








生物技术通报 ›› 2024, Vol. 40 ›› Issue (1): 207-221.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0583
收稿日期:2023-06-20
出版日期:2024-01-26
发布日期:2024-02-06
通讯作者:
岳洁瑜,女,博士,副研究员,研究方向:植物逆境生物学;E-mail: skyyjy@tjnu.edu.cn作者简介:焦进兰,女,硕士研究生,研究方向:植物逆境生物学;E-mail: 2330876687@qq.com
基金资助:
JIAO Jin-lan( ), WANG Wen-wen, JIE Xin-rui, WANG Hua-zhong, YUE Jie-yu(
), WANG Wen-wen, JIE Xin-rui, WANG Hua-zhong, YUE Jie-yu( )
)
Received:2023-06-20
Published:2024-01-26
Online:2024-02-06
摘要:
【目的】探究自噬参与调控外源钙维持盐胁迫下小麦幼苗稳态的机制,为进一步揭示植物盐胁迫应答机制提供分子证据。【方法】以小麦品种河农6425为材料,通过水培培养小麦幼苗,利用叶绿素荧光、组织原位染色、荧光定量PCR、激光共聚焦显微镜等生理及分子生物学技术开展研究。【结果】150 mmol/L NaCl胁迫显著抑制小麦幼苗生长,导致光系统II(photosystem II, PSII)受损,抗氧化能力减弱,细胞活力降低,自噬活性升高。随着NaCl处理时间的延长,小麦幼苗根和叶片中的自噬小体显著增多,细胞程序性死亡(programmed cell death, PCD)水平升高。正常条件下,外源施加Ca2+对小麦幼苗根和叶片中自噬小体数量没有明显影响。而在NaCl胁迫下,外源Ca2+处理能够在一定时间内维持NaCl胁迫诱导的自噬维持在促存活的水平,减少PCD。【结论】Ca2+通过调控小麦幼苗根和叶片的自噬水平限制PCD的规模,缓解NaCl胁迫对小麦幼苗的损伤。
焦进兰, 王文文, 介欣芮, 王华忠, 岳洁瑜. 外源钙缓解小麦幼苗盐胁迫的作用机制[J]. 生物技术通报, 2024, 40(1): 207-221.
JIAO Jin-lan, WANG Wen-wen, JIE Xin-rui, WANG Hua-zhong, YUE Jie-yu. Mechanism of Exogenous Calcium Alleviating Salt Stress Toxicity in Wheat Seedlings[J]. Biotechnology Bulletin, 2024, 40(1): 207-221.
| 成分Content | 浓度Concentration/(mg·L-1) |
|---|---|
| Ca(NO3)2·4H2O | 165 |
| KNO3 | 51 |
| NH4NO3 | 8 |
| KH2PO4 | 27 |
| MgSO4 | 49 |
| 微量元素 | 1 |
| 铁盐溶液 | 1 |
表1 营养液配置表
Table 1 Nutrient solution formula
| 成分Content | 浓度Concentration/(mg·L-1) |
|---|---|
| Ca(NO3)2·4H2O | 165 |
| KNO3 | 51 |
| NH4NO3 | 8 |
| KH2PO4 | 27 |
| MgSO4 | 49 |
| 微量元素 | 1 |
| 铁盐溶液 | 1 |
| 基因Gene | 引物序列Primer's sequence(5'-3') |
|---|---|
| TaCDPK27 | F: GCCGCCTTCCAATACTTT R: TTATCCTGATCTACTTCGCCTA |
| TaATG2 | F:TGTATCCAGATGGGGGTGTT R:GGAACTTAAGCTGCCCTTGA |
| TaATG5 | F: CCAGAAAGGCCATGGAATCTAAC R: GCCTCTTTCAGGGAATTGTTGTA |
| TaATG7 | F: TGACGTTATCGCTCCTGTTG R: ACAGCTGCTCGAGGAATAGC |
| TaATG10 | F: TATTACTCGAGAGGAGCATCCCCAC R: GATTTTCAATCCTACTGCCTGACCG |
| TaATG8 | F: GGAAAGGAGGCAAGCTGAA R: GCATCTCGTTAGGGACAAGGTA |
| Tubulin | F: GTGGAACTGGCTCTGGC R: CGCTCAATGTCAAGGGA |
表2 RT-qPCR引物
Table 2 Primers used for RT-qPCR
| 基因Gene | 引物序列Primer's sequence(5'-3') |
|---|---|
| TaCDPK27 | F: GCCGCCTTCCAATACTTT R: TTATCCTGATCTACTTCGCCTA |
| TaATG2 | F:TGTATCCAGATGGGGGTGTT R:GGAACTTAAGCTGCCCTTGA |
| TaATG5 | F: CCAGAAAGGCCATGGAATCTAAC R: GCCTCTTTCAGGGAATTGTTGTA |
| TaATG7 | F: TGACGTTATCGCTCCTGTTG R: ACAGCTGCTCGAGGAATAGC |
| TaATG10 | F: TATTACTCGAGAGGAGCATCCCCAC R: GATTTTCAATCCTACTGCCTGACCG |
| TaATG8 | F: GGAAAGGAGGCAAGCTGAA R: GCATCTCGTTAGGGACAAGGTA |
| Tubulin | F: GTGGAACTGGCTCTGGC R: CGCTCAATGTCAAGGGA |

图1 不同浓度CaCl2对NaCl胁迫下小麦幼苗生长影响 A:不同浓度CaCl2对小麦幼苗生长的影响;B:不同浓度CaCl2对NaCl胁迫下小麦幼苗生长影响;C:NaCl胁迫下不同浓度CaCl2处理后小麦幼苗根长测量值; D:NaCl胁迫下不同浓度CaCl2处理后小麦幼苗叶宽测量值。不同字母表示各处理组数据差异显著(n=3,P<0.05)。CK表示对照;Ca表示外源添加CaCl2处理;Na表示外源添加NaCl处理。下同
Fig. 1 Effects of different concentrations of CaCl2 on wheat seedling growth under NaCl stress A: Effects of different concentrations of CaCl2 on wheat seedling growth. B: Effects of different concentrations of CaCl2 on wheat seedling growth under NaCl stress. C: Root length of wheat seedlings treated with different concentrations of CaCl2 under NaCl stress. D: Leaf width of wheat seedlings treated with different concentrations of CaCl2 under NaCl stress. Different letters indicate significant difference in data of each treatment group(n=3, P<0.05). CK in the figure indicates the control check. Ca indicates the treatment with exogenous addition of CaCl2. Na indicates the treatment with exogenous addition of NaCl. The same below

图2 NaCl胁迫对小麦幼苗叶片生长机理影响 A:DAB染色检测小麦幼苗叶片H2O2积累情况;B:NBT染色检测小麦幼苗叶片O2·-积累量;C:伊文思蓝染色检测小麦幼苗叶片细胞活力。Bar=1 000 µm
Fig. 2 Effects of NaCl stress on the growth mechanism of wheat seedling leaves A: DAB staining was used to detect H2O2 accumulation in wheat seedling leaves. B: NBT staining was used to detect O2·- accumulation in wheat seedling leaves. C: Evans Blue staining was used to detect cell viability in wheat seedling leaves. Bar=1 000 µm
| 不同处理组 Different treatment group | 根长Root length | 株高Plant height | 叶长Leaf Length | 叶宽Leaf width |
|---|---|---|---|---|
| CK | 31.17±1.144a | 27.07±0.249a | 14.68±0.130a | 0.40±0.012a |
| Ca | 31.83±0.450a | 27.33±0.170a | 14.80±0.294a | 0.41±0.005a |
| La | 29.83±0.464b | 25.60±0.616b | 14.27±0.125b | 0.38±0.008b |
| Na | 21.70±0.497d | 21.73±0.403d | 13.47±0.047d | 0.33±0.014d |
| Ca+Na | 23.43±0.205c | 23.60±0.216c | 13.90±0.082c | 0.35±0.005c |
| La+Na | 18.20±0.589e | 19.60±1.393e | 13.01±0.084e | 0.31±0.005e |
表3 外源Ca2+ 对NaCl胁迫下小麦幼苗根长、株高、叶长、叶宽的影响
Table 3 Effects of exogenous Ca2+ on the root length, plant height, leaf length and leaf width of wheat seedlings under NaCl stress
| 不同处理组 Different treatment group | 根长Root length | 株高Plant height | 叶长Leaf Length | 叶宽Leaf width |
|---|---|---|---|---|
| CK | 31.17±1.144a | 27.07±0.249a | 14.68±0.130a | 0.40±0.012a |
| Ca | 31.83±0.450a | 27.33±0.170a | 14.80±0.294a | 0.41±0.005a |
| La | 29.83±0.464b | 25.60±0.616b | 14.27±0.125b | 0.38±0.008b |
| Na | 21.70±0.497d | 21.73±0.403d | 13.47±0.047d | 0.33±0.014d |
| Ca+Na | 23.43±0.205c | 23.60±0.216c | 13.90±0.082c | 0.35±0.005c |
| La+Na | 18.20±0.589e | 19.60±1.393e | 13.01±0.084e | 0.31±0.005e |

图4 外源Ca2+对NaCl胁迫下小麦幼苗根和叶片中TaCDPK27表达情况的影响
Fig. 4 Effects of exogenous Ca2+ on TaCDPK27 expression in the roots and leaves of wheat seedlings under NaCl stress
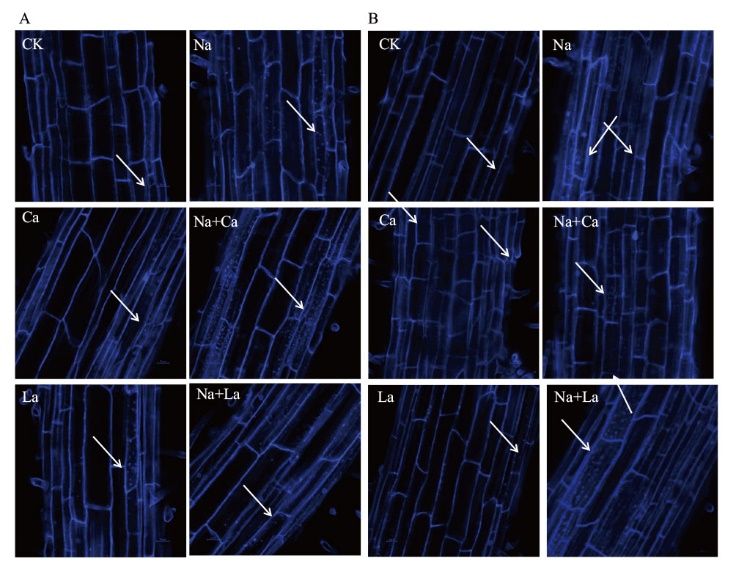
图6 MDC染色检测外源Ca2+ 对NaCl胁迫下小麦幼苗根自噬活性影响(标尺=100 µm) A:MDC染色检测外源Ca2+ 对NaCl胁迫6 h小麦幼苗根自噬活性影响; B:MDC染色检测外源Ca2+ 对NaCl胁迫144 h小麦幼苗根自噬活性影响
Fig. 6 Effects of exogenous Ca2+ on the autophagy activities of wheat seedling roots under NaCl stress by MDC staining(Bar=100 µm) A: MDC staining was used to detect the effects of exogenous Ca2+ on autophagy activity in the roots of wheat seedlings under NaCl stress for 6 h. B: MDC staining was used to detect the effects of exogenous Ca2+ on the autophagy activity in the roots of 144 h wheat seedlings under NaCl stress
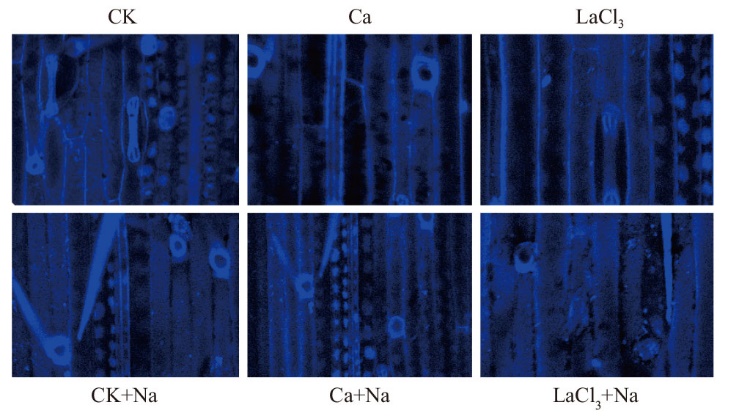
图7 MDC染色检测外源Ca2+ 对NaCl胁迫144 h小麦幼苗叶片自噬活性的影响(标尺=100 µm)
Fig. 7 Effects of exogenous Ca2+ on the autophagy activities of wheat seedling leaves under NaCl stress at 144 h by MDC staining(Bar=100 µm)

图8 RT-qPCR检测外源Ca2+对NaCl胁迫下小麦幼苗根和叶片中TaATGs表达的影响 A:外源Ca2+对NaCl胁迫下小麦幼苗根中TaATGs表达的影响;B:外源Ca2+对NaCl胁迫下小麦幼苗叶片TaATGs表达的影响
Fig. 8 Effects of exogenous Ca2+ on TaATGs expression in the roots and leaves of wheat seedlings under NaCl stress by RT-qPCR A: Effects of exogenous Ca2+ on TaATGs expression in wheat seedling roots under NaCl stress. B: Effects of exogenous Ca2+ on TaATGs expression in wheat seedling leaves under NaCl stress
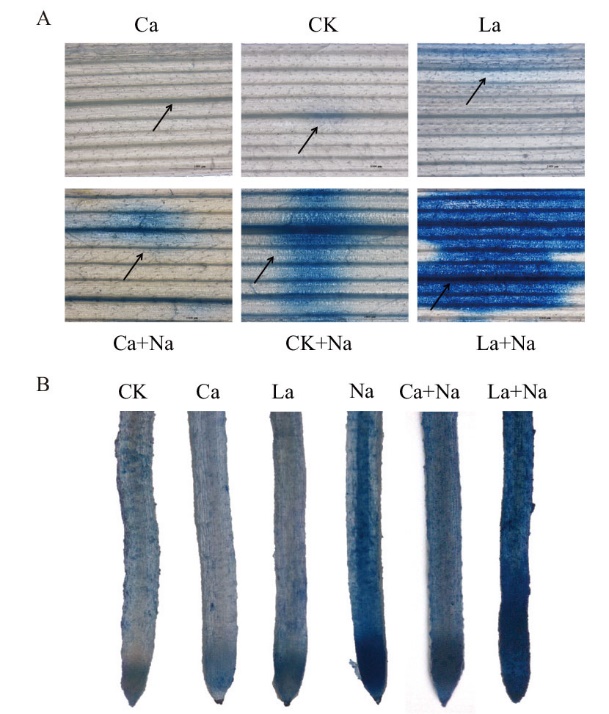
图9 伊文思蓝染色检测外源Ca2+对NaCl胁迫下小麦幼苗根和叶片细胞活力的影响(标尺=1 000 µm) A:外源Ca2+对NaCl胁迫下小麦幼苗叶片细胞活力的影响;B:外源Ca2+对NaCl胁迫下小麦幼苗根细胞活力的影响。图中箭头表示细胞死亡区域
Fig. 9 Effects of exogenous Ca2+ on cell viability in the roots and leaves of wheat seedlings under NaCl stress by Evans Blue staining(Bar=1 000 µm) A: Effects of exogenous Ca2+ on the cell viability of wheat seedling leaves under NaCl stress. B: Effects of exogenous Ca2+ on the cell viability of wheat seedling roots under NaCl stress. The arrows in the figures indicatethe area of cell death
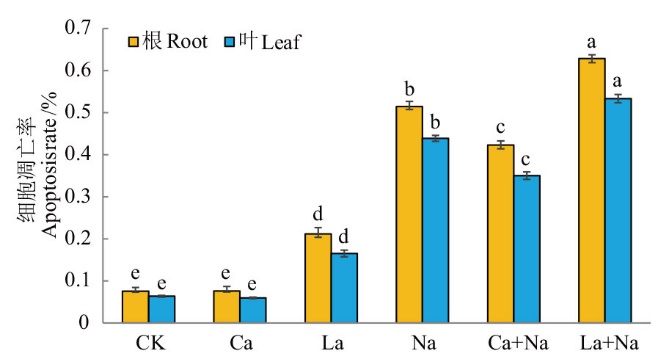
图10 TUNEL染色检测外源Ca2+ 对NaCl胁迫下小麦幼苗根和叶片PCD的影响
Fig. 10 Effects of exogenous Ca2+ on PCD in the roots and leaves of wheat seedlings under NaCl stress by TUNEL staining

图11 DAB与NBT染色检测外源Ca2+对NaCl胁迫下小麦幼苗H2O2与O2·-累积量的影响(标尺=1 000 µm) A和B分别为DAB染色检测小麦幼苗叶片和根中H2O2分布;C和D分别为NBT染色检测小麦幼苗叶片和根中O2·-分布
Fig. 11 Effects of exogenous Ca2+ on H2O2 and O2·- accumulation in wheat seedlings under NaCl stress by DAB and NBT staining(Bar=1 000 µm) A and B showed the distribution of H2O2 in the leaves and roots of wheat seedlings detected by DAB staining. C and D showed the distribution of O2·- in the leaves and roots of wheat seedlings detected by NBT staining
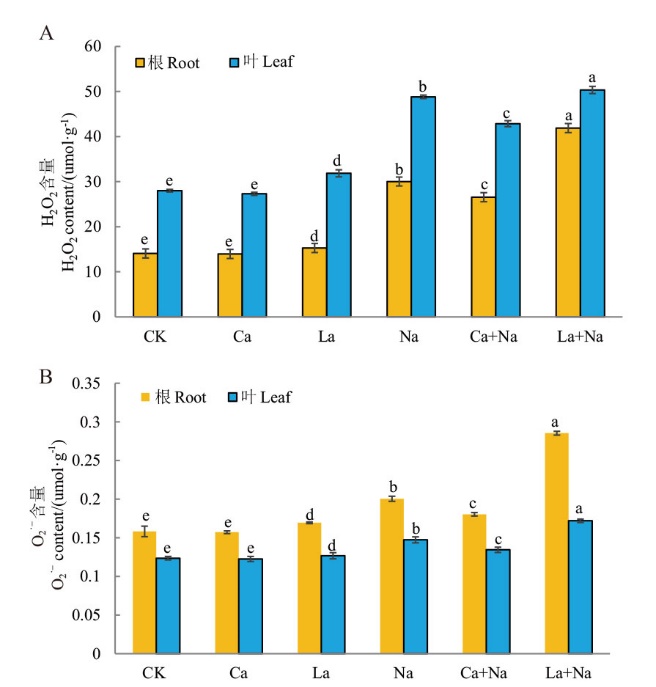
图12 外源Ca2+对NaCl胁迫下小麦幼苗根和叶片H2O2与O2·-含量的影响 A:小麦幼苗根与叶片中H2O2含量;B:小麦幼苗根与叶片中O2·-含量
Fig. 12 Effects of exogenous Ca2+ on H2O2 and O2·- contents in the roots and leaves of wheat seedlings under NaCl stress A: The contents of H2O2 in the roots and leaves of wheat seedlings. B: The contents of O2·- in the roots and leaves of wheat seedlings
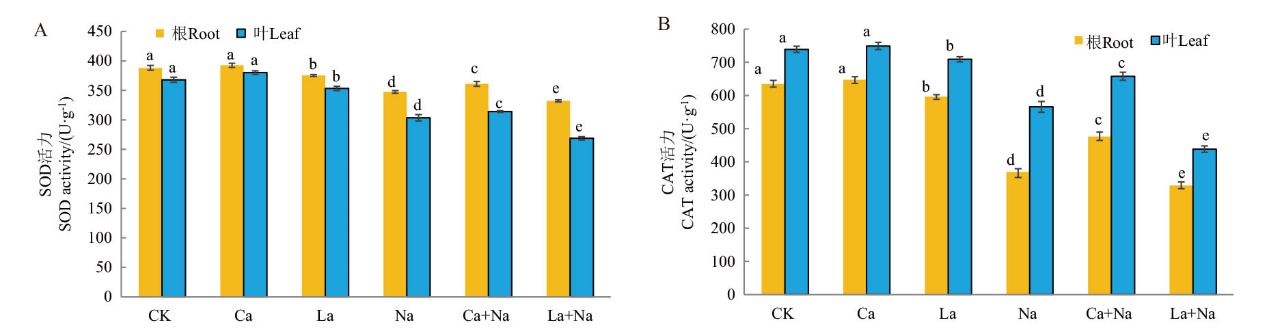
图14 外源Ca2+ 对NaCl胁迫下小麦幼苗SOD、CAT活性的影响 A:外源Ca2+对NaCl胁迫下小麦幼苗根与叶片中SOD含量的影响;B:外源Ca2+对NaCl胁迫下小麦幼苗根与叶片中CAT含量的影响
Fig. 14 Effects of exogenous Ca2+ on the SOD and CAT activities of wheat seedlings under NaCl stress A: The effects of exogenous Ca2+ on SOD content in the roots and leaves of wheat seedlings under NaCl stress. B: The effects of exogenous Ca2+ on CAT content in the roots and leaves of wheat seedlings under NaCl stress
| 处理天数 Treatment days/d | 处理组 Treatment group | Fv/Fm | Y(Ⅱ) | qP | F0 | NPQ | ETR |
|---|---|---|---|---|---|---|---|
| 5 | CK | 0.796±0.001a | 0.393±0.011a | 0.621±0.044a | 0.046±0.001a | 1.227±0.039e | 59.2±3.767a |
| Ca | 0.797±0.004a | 0.392±0.004a | 0.624±0.007a | 0.046±0.002a | 1.229±0.107e | 58.6±2.546a | |
| La | 0.792±0.004b | 0.386±0.048b | 0.608±0.054b | 0.044±0.003b | 1.363±0.151d | 55.9±1.543b | |
| Na | 0.787±0.001d | 0.281±0.007d | 0.474±0.008d | 0.038±0.003d | 1.731±0.099b | 40.2±0.834d | |
| Ca+Na | 0.789±0.007c | 0.309±0.037c | 0.497±0.045c | 0.041±0.001c | 1.566±0.036c | 43.2±3.982c | |
| La+Na | 0.784±0.003e | 0.254±0.036e | 0.383±0.019e | 0.035±0.002e | 1.782±0.037a | 35.2±4.626e | |
| 10 | CK | 0.794±0.001a | 0.379±0.007a | 0.628±0.002a | 0.055±0.003a | 1.341±0.129e | 53.3±1.296a |
| Ca | 0.792±0.001a | 0.383±0.025a | 0.633±0.016a | 0.053±0.007a | 1.339±0.024e | 54.6±1.964a | |
| La | 0.779±0.001b | 0.342±0.025b | 0.55±0.011b | 0.047±0.003b | 1.404±0.099d | 46.9±1.438b | |
| Na | 0.778±0.002d | 0.265±0.024d | 0.459±0.033d | 0.035±0.002d | 1.595±0.117b | 36.8±3.432d | |
| Ca+Na | 0.782±0.002c | 0.321±0.033c | 0.546±0.045c | 0.039±0.002c | 1.541±0.043c | 44.3±4.613c | |
| La+Na | 0.765±0.003e | 0.238±0.017e | 0.454±0.035e | 0.033±0.003e | 2.056±0.125a | 32.9±2.286e |
表4 外源Ca2+对NaCl胁迫5 d和10 d小麦幼苗叶绿素荧光参数的影响
Table 4 Effects of exogenous Ca2+ on the chlorophyll fluorescence parameters of wheat seedlings after 5 d and 10 d of NaCl stress
| 处理天数 Treatment days/d | 处理组 Treatment group | Fv/Fm | Y(Ⅱ) | qP | F0 | NPQ | ETR |
|---|---|---|---|---|---|---|---|
| 5 | CK | 0.796±0.001a | 0.393±0.011a | 0.621±0.044a | 0.046±0.001a | 1.227±0.039e | 59.2±3.767a |
| Ca | 0.797±0.004a | 0.392±0.004a | 0.624±0.007a | 0.046±0.002a | 1.229±0.107e | 58.6±2.546a | |
| La | 0.792±0.004b | 0.386±0.048b | 0.608±0.054b | 0.044±0.003b | 1.363±0.151d | 55.9±1.543b | |
| Na | 0.787±0.001d | 0.281±0.007d | 0.474±0.008d | 0.038±0.003d | 1.731±0.099b | 40.2±0.834d | |
| Ca+Na | 0.789±0.007c | 0.309±0.037c | 0.497±0.045c | 0.041±0.001c | 1.566±0.036c | 43.2±3.982c | |
| La+Na | 0.784±0.003e | 0.254±0.036e | 0.383±0.019e | 0.035±0.002e | 1.782±0.037a | 35.2±4.626e | |
| 10 | CK | 0.794±0.001a | 0.379±0.007a | 0.628±0.002a | 0.055±0.003a | 1.341±0.129e | 53.3±1.296a |
| Ca | 0.792±0.001a | 0.383±0.025a | 0.633±0.016a | 0.053±0.007a | 1.339±0.024e | 54.6±1.964a | |
| La | 0.779±0.001b | 0.342±0.025b | 0.55±0.011b | 0.047±0.003b | 1.404±0.099d | 46.9±1.438b | |
| Na | 0.778±0.002d | 0.265±0.024d | 0.459±0.033d | 0.035±0.002d | 1.595±0.117b | 36.8±3.432d | |
| Ca+Na | 0.782±0.002c | 0.321±0.033c | 0.546±0.045c | 0.039±0.002c | 1.541±0.043c | 44.3±4.613c | |
| La+Na | 0.765±0.003e | 0.238±0.017e | 0.454±0.035e | 0.033±0.003e | 2.056±0.125a | 32.9±2.286e |
| 处理组Treatment group | 5 d | 10 d |
|---|---|---|
| CK | 0.785±0.021a | 0.966±0.027a |
| Ca | 0.793±0.019a | 0.985±0.061a |
| La | 0.686±0.009b | 0.872±0.048b |
| Na | 0.531±0.037d | 0.640±0.012d |
| Ca+Na | 0.620±0.008c | 0.746±0.032c |
| La+Na | 0.336±0.048e | 0.538±0.014e |
表5 外源Ca2+对NaCl胁迫下小麦幼苗叶绿素含量的影响
Table 5 Effects of exogenous Ca2+ on the chlorophyll contents of wheat seedlings under NaCl stress
| 处理组Treatment group | 5 d | 10 d |
|---|---|---|
| CK | 0.785±0.021a | 0.966±0.027a |
| Ca | 0.793±0.019a | 0.985±0.061a |
| La | 0.686±0.009b | 0.872±0.048b |
| Na | 0.531±0.037d | 0.640±0.012d |
| Ca+Na | 0.620±0.008c | 0.746±0.032c |
| La+Na | 0.336±0.048e | 0.538±0.014e |
| [1] | 豆昕桐, 王英杰, 王华忠, 等. 耐盐和盐敏感型小麦品种对NaCl胁迫的生理响应及耐盐性差异[J]. 生态学报, 2021, 41(12): 4976-4992. |
| Dou XT, Wang YJ, Wang HZ, et al. Physiological response and tolerance difference of two wheat varieties to NaCl stress[J]. Acta Ecol Sin, 2021, 41(12): 4976-4992. | |
| [2] |
孔德真, 段震宇, 王刚, 等. 盐、碱胁迫下高丹草苗期生理特征及转录组学分析[J]. 生物技术通报, 2023, 39(6): 199-207.
doi: 10.13560/j.cnki.biotech.bull.1985.2022-0758 |
| Kong DZ, Duan ZY, Wang G, et al. Physiological characteristics and transcriptome analysis of Sorghum bicolor×S. Sudanense seedlings under salt-alkali stress[J]. Biotechnol Bull, 2023, 39(6): 199-207. | |
| [3] |
Lamers J, van der Meer T, Testerink C. How plants sense and respond to stressful environments[J]. Plant Physiol, 2020, 182(4): 1624-1635.
doi: 10.1104/pp.19.01464 pmid: 32132112 |
| [4] |
Van Zelm E, Zhang YX, Testerink C. Salt tolerance mechanisms of plants[J]. Annu Rev Plant Biol, 2020, 71: 403-433.
doi: 10.1146/annurev-arplant-050718-100005 pmid: 32167791 |
| [5] |
Zheng QW, Li GG, Wang HY, et al. The relationship between ethylene-induced autophagy and reactive oxygen species in Arabidopsis root cells during the early stages of waterlogging stress[J]. PeerJ. 2023, 11: e15404.
doi: 10.7717/peerj.15404 URL |
| [6] |
Yue JY, Wang YJ, Jiao JL, et al. Silencing of ATG2 and ATG7 promotes programmed cell death in wheat via inhibition of autophagy under salt stress[J]. Ecotoxicol Environ Saf, 2021, 225: 112761.
doi: 10.1016/j.ecoenv.2021.112761 URL |
| [7] |
Jiang L, Zheng X, Liu Y, et al. Plant protein P3IP participates in the regulation of autophagy in Nicotiana benthamiana[J]. Plant Signal Behav, 2021, 16(3): 1861768.
doi: 10.1080/15592324.2020.1861768 URL |
| [8] | Li YB, Cui DZ, Sui XX, et al. Autophagic survival precedes programmed cell death in wheat seedlings exposed to drought stress[J]. Int J Mol Sci, 2019, 20(22): E5777. |
| [9] |
Jeon HS, Jang E, Kim J, et al. Pathogen-induced autophagy regulates monolignol transport and lignin formation in plant immunity[J]. Autophagy, 2023, 19(2): 597-615.
doi: 10.1080/15548627.2022.2085496 URL |
| [10] |
Wang P, Nolan TM, Yin YH, et al. Identification of transcription factors that regulate ATG8 expression and autophagy in Arabidopsis[J]. Autophagy, 2020, 16(1): 123-139.
doi: 10.1080/15548627.2019.1598753 URL |
| [11] |
Luo LM, Zhang PP, Zhu RH, et al. Autophagy is rapidly induced by salt stress and is required for salt tolerance in Arabidopsis[J]. Front Plant Sci, 2017, 8: 1459.
doi: 10.3389/fpls.2017.01459 URL |
| [12] |
Mockevičiūtė R, Jurkonienė S, Šveikauskas V, et al. Probiotics, roline and calcium induced protective responses of Triticum aestivum under drought stress[J]. Plants, 2023, 12(6): 1301.
doi: 10.3390/plants12061301 URL |
| [13] | Farré JC, Subramani S. Mechanistic insights into selective autophagy pathways: lessons from yeast[J]. Nat Rev Mol cell biol, 2016, 17(9): 537-552. |
| [14] |
Kou LP, Yang TB, Luo YG, et al. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens[J]. Postharvest Bio Technol, 2014, 87(1): 70-78.
doi: 10.1016/j.postharvbio.2013.08.004 URL |
| [15] | 刘云芬, 彭华, 王薇薇, 等. 植物耐盐性生理与分子机制研究进展[J]. 江苏农业科学, 2019, 47(12): 30-36. |
| Liu YF, Peng H, Wang WW, et al. Advances in progress on physiological and molecular mechanisms of salt tolerance in plants[J]. Jiangsu Agric Sci, 47(12): 30-36. | |
| [16] | 赵瑞, 陈少良. 杨树耐盐性调控的离子平衡与活性氧平衡信号网络[J]. 中国科学: 生命科学, 2020, 50(2): 167-175. |
|
Zhao R, Chen SL. The salt-stress signaling network involved in the regulation of ionic and ROS homeostasis in poplar[J]. Sci Sin Vitae, 2020, 50(2): 167-175. (in Chinese)
doi: 10.1360/SSV-2019-0187 URL |
|
| [17] | 李青松, 王林权, 周春菊, 等. 钙离子及钙调蛋白对不同温度型冬小麦盐分吸收与累积的影响[J]. 西北植物学报, 2009, 29(5): 975-982. |
| Li QS, Wang LQ, Zhou CJ, et al. Function of calcium ion and calmodulin on sodium uptake and accumulation of winter wheat with different canopy temperature[J]. Acta Bot Boreali-Occidentalia Sin, 2009, 29(5): 975-982. | |
| [18] |
Steinhorst L, He GF, Moore LK, et al. A Ca2+-sensor switch for tolerance to elevated salt stress in Arabidopsis[J]. Dev Cell, 2022, 57(17): 2081-2094. e7.
doi: 10.1016/j.devcel.2022.08.001 pmid: 36007523 |
| [19] | 周子馨, 兰海燕. 植物CDPK信号转导途径及其互作组分的功能研究进展[J]. 新疆大学学报: 自然科学版, 2021(6): 705-714. |
| Zhou ZX, Lan HY. CDPK signaling pathway and its interaction components in plants[J]. J Xinjiang Univ Nat Sci Ed Chin Engl, 2021(6): 705-714. | |
| [20] |
Asano T, Hayashi N, Kobayashi M, et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance[J]. Plant J, 2012, 69(1): 26-36.
doi: 10.1111/tpj.2011.69.issue-1 URL |
| [21] | Dubrovina AS, Kiselev KV, Khristenko VS, et al. VaCPK20, a calcium-dependent protein kinase gene of wild grapevine vitis amurensis rupr., mediates cold and drought stress tolerance[J]. J Plant Physiol, 2015, 185: 1-12. |
| [22] |
Medina DL, Di Paola S, Peluso I, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB[J]. Nat Cell Biol, 2015, 17(3): 288-299.
pmid: 25720963 |
| [23] | 赵楠, 肖雪, 曹兰秀. 内质网应激在细胞自噬调控中的作用及其分子机制[J]. 生理科学进展, 2023, 54(5):426-432. |
| Zhao N, Xiao X, Cao LX. The role and molecular mechanisms of endoplasmic reticulum stress in autophagy regulation[J]. Progress in Physiological Sciences, 2023, 54(5):426-432. | |
| [24] |
Sun X, Pan B, Wang Y, et al. Exogenous calcium improved resistance to Botryosphaeria dothidea by increasing autophagy activity and salicylic acid level in pear[J]. Mol plant microbe interact, 2020, 33(9): 1150-1160.
doi: 10.1094/MPMI-04-20-0101-R URL |
| [25] | 张丽霞, 郭晓彦, 史鹏飞, 等. 旺长期水分胁迫对红麻叶片中叶绿素和胡萝卜素含量的影响[J]. 中国麻业科学, 2021, 43(2): 80-87. |
| Zhang LX, Guo XY, Shi PF, et al. Effect of water stress on the chlorophyll content and carotenoid content of kenaf leaves during vigorous growing stage[J]. Plant Fiber Sci China, 2021, 43(2): 80-87. | |
| [26] | Khalid AR, Lv X, Naeem M, et al. Autophagy related gene(ATG3)is a key regulator for cell growth, development, and virulence of Fusarium oxysporum[J]. Genes: Basel, 2019, 10(9): E658. |
| [27] | 肖畅, 彭婷. 植物应答非生物胁迫的信号转导途径研究进展[J]. 陕西农业科学, 2020, 66(2): 72-81. |
| Xiao C, Peng T. Research progress on signal transduction pathway of plants in physical response to abiotic stress[J]. Shaanxi J Agric Sci, 2020, 66(2): 72-81. | |
| [28] | 郭凤丹, 管仁伟, 赵秋晨, 等. 药用植物盐胁迫响应机理研究进展[J]. 山东农业科学, 2022, 54(9): 148-157. |
| Guo FD, Guan RW, Zhao QC, et al. Research progress on salt stress response mechanism of medicinal plants[J]. Shandong Agric Sci, 2022, 54(9): 148-157. | |
| [29] | 吴华鑫, 赵野, 王浩宇, 等. 外源Ca2+对西伯利亚白刺盐胁迫伤害的缓解效应[J]. 山东农业科学, 2022, 54(8): 73-78. |
| Wu HX, Zhao Y, Wang HY, et al. Relieving effect of exogenous Ca2+ on salt stress injury to Nitraria sibirica[J]. Shandong Agric Sci, 2022, 54(8): 73-78. | |
| [30] |
Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity[J]. Plant J, 1997, 12(5): 1067-1078.
doi: 10.1046/j.1365-313x.1997.12051067.x pmid: 9418048 |
| [31] | 李辉, 杨宁, 刘锐锐, 等. 磷酸肌醇特异性磷脂酶C在植物生长发育中的作用[J]. 中国细胞生物学学报, 2020, 42(12): 2215-2226. |
| Li H, Yang N, Liu RR, et al. The role of phosphoinositide-specific PLC in plant growth and development[J]. Chin J Cell Biol 2020, 42(12): 2215-2226 | |
| [32] | 谢美娟, 曹高燚, 包曙光, 等. 植物钙依赖蛋白激酶的结构、表达特性及其生物学功能[J]. 分子植物育种, 2023, 21(19):6413-6421. |
| Xie MJ, Cao GY, Bao SG, et al. Structure, expression and biological functions of calcium-dependent protein kinases in plants[J]. Molecular Plant Breeding, 2023, 21(19):6413-6421. | |
| [33] | 景红娟, 周广舟, 谭晓荣, 等. 活性氧对植物自噬调控的研究进展[J]. 植物学报, 2012, 47(5): 534-542. |
| Jing HJ, Zhou GZ, Tan XR, et al. Research progress in regulation of reactive oxygen species in plant autophagy[J]. Bull Bot, 2012, 47(5): 534-542, | |
| [34] |
Wang Y, Shen C, Jiang Q, et al. Seed priming with calcium chloride enhances stress tolerance in rice seedlings[J]. Plant Sci, 2022, 323: 111381.
doi: 10.1016/j.plantsci.2022.111381 URL |
| [35] | 张杨, 孙弯弯, 陆丽丹, 等. 细胞自噬与凋亡相互作用分子机制的研究进展[J]. 基础医学与临床, 2021, 41(9): 1342-1346. |
| Zhang Y, Sun WW, Lu LD, et al. Research progress on molecular mechanism of the interaction between autophagy and apoptosis[J]. Basic Clin Med, 2021, 41(9): 1342-1346. | |
| [36] | 李喜豹, 赖敏怡, 梁山, 等. 植物细胞自噬基因的功能与转录调控机制[J]. 植物学报, 2021, 56(2): 201-217. |
| Li XB, Lai MY, Liang S, et al. Function and transcriptional regulation of autophagy-related genes in plants[J]. Bull Bot, 2021, 56(2): 201-217. | |
| [37] |
Zhou SM, Hong Q, Li Y, et al. Autophagy contributes to regulate the ROS levels and PCD progress in TMV-infected tomatoes[J]. Plant Sci, 2018, 269: 12-19.
doi: S0168-9452(17)30587-3 pmid: 29606209 |
| [38] | 李浩冬, 李良云, 桂馨墨, 等. 基于自噬探讨Atg5参与肝脏疾病调控的研究进展[J]. 生理科学进展, 2021, 52(5): 395-400. |
| Li HD, Li LY, Lin XM, et al. Research progress of ATG5 involved in the regulation of liver diseases based on autophagy[J]. Prog Physiol Sci, 2021, 52(5): 395-400. | |
| [39] |
李永波, 崔德周, 黄琛, 等. 高度特异性小麦ATG8抗体的研制及其在细胞自噬检测中的应用[J]. 作物学报, 2022, 48(9): 2390-2399.
doi: 10.3724/SP.J.1006.2022.11070 |
| Li YB, Cui DZ, Huang C, et al. Preparation of highly specific wheat ATG8 antibody and its application in the detection of autophagy[J]. Acta Agr Sin, 2022, 48(9): 2390-2399. | |
| [40] |
Yue JY, Wang YJ, Jiao JL, et al. The metacaspase TaMCA-Id negatively regulates salt-induced programmed cell death and functionally links with autophagy in wheat[J]. Front Plant Sci, 2022, 13: 904933.
doi: 10.3389/fpls.2022.904933 URL |
| [41] | 于颖, 龙聪. 动脉粥样硬化中自噬与凋亡相互作用的研究进展[J]. 心血管病学进展, 2022, 43(5): 454-458. |
| Yu Y, Long C. Crosstalk between autophagy and apoptosis in atherosclerosis[J]. Adva Cardiovascular Dis, 2022, 43(5): 454-458. |
| [1] | 王雨晴, 马子奇, 侯嘉欣, 宗钰琪, 郝晗睿, 刘国元, 魏辉, 连博琳, 陈艳红, 张健. 盐胁迫下植物根系分泌物的成分分析与生态功能研究进展[J]. 生物技术通报, 2024, 40(1): 12-23. |
| [2] | 张怡, 张心如, 张金珂, 胡利宗, 上官欣欣, 郑晓红, 胡娟娟, 张聪聪, 穆桂清, 李成伟. 小麦镉胁迫响应基因TaMYB1的功能分析[J]. 生物技术通报, 2024, 40(1): 194-206. |
| [3] | 常泸尹, 王中华, 李凤敏, 高梓源, 张辉红, 王祎, 李芳, 韩燕来, 姜瑛. 玉米根际多功能促生菌的筛选及其对冬小麦-夏玉米轮作体系产量提升效果[J]. 生物技术通报, 2024, 40(1): 231-242. |
| [4] | 温晓蕾, 李建嫄, 李娜, 张娜, 杨文香. 小麦叶锈菌与小麦互作的酵母双杂交cDNA文库构建与应用[J]. 生物技术通报, 2023, 39(9): 136-146. |
| [5] | 韩志阳, 贾子苗, 梁秋菊, 王轲, 唐华丽, 叶兴国, 张双喜. 二套小麦-簇毛麦染色体附加系苗期耐盐性及籽粒硒和叶酸的含量[J]. 生物技术通报, 2023, 39(8): 185-193. |
| [6] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [7] | 魏茜雅, 秦中维, 梁腊梅, 林欣琪, 李映志. 褪黑素种子引发处理提高朝天椒耐盐性的作用机制[J]. 生物技术通报, 2023, 39(7): 160-172. |
| [8] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [9] | 扈丽丽, 林柏荣, 王宏洪, 陈建松, 廖金铃, 卓侃. 最短尾短体线虫转录组及潜在效应蛋白分析[J]. 生物技术通报, 2023, 39(3): 254-266. |
| [10] | 杜清洁, 周璐瑶, 杨思震, 张嘉欣, 陈春林, 李娟起, 李猛, 赵士文, 肖怀娟, 王吉庆. 过表达CaCP1提高转基因烟草对盐胁迫的敏感性[J]. 生物技术通报, 2023, 39(2): 172-182. |
| [11] | 叶红, 王玉昆. 植物PRR免疫受体功能研究进展[J]. 生物技术通报, 2023, 39(12): 1-15. |
| [12] | 汪明滔, 刘建伟, 赵春钊. 植物调控盐胁迫下细胞壁完整性的分子机制[J]. 生物技术通报, 2023, 39(11): 18-27. |
| [13] | 张玉娟, 黎冬华, 宫慧慧, 崔新晓, 高春华, 张秀荣, 游均, 赵军胜. 芝麻NAC转录因子基因SiNAC77的克隆及耐盐功能分析[J]. 生物技术通报, 2023, 39(11): 308-317. |
| [14] | 徐扬, 丁红, 张冠初, 郭庆, 张智猛, 戴良香. 盐胁迫下花生种子萌发期代谢组学分析[J]. 生物技术通报, 2023, 39(1): 199-213. |
| [15] | 孔德真, 聂迎彬, 崔凤娟, 桑伟, 徐红军, 田笑明. 杂交小麦制种研究现状及展望[J]. 生物技术通报, 2023, 39(1): 95-103. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||