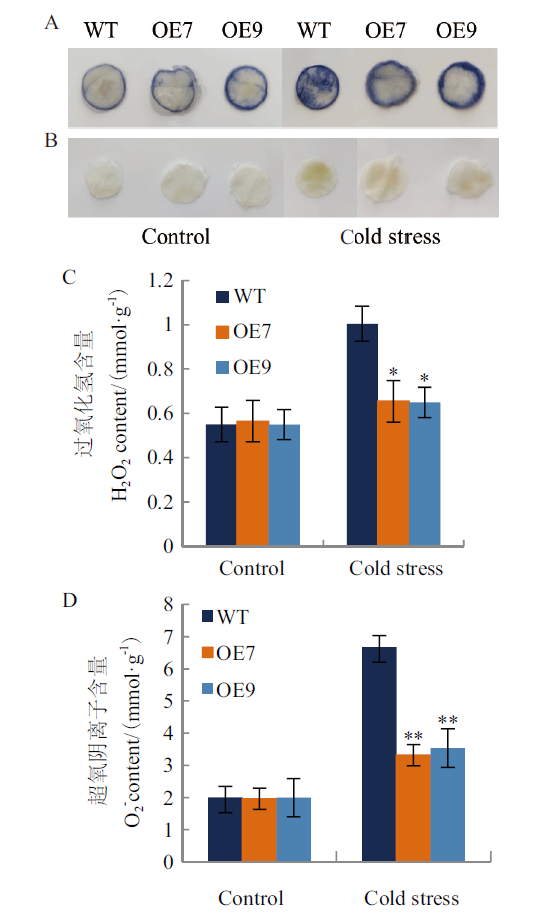
图9 在冷胁迫处理下转基因烟草与野生型烟草叶片H2O2和O2-的定性及定量分析 A:NBT染色;B:DAB染色;C:H2O2的含量; D:O2-的含量
Fig. 9 Qualitative and quantitative analysis of H2O2 and O2- in transgenic and wild-type tobacco leaves under cold stress A: NBT staining; B: DAB staining; C: H2O2 content; D: O2- content
| [1] |
Jiao P, Jiang ZZ, Wei XT, et al. Overexpression of the homeobox-leucine zipper protein ATHB-6 improves the drought tolerance of maize(Zea mays L.)[J]. Plant Sci, 2022, 316: 111159.
doi: 10.1016/j.plantsci.2021.111159 URL |
| [2] |
Zhang YC, Liu P, Wang C, et al. Genome-wide association study uncovers new genetic loci and candidate genes underlying seed chilling-germination in maize[J]. PeerJ, 2021, 9: e11707.
doi: 10.7717/peerj.11707 URL |
| [3] |
Jiang SQ, Zhang HB, Ni PZ, et al. Genome-wide association study dissects the genetic architecture of maize husk tightness[J]. Front Plant Sci, 2020, 11: 861.
doi: 10.3389/fpls.2020.00861 pmid: 32695127 |
| [4] |
Bilska-Kos A, Solecka D, Dziewulska A, et al. Low temperature caused modifications in the arrangement of cell wall pectins due to changes of osmotic potential of cells of maize leaves(Zea mays L.)[J]. Protoplasma, 2017, 254(2): 713-724.
doi: 10.1007/s00709-016-0982-y pmid: 27193139 |
| [5] |
Li Z, Xu JG, Gao Y, et al. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize(Zea mays L.) seed germination[J]. Front Plant Sci, 2017, 8: 1153.
doi: 10.3389/fpls.2017.01153 URL |
| [6] |
Li M, Lin L, Zhang YH, et al. ZmMYB31, a R2R3-MYB transcription factor in maize, positively regulates the expression of CBF genes and enhances resistance to chilling and oxidative stress[J]. Mol Biol Rep, 2019, 46(4): 3937-3944.
doi: 10.1007/s11033-019-04840-5 pmid: 31037550 |
| [7] |
Li XY, Li LJ, Zuo SY, et al. Differentially expressed ZmASR genes associated with chilling tolerance in maize(Zea mays)varieties[J]. Funct Plant Biol, 2018, 45(12): 1173-1180.
doi: 10.1071/FP17356 URL |
| [8] |
Ahmad S, Kamran M, Zhou XB, et al. Melatonin improves the seed filling rate and endogenous hormonal mechanism in grains of summer maize[J]. Physiol Plant, 2021, 172(2): 1059-1072.
doi: 10.1111/ppl.13282 pmid: 33206390 |
| [9] |
Jiao P, Jin SY, Chen NN, et al. Improvement of cold tolerance in maize(Zea mays L.) using Agrobacterium-mediated transformation of ZmSAMDC gene[J]. GM Crops Food, 2022, 13(1): 131-141.
doi: 10.1080/21645698.2022.2097831 URL |
| [10] |
Liu Y, Liang JN, Sun LP, et al. Group 3 LEA protein, ZmLEA3, is involved in protection from low temperature stress[J]. Front Plant Sci, 2016, 7: 1011.
doi: 10.3389/fpls.2016.01011 pmid: 27471509 |
| [11] |
Wang XC, Zhang M, Xie BH, et al. Functional characteristics analysis of dehydrins in Larix kaempferi under osmotic stress[J]. Int J Mol Sci, 2021, 22(4): 1715.
doi: 10.3390/ijms22041715 URL |
| [12] |
Yu YL, Li YJ, Jia FJ, et al. ZmFKBP20-1 improves the drought and salt tolerance of transformed Arabidopsis[J]. J Plant Biol, 2017, 60(6): 558-570.
doi: 10.1007/s12374-017-0262-1 URL |
| [13] |
Li QL, Zhang XC, Lv Q, et al. Physcomitrella patens dehydrins(PpDHNA and PpDHNC)confer salinity and drought tolerance to transgenic Arabidopsis plants[J]. Front Plant Sci, 2017, 8: 1316.
doi: 10.3389/fpls.2017.01316 URL |
| [14] | 潘潇潇, 胡慧芳, 陈楠, 等. 脱水素在植物非生物胁迫中的作用研究进展[J]. 农业生物技术学报, 2022, 30(3): 594-605. |
| Pan XX, Hu HF, Chen N, et al. Research progress on the role of dehydrin in plant abiotic stress[J]. J Agric Biotechnol, 2022, 30(3): 594-605. | |
| [15] |
Dong J, Cao L, Zhang XY, et al. An R2R3-MYB transcription factor RmMYB108 responds to chilling stress of Rosa multiflora and conferred cold tolerance of Arabidopsis[J]. Front Plant Sci, 2021, 12: 696919.
doi: 10.3389/fpls.2021.696919 URL |
| [16] |
Habib I, Shahzad K, Rauf M, et al. Dehydrin responsive HVA1 driven inducible gene expression enhanced salt and drought tolerance in wheat[J]. Plant Physiol Biochem, 2022, 180: 124-133.
doi: 10.1016/j.plaphy.2022.03.035 URL |
| [17] | Antonić DD, Subotić AR, Dragićević MB, et al. Effects of exogenous salicylic acid on drought response and characterization of dehydrins in Impatiens walleriana[J]. Plants(Basel), 2020, 9(11): 1589. |
| [18] |
Meng YC, Zhang HF, Pan XX, et al. CaDHN3, a pepper(Capsicum annuum L.) dehydrin gene enhances the tolerance against salt and drought stresses by reducing ROS accumulation[J]. Int J Mol Sci, 2021, 22(6): 3205.
doi: 10.3390/ijms22063205 URL |
| [19] |
张彤彤, 郑登俞, 吴忠义, 等. 玉米NF-Y转录因子基因ZmNF-YB13响应干旱和盐胁迫的功能分析[J]. 生物技术通报, 2022, 38(10):115-123.
doi: 10.13560/j.cnki.biotech.bull.1985.2022-0066 |
| Zhang TT, Zhen DY, Wu ZY, et al. Functional analysis of ZmNF-YB13 response to drought and salt stress[J]. Biotechnol Bull, 2022, 38(10):115-123. | |
| [20] |
张云川, 林熠轩, 曹新文, 等. 橡胶草TkDREB2基因的克隆以及在烟草中的抗旱功能分析[J]. 生物技术通报, 2021, 37(11): 212-224.
doi: 10.13560/j.cnki.biotech.bull.1985.2021-0108 |
| Zhang YC, Lin YX, Cao XW, et al. TkDREB2 clone from Taraxa-cum kok-saghyz and drought tolerance analysis of transgenic Nico-tiana tabacum[J]. Biotechnol Bull, 2021, 37(11): 212-224. | |
| [21] | 金时酉, 刘畅, 焦鹏, 等. 玉米抗冷相关基因ZmSAMDC克隆及生物信息学分析[J]. 吉林农业大学学报, 2021, 43(6): 651-656. |
| Jin SY, Liu C, Jiao P, et al. Cloning and bioinformatics analysis of ZmSAMDC gene related to maize cold resistance[J]. J Jilin Agric Univ, 2021, 43(6): 651-656. | |
| [22] |
刘桐羽, 刘梦彤, 周洋洋, 等. 玉米ZmTCP14基因的筛选、生物信息学分析及植物表达载体的构建[J]. 吉林农业大学学报, 2022. DOI: 10.13327/j.jjlau.2021.1657.
doi: 10.13327/j.jjlau.2021.1657 |
|
Liu TY, Liu MT, Zhou YY, et al. Screening, bioinformatics analysis and construction of plant expression vector of ZmTCP14 gene in maize[J]. J Jilin Agric Univ, 2022. DOI: 10.13327/j.jjlau.2021.1657.
doi: 10.13327/j.jjlau.2021.1657 |
|
| [23] |
Nkomo M, Gokul A, Ndimba R, et al. Piperonylic acid alters growth, mineral content accumulation and reactive oxygen species-scavenging capacity in chia seedlings[J]. AoB PLANTS, 2022, 14(3): plac025.
doi: 10.1093/aobpla/plac025 URL |
| [24] | Bao F, Du DL, An Y, et al. Overexpression of Prunus mume dehydrin genes in tobacco enhances tolerance to cold and drought[J]. Front Plant Sci, 2017, 8: 151. |
| [25] |
Falavigna V, Malabarba J, Silveira CP, et al. Characterization of the nucellus-specific dehydrin MdoDHN11 demonstrates its involvement in the tolerance to water deficit[J]. Plant Cell Rep, 2019, 38(9): 1099-1107.
doi: 10.1007/s00299-019-02428-8 pmid: 31127322 |
| [26] |
Ju HN, Li DX, Li DQ, et al. Overexpression of ZmDHN11 could enhance transgenic yeast and tobacco tolerance to osmotic stress[J]. Plant Cell Rep, 2021, 40(9): 1723-1733.
doi: 10.1007/s00299-021-02734-0 |
| [27] |
Zhang HF, Liu SY, Ma JH, et al. CaDHN4, a salt and cold stress-responsive dehydrin gene from pepper decreases abscisic acid sensitivity in Arabidopsis[J]. Int J Mol Sci, 2019, 21(1): 26.
doi: 10.3390/ijms21010026 URL |
| [28] | 董程, 杨景松, 刘紫嫣, 等. 红花非生物胁迫相关CtDHN1基因的克隆及功能研究[J]. 中国油料作物学报, 2020, 42(1): 85-90. |
| Dong C, Yang JS, Liu ZY, et al. Cloning and functional analysis of CtDHN1 gene related to abiotic stress in Carthamus tinctori-us[J]. Chin J Oil Crop Sci, 2020, 42(1): 85-90. | |
| [29] |
Wang HY, Li ZB, Ren HB, et al. Regulatory interaction of BcWRKY33A and BcHSFA4A promotes salt tolerance in non-heading Chinese cabbage[Brassica campestris(syn. Brassica rapa)ssp. chinensis[J]. Hortic Res, 2022, 9: uhac113.
doi: 10.1093/hr/uhac113 URL |
| [30] | Xiong J, Zhang WX, Zheng D, et al. ZmLBD5 increases drought sensitivity by suppressing ROS accumulation in Arabidopsis[J]. Plants(Basel), 2022, 11(10): 1382. |
| [31] |
Yu YL, Zhen SM, Wang S, et al. Comparative transcriptome analysis of wheat embryo and endosperm responses to ABA and H2O2 stresses during seed germination[J]. BMC Genomics, 2016, 17: 97.
doi: 10.1186/s12864-016-2416-9 URL |
| [1] | 王宝宝, 王海洋. 理想株型塑造之于玉米耐密改良[J]. 生物技术通报, 2023, 39(8): 11-30. |
| [2] | 张道磊, 甘雨军, 乐亮, 普莉. 玉米产量性状的表观遗传调控机制和育种应用[J]. 生物技术通报, 2023, 39(8): 31-42. |
| [3] | 冷燕, 马晓薇, 陈光, 任鹤, 李翔. 玉米高产竞赛助力中国玉米种业振兴[J]. 生物技术通报, 2023, 39(8): 4-10. |
| [4] | 王天依, 王荣焕, 王夏青, 张如养, 徐瑞斌, 焦炎炎, 孙轩, 王继东, 宋伟, 赵久然. 玉米矮秆基因与矮秆育种研究[J]. 生物技术通报, 2023, 39(8): 43-51. |
| [5] | 刘月娥, 徐田军, 蔡万涛, 吕天放, 张勇, 薛洪贺, 王荣焕, 赵久然. 我国玉米超高产研究现状与展望[J]. 生物技术通报, 2023, 39(8): 52-61. |
| [6] | 张勇, 徐田军, 吕天放, 邢锦丰, 刘宏伟, 蔡万涛, 刘月娥, 赵久然, 王荣焕. 种植密度对夏播玉米茎秆质量和根系表型性状的影响[J]. 生物技术通报, 2023, 39(8): 70-79. |
| [7] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [8] | 朱少喜, 金肇阳, 葛建镕, 王蕊, 王凤格, 路运才. 基于KASP平台的转基因玉米高通量特异性检测方法[J]. 生物技术通报, 2023, 39(6): 133-140. |
| [9] | 马玉倩, 孙东辉, 岳浩峰, 辛佳瑜, 刘宁, 曹志艳. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135. |
| [10] | 李圣彦, 李香银, 李鹏程, 张明俊, 张杰, 郎志宏. 转基因玉米2HVB5的性状鉴定及遗传稳定性分析[J]. 生物技术通报, 2023, 39(1): 21-30. |
| [11] | 李东阳, 肖冰, 王晨尧, 杨现明, 梁晋刚, 吴孔明. 转基因抗虫耐除草剂玉米瑞丰125 Cry1Ab/Cry2Aj杀虫蛋白的时空表达分析[J]. 生物技术通报, 2023, 39(1): 31-39. |
| [12] | 李鹏程, 张明俊, 王银晓, 李香银, 李圣彦, 郎志宏. 转基因玉米HGK60在不同遗传背景下抗虫性鉴定及农艺性状分析[J]. 生物技术通报, 2023, 39(1): 40-47. |
| [13] | 金云倩, 王彬, 郭书磊, 赵霖熙, 韩赞平. 赤霉素调控玉米种子活力的研究进展[J]. 生物技术通报, 2023, 39(1): 84-94. |
| [14] | 王松, 简晓平, 潘婉舒, 张永光, 王涛, 游玲. 玉米小曲酒糟发酵饲料对育肥猪肠道菌群的影响[J]. 生物技术通报, 2022, 38(9): 248-257. |
| [15] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||