








生物技术通报 ›› 2025, Vol. 41 ›› Issue (11): 143-152.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0690
• 未来食品工程专题 • 上一篇
何听雨( ), 逄雨, 张远洋, 孙雪(
), 逄雨, 张远洋, 孙雪( ), 李玉, 路福平, 李庆刚(
), 李玉, 路福平, 李庆刚( )
)
收稿日期:2025-06-30
出版日期:2025-11-26
发布日期:2025-12-09
通讯作者:
李庆刚,博士,教授,研究方向 :应用微生物与酶工程;E-mail: liqinggang@tust.edu.cn作者简介:何听雨,硕士研究生,研究方向 :应用微生物与酶工程;E-mail: hetingyu2023@163.com
基金资助:
HE Ting-yu( ), PANG Yu, ZHANG Yuan-yang, SUN Xue(
), PANG Yu, ZHANG Yuan-yang, SUN Xue( ), LI Yu, LU Fu-ping, LI Qing-gang(
), LI Yu, LU Fu-ping, LI Qing-gang( )
)
Received:2025-06-30
Published:2025-11-26
Online:2025-12-09
摘要:
目的 利用微生物合成乳酰-N-三糖 Ⅱ(lacto-N-triose, LNT Ⅱ)是实现其工业化生产的可行方法,但目前由于异源酶的表达较差、途径关键酶及限速酶表达不平衡和前体物质合成不足等问题,LNT Ⅱ的产量仍然较低,本研究构建LNT Ⅱ高产菌株,提高LNT Ⅱ的合成能力。 方法 对比不同促溶蛋白标签对关键酶β-1,3-N-乙酰葡糖胺转移酶LgtA可溶性表达的影响,并精准调控LgtA和限速酶谷氨酰胺-果糖-6-磷酸氨基转移酶GlmS的表达强度,同时对不同来源的谷氨酰胺合成酶GlnA进行筛选,显著提升异源酶的可溶性表达,均衡关键途径酶的表达水平并强化前体供给,最后对LNT Ⅱ发酵培养基成分,包括甘油、IPTG、甜菜碱和乳清酸添加量进行优化。 结果 将MBP与LgtA融合后,LgtA的溶解度显著提高;通过RBS T7调节关键酶LgtA和限速酶GlmS的翻译强度、表达SGlnAE304A后有利于LNT Ⅱ的合成和菌株的生长;在甘油添加量为15 mL/L、IPTG添加量为0.1 mmol/L、甜菜碱添加量为3 g/L、乳清酸添加量3 g/L的培养条件下,LNT Ⅱ的产量由4.37 g/L提高至14.12 g/L。 结论 本研究对LNT Ⅱ生产菌株的代谢工程改造与发酵条件优化,显著提高了LNT Ⅱ的生产水平,并为在大肠杆菌中合成其他种类的HMOs提供了参考。
何听雨, 逄雨, 张远洋, 孙雪, 李玉, 路福平, 李庆刚. 高产乳酰-N-三糖Ⅱ大肠杆菌菌株的构建[J]. 生物技术通报, 2025, 41(11): 143-152.
HE Ting-yu, PANG Yu, ZHANG Yuan-yang, SUN Xue, LI Yu, LU Fu-ping, LI Qing-gang. Construction of a High-production Lacto -N-triose Ⅱ-producing Escherichia coli Strain[J]. Biotechnology Bulletin, 2025, 41(11): 143-152.
融合蛋白名称 Name of fusion protein | 理论等电点 Theoretical isoelectric point | 负电荷氨基酸残基总数 Total number of negatively charged amino acid residues | 正电荷氨基酸残基总数 Total number of positively charged amino acid residues | 总平均亲水性 Overall average hydrophilicity |
|---|---|---|---|---|
| TrxA-Nm58LgtAR13H,L24M,R205C | 6.48 | 64 | 61 | -0.412 |
| SUMO-Nm58LgtAR13H,L24M,R205C | 6.38 | 69 | 65 | -0.621 |
| GST-Nm58LgtAR13H,L24M,R205C | 6.99 | 81 | 80 | -0.478 |
| MBP-Nm58LgtAR13H,L24M,R205C | 6.24 | 99 | 93 | -0.441 |
| AHP-Nm58LgtAR13H,L24M,R205C | 7.75 | 50 | 51 | -0.552 |
表1 融合蛋白的理化性质
Table 1 Physicochemical properties of fusion proteins
融合蛋白名称 Name of fusion protein | 理论等电点 Theoretical isoelectric point | 负电荷氨基酸残基总数 Total number of negatively charged amino acid residues | 正电荷氨基酸残基总数 Total number of positively charged amino acid residues | 总平均亲水性 Overall average hydrophilicity |
|---|---|---|---|---|
| TrxA-Nm58LgtAR13H,L24M,R205C | 6.48 | 64 | 61 | -0.412 |
| SUMO-Nm58LgtAR13H,L24M,R205C | 6.38 | 69 | 65 | -0.621 |
| GST-Nm58LgtAR13H,L24M,R205C | 6.99 | 81 | 80 | -0.478 |
| MBP-Nm58LgtAR13H,L24M,R205C | 6.24 | 99 | 93 | -0.441 |
| AHP-Nm58LgtAR13H,L24M,R205C | 7.75 | 50 | 51 | -0.552 |
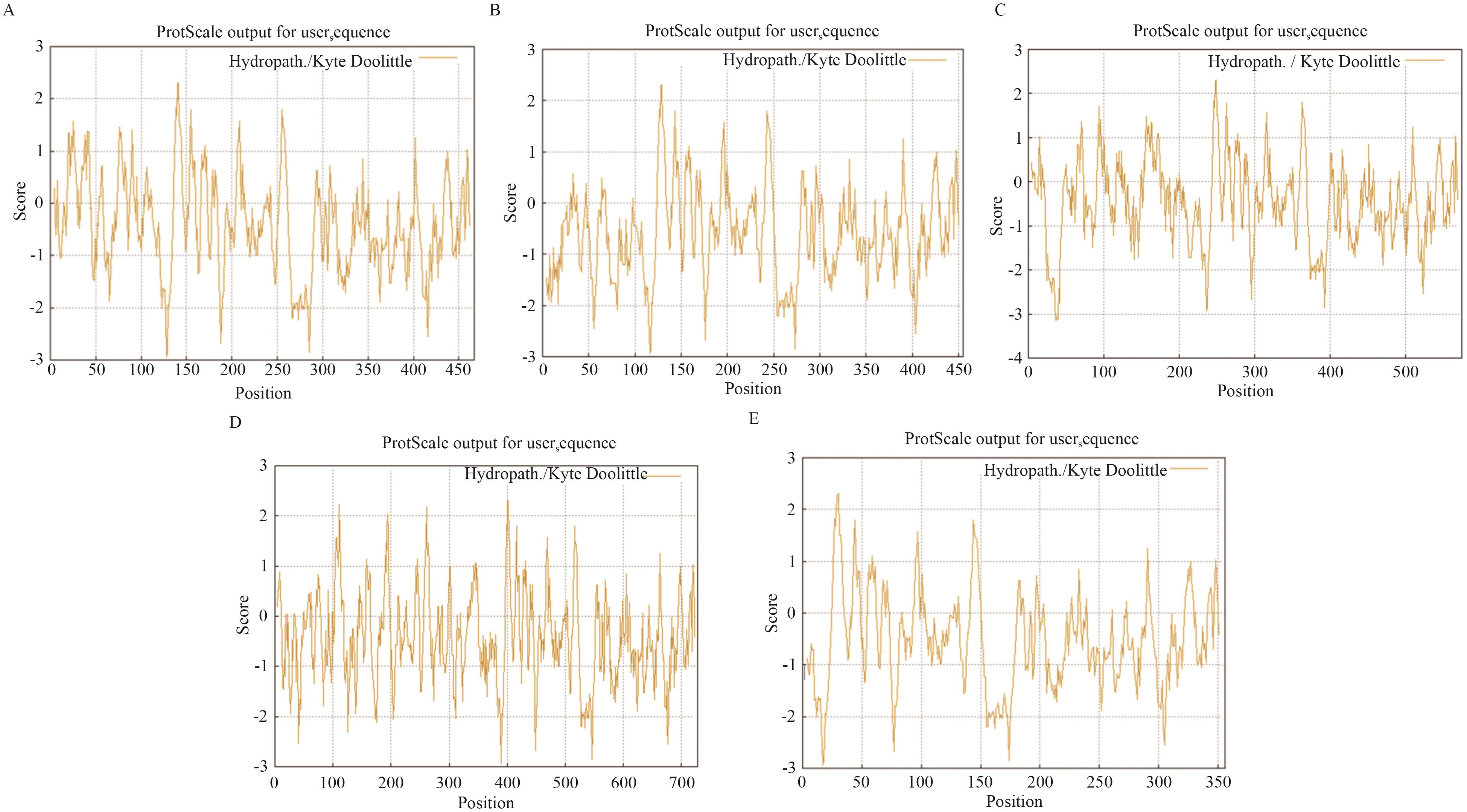
图1 融合蛋白的亲疏水性能分析A-E:分别表示添加促溶标签的融合蛋白TrxA-Nm58LgtAR13H,L24M,R205C、SUMO-Nm58LgtAR13H,L24M,R205C、GST-Nm58LgtAR13H,L24M,R205C、MBP-Nm58LgtAR13H,L24M,R205C和AHP-Nm58LgtAR13H,L24M,R205C的亲疏水性分析,图中纵坐标Score代表疏水性值。正值表示疏水性区域,负值表示亲水性区域。横坐标代表氨基酸在该蛋白序列中的位置
Fig. 1 Analysis of hydrophilicity and hydrophobicity of fusion proteinsA-E: Indicate the hydrophilicity analysis of the fusion proteins TrxA-Nm58LgtAR13H,L24M,R205C, SUMO-Nm58LgtAR13H,L24M,R205C, GST-Nm58LgtAR13H,L24M,R205C, MBP-Nm58LgtAR13H,L24M,R205C and AHP-Nm58LgtAR13H,L24M,R205C with solubility-promoting tags, respectively. In the figures, the vertical axis score indicates the hydrophobicity value. Positive values indicate hydrophobic regions, and negative values indicate hydrophilic regions. The horizontal axis indicates the position of the amino acid in the protein sequence
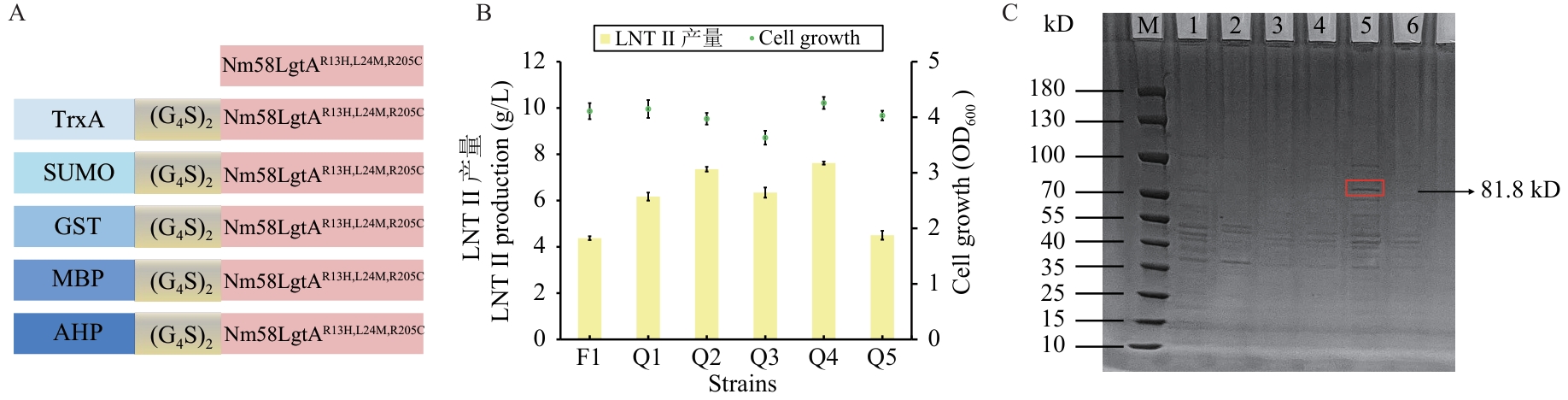
图2 不同促溶蛋白标签对LNT Ⅱ产量的影响A:融合蛋白示意图;B:Q1-Q5的菌株生长情况和LNT Ⅱ产量;C:融合蛋白胶图
Fig. 2 Influence of different solubility-promoting protein tags on the production of LNT ⅡA: Schematic diagram of fusion protein; B: growth and LNT Ⅱ production of strains Q1 to Q5; C: fusion protein gel diagram. M: Marker; 1: Nm58LgtAR13H,L24M,R205C; 2: TrxA-Nm58LgtAR13H,L24M,R205C; 3: SUMO-Nm58LgtAR13H,L24M,R205C; 4: GST-Nm58LgtAR13H,L24M,R205C; 5: MBP-Nm58LgtAR13H,L24M,R205C; 6: AHP-Nm58LgtAR13H,L24M,R205C

图4 LgtA和GlmS*的表达强度对LNT Ⅱ合成的影响A:质粒过表达MBP-Nm58LgtAR13H,L24M,R205C和GlmS*与不同RBS组合示意图;B:Z1-Z36的菌株生长情况和LNT Ⅱ产量
Fig. 4 Influences of the expression intensities of LgtA and GlmS* on the synthesis of LNT ⅡA: Schematic diagrams of plasmids overexpressing MBP-Nm58LgtAR13H,L24M,R205C and GlmS* with various RBSs. B: Growth and LNT Ⅱ production of strains Z1 to Z36
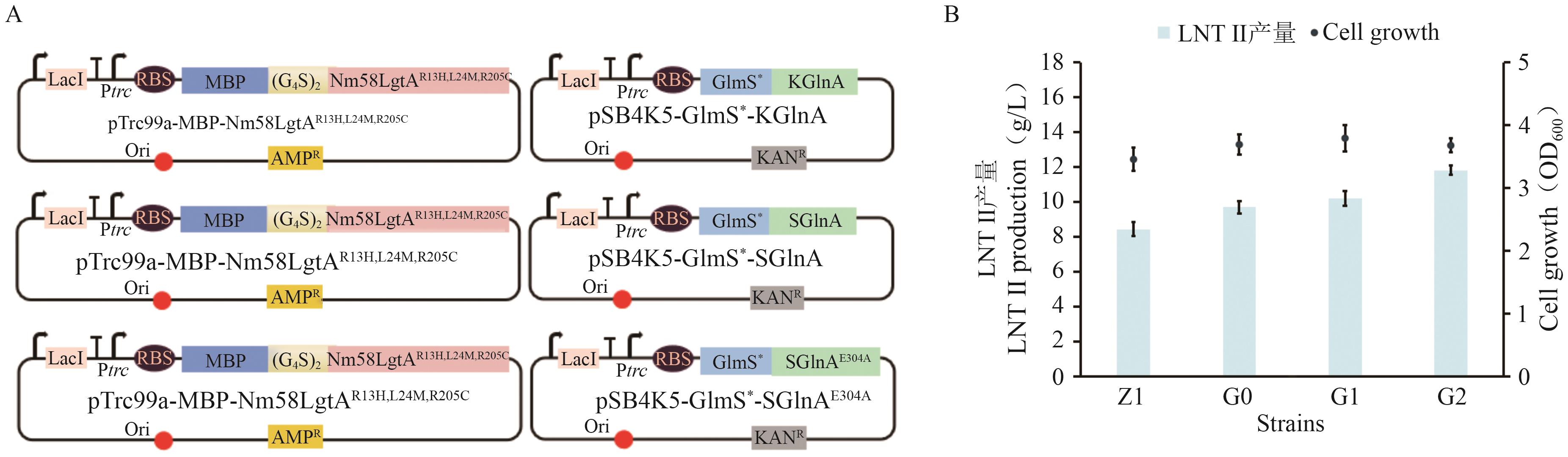
图5 不同来源的GlnA对LNT Ⅱ合成的影响A:过表达不同来源GlnA示意图;B:G0-G2的菌株生长情况和LNT Ⅱ产量
Fig. 5 Influences of GlnA from different sources on the synthesis of LNT ⅡA: Schematic diagram of overexpressing GlnA from different sources. B: Growth and LNT Ⅱ production of strains G0 to G2
| [1] | 胡多多. 代谢工程改造大肠杆菌生产乳酰-N-三糖Ⅱ [D]. 无锡: 江南大学, 2022. |
| Hu DD. Production of lactoyl-N-trisaccharide Ⅱ by metabolic engineering transformation of Escherichia coli [D]. Wuxi: Jiangnan University, 2022. | |
| [2] | 孟佳炜, 朱莺莺, 罗国聪, 等. 乳酰-N-新四糖的生理功能、生物合成及其衍生化研究进展 [J]. 中国食品学报, 2022, 22(3): 320-328. |
| Meng JW, Zhu YY, Luo GC, et al. Recent advances on physiological function, biosynthesis, and derivatization of Lacto-N-neotetraose [J]. J Chin Inst Food Sci Technol, 2022, 22(3): 320-328. | |
| [3] | 李凤仪. 利用工程化乳酸克鲁维酵母实现人乳寡糖乳-N-二糖和乳-N-三糖的高效合成 [D]. 济南:山东大学, 2024. |
| Li FY. Engineering Kluyveromyces lactis for producing human milk oligosaccharides lacto-N-biose and lacto-N-triose Ⅱ efficiently [D]. Jinan: Shandong University, 2024. | |
| [4] | Blixt O, van Die I, Norberg T, et al. High-level expression of the Neisseria meningitidis lgtA gene in Escherichia coli and characterization of the encoded N-acetylglucosaminyltransferase as a useful catalyst in the synthesis of GlcNAc beta 1—>3Gal and GalNAc beta 1—>3Gal linkages [J]. Glycobiology, 1999, 9(10): 1061-1071. |
| [5] | 刘丹, 梁山泉, 闫巧娟, 等. 基于模块优化强化大肠杆菌合成乳糖-N-新四糖的研究 [J]. 食品科学技术学报, 2024, 42(2): 75-83. |
| Liu D, Liang SQ, Yan QJ, et al. Study on enhancement of lacto-N-neotetraose synthesis in Escherichia coli based on module optimization [J]. J Food Sci Technol, 2024, 42(2): 75-83. | |
| [6] | Hu DD, Wu H, Zhu YY, et al. Engineering Escherichia coli for highly efficient production of lacto-N-triose Ⅱ from N-acetylglucosamine, the monomer of chitin [J]. Biotechnol Biofuels, 2021, 14(1): 198. |
| [7] | Lin L, Gong MY, Liu YF, et al. Combinatorial metabolic engineering of Escherichia coli for de novo production of 2'-fucosyllactose [J]. Bioresour Technol, 2022, 351: 126949. |
| [8] | 胡苗苗. 代谢工程改造大肠杆菌产乳酰-N-新四糖和乳酰-N-四糖 [D]. 无锡: 江南大学, 2024. |
| Hu MM. Metabolic engineering of Escherichia coli for the production of lacto-N-neotetraose and lacto-N-tetraose [D]. Wuxi: Jiangnan University, 2024. | |
| [9] | 吴凤礼, 王晓霜, 宋富强, 等. 芳香族化合物微生物代谢工程研究进展 [J]. 生物工程学报, 2021, 37(5): 1771-1793. |
| Wu FL, Wang XS, Song FQ, et al. Advances in metabolic engineering for the production of aromatic chemicals [J]. Chin J Biotechnol, 2021, 37(5): 1771-1793. | |
| [10] | Fierfort N, Samain E. Genetic engineering of Escherichia coli for the economical production of sialylated oligosaccharides [J]. J Biotechnol, 2008, 134(3/4): 261-265. |
| [11] | Li JZ, He TY, Zhao JJ, et al. Combination of metabolic engineering and high-throughput screening to realize high-producing lacto-N-triose Ⅱ in Escherichia coli [J]. J Agric Food Chem, 2025, 73(28): 17769-17775. |
| [12] | Yu J, Shin J, Park M, et al. Engineering of α-1, 3-fucosyltransferases for production of 3-fucosyllactose in Escherichia coli [J]. Metab Eng, 2018, 48: 269-278. |
| [13] | Choi YH, Kim JH, Park BS, et al. Solubilization and iterative saturation mutagenesis of α1, 3-fucosyltransferase from Helicobacter pylori to enhance its catalytic efficiency [J]. Biotechnol Bioeng, 2016, 113(8): 1666-1675. |
| [14] | Wray LV Jr, Fisher SH. Functional roles of the conserved Glu304 loop of Bacillus subtilis glutamine synthetase [J]. J Bacteriol, 2010, 192(19): 5018-5025. |
| [15] | 黎玉. 基于多细胞耦合催化策略的乳酰-N-三糖 Ⅱ合成研究[D]. 无锡:江南大学, 2023. |
| Li Y. Synthesis of lacto-N-triose Ⅱ based onmulti-cell coupling catalysis strategy [D]. Wuxi: Jiangnan University, 2023. | |
| [16] | 张景, 潘玲, 毛威威, 等. 鸡HNF 3β重组蛋白在大肠杆菌中的高效表达及其抗体制备 [J]. 农业生物技术学报, 2022, 30(4): 809-816. |
| Zhang J, Pan L, Mao WW, et al. High-level expression of chicken(Gallus gallus) HNF 3β recombinant protein in Escherichia coli and preparation of its antibody [J]. J Agric Biotechnol, 2022, 30(4): 809-816. | |
| [17] | 苗朝悦, 杜乐, 王佳琦, 等. 重组蛋白质在大肠杆菌体系中的可溶性表达策略 [J]. 中国生物工程杂志, 2023, 43(9): 33-45. |
| Miao ZY, Du L, Wang JQ, et al. Soluble expression strategies for production of recombinant proteins in Escherichia coli [J]. China Biotechnol, 2023, 43(9): 33-45. | |
| [18] | 田顺立, 郑春阳. 重组角质细胞生长因子(KGF)的原核表达及优化 [J]. 安徽农业科学, 2018, 46 (33): 71-74. |
| Tian SL, Zheng CY. Expression and optimization of recombinant keratinocyte growth factor(KGF) in E.coli [J]. J Anhui Agric Sci, 2018, 46(33): 71-74. | |
| [19] | 周生瑞, 郑依琳, 候志亮, 等. 多策略组合优化大肠杆菌高效表达贻贝足丝蛋白Mcofp-3 [J/OL]. 食品与发酵工业, 2025. DOI: 10.13995/j.cnki.11-1802/ts.042554 . |
| Zhou SR, Zheng YL, Hou ZL, et al. Optimization of multi-strategy combination for efficient expression of mussel foot protein mcofp-3 in E . coli [J/OL]. Food Ferment Ind, 2025. DOI: 10.13995/j.cnki.11-1802/ts.042554 . | |
| [20] | Waugh DS. The remarkable solubility-enhancing power of Escherichia coli maltose-binding protein [J]. Postepy Biochem, 2016, 62(3): 377-382. |
| [21] | Li QG, Liu C, He JH, et al. Construction and application of 3-fucosyllactose whole-cell biosensor for high-throughput screening of overproducers [J]. Bioresour Technol, 2024, 402: 130798. |
| [22] | 朱莺莺. 基于代谢工程的乳酰-N-新四糖和乳酰-N-四糖的高效生物合成研究 [D]. 无锡:江南大学, 2024. |
| Zhu YY. Efficient biosynthesis of lacto-N-neotetraose and lacto-N-tetraose based on metabolic engineering [D]. Wuxi: Jiangnan University, 2024. | |
| [23] | 王源. 代谢工程改造枯草芽孢杆菌合成番茄红素 [D]. 无锡:江南大学, 2025. |
| Wang Y. Metabolic engineering for the synthesis of lycopene in Bacillus subtilis [D]. Wuxi: Jiangnan University, 2025. | |
| [24] | Sun X, Peng ZT, Li C, et al. Combinatorial metabolic engineering and tolerance evolving of Escherichia coli for high production of 2'-fucosyllactose [J]. Bioresour Technol, 2023, 372: 128667. |
| [25] | Lv QL, Hu MK, Tian LZ, et al. Enhancing l-glutamine production in Corynebacterium glutamicum by rational metabolic engineering combined with a two-stage pH control strategy [J]. Bioresour Technol, 2021, 341: 125799. |
| [26] | 杨佳瑶, 唐霓, 汪凯. 己糖胺生物合成途径代谢酶与OGT介导的O-GlcNAc修饰在肿瘤中的研究进展[J]. 重庆医科大学学报, 2023, 48 (11): 1290-1295. |
| Yang JY, Tang N, Wang K. Advances in metabolic enzymes in the hexosamine biosynthetic pathway and OGT-mediated O-GlcNAc modification in tumors [J]. J Chongqing Med Univ, 2023, 48 (11): 1290-1295. | |
| [27] | Zhu YY, Luo GC, Li ZY, et al. Efficient biosynthesis of lacto-N-neotetraose by a novel β-1, 4-galactosyltransferase from Aggregatibacter actinomycetemcomitans NUM4039 [J]. Enzyme Microb Technol, 2022, 153: 109912. |
| [28] | 熊赢, 徐宁, 黄君慧, 等. 灭螺真菌橘灰青霉菌Z12株培养基及发酵条件优化[J]. 中国血吸虫病防治杂志, 2023, 35(2): 137-146, 205. |
| Xiong Y, Xu N, Huang JH, et al. Optimization of the medium and fermentation condition for the Penicillium aurantiocandidum Z12 strain with molluscicidal actions against Oncomelania hupensis [J]. Chin J Schisto Control, 2023, 35(2): 137-146, 205. | |
| [29] | Xu FX, Cao H, Cui XW, et al. Optimization of fermentation condition for echinacoside yield improvement with Penicillium sp. H1, an endophytic fungus isolated from Ligustrum lucidum ait using response surface methodology [J]. Molecules, 2018, 23(10): 2586. |
| [30] | 骆叶姣, 胡苗苗, 李梦丽, 等. 乳酰-N-新四糖的生物合成菌株构建及发酵条件研究 [J]. 食品与发酵工业, 2023, 49(21): 23-29. |
| Luo YJ, Hu MM, Li ML, et al. Construction of lacto-N-neotetraose biosynthesis strain and study on fermentation conditions [J]. Food Ferment Ind, 2023, 49(21): 23-29. | |
| [31] | Gomes L, Monteiro G, Mergulhão F. The impact of IPTG induction on plasmid stability and heterologous protein expression by Escherichia coli biofilms [J]. Int J Mol Sci, 2020, 21(2): 576. |
| [32] | 张天鹏, 杨兴洪. 甜菜碱提高植物抗逆性及促进生长发育研究进展 [J]. 植物生理学报, 2017, 53(11): 1955-1962. |
| Zhang TP, Yang XH. Research on the mechanism of glycinebetaine regulating plants stress resistance and development [J]. Plant Physiol J, 2017, 53(11): 1955-1962. | |
| [33] | 赵琳琳. 脱氮假单胞菌产维生素B12发酵过程中甜菜碱作用机理及其补料策略的研究 [D]. 广州:华南理工大学, 2012. |
| Zhao LL. Roles of betaine in vitamin B12 fermentation by Pseudomonas denitrificans and its feeding strategy optimization [D]. Guangzhou: South China University of Technology, 2012. | |
| [34] | 范晓光, 张通, 李杰, 等. 甜菜碱在微生物中的代谢及应用研究进展 [J]. 发酵科技通讯, 2018, 47(3): 151-156, 188. |
| Fan XG, Zhang T, Li J, et al. Betaine metabolism in microorganism and its application [J]. Bull Ferment Sci Technol, 2018, 47(3): 151-156, 188. | |
| [35] | Zhang MW, Zhang K, Liu TL, et al. High-level production of lacto- N-neotetraose in Escherichia coli by stepwise optimization of the biosynthetic pathway [J]. J Agric Food Chem, 2023, 71(43): 16212-16220. |
| [1] | 闫梦阳, 梁晓阳, 戴君昂, 张妍, 关团, 张辉, 刘良波, 孙志华. 阿莫西林降解菌的筛选及降解机制研究[J]. 生物技术通报, 2025, 41(9): 314-325. |
| [2] | 黄旭升, 周雅莉, 柴旭东, 闻婧, 王计平, 贾小云, 李润植. 紫苏质体型PfLPAT1B基因的克隆及其在油脂合成中的功能分析[J]. 生物技术通报, 2025, 41(7): 226-236. |
| [3] | 魏敏华, 李晓童, 姜亚文, 周飘飘, 汪凯, 孙浩, 芦楠, 张成林. |
| [4] | 杨熠辰, 朱宏宇, 苏小运, 王苑, 罗会颖, 田健, 姚斌, 黄火清, 张杰. 以果葡糖浆为底物高效合成肌醇细胞工厂的构建[J]. 生物技术通报, 2025, 41(11): 121-133. |
| [5] | 饶峻, 赵晨, 李端华, 廖豪, 黄加雨, 王辂. 自诱导策略在麦角硫因生物合成中的应用[J]. 生物技术通报, 2025, 41(1): 333-346. |
| [6] | 张静安, 胡孝龙, 曹蓓蓓, 廖敏, 束长龙, 张杰, 王奎, 操海群. 苏云金芽胞杆菌可视化快速表达载体的构建与特性分析[J]. 生物技术通报, 2025, 41(1): 95-102. |
| [7] | 王周, 余杰, 王金华, 王永泽, 赵筱. 厌氧表达乳酸脱氢酶以提高大肠杆菌产D-乳酸光学纯度[J]. 生物技术通报, 2024, 40(5): 290-299. |
| [8] | 庄棵, 梁至轩, 何英婷, 谢秋玲. 大肠杆菌DH5α通过外膜囊泡传递抗生素抗性基因AmpR[J]. 生物技术通报, 2024, 40(12): 275-281. |
| [9] | 杨红艳, 韩筱, 杨建军. pDNA质粒在一次性生物反应器中的放大生产研究[J]. 生物技术通报, 2024, 40(1): 168-175. |
| [10] | 程亚楠, 张文聪, 周圆, 孙雪, 李玉, 李庆刚. 乳酸乳球菌生产2'-岩藻糖基乳糖的途径构建及发酵培养基优化[J]. 生物技术通报, 2023, 39(9): 84-96. |
| [11] | 陈彩萍, 任昊, 龙腾飞, 何冰, 鲁兆祥, 孙坚. 大肠杆菌Nissle 1917对炎症性肠病治疗作用的研究进展[J]. 生物技术通报, 2023, 39(6): 109-118. |
| [12] | 吴莉丹, 冉雪琴, 牛熙, 黄世会, 李升, 王嘉福. 猪源致病性大肠杆菌基因组比较与毒力因子分析[J]. 生物技术通报, 2023, 39(12): 287-299. |
| [13] | 侯炜辰, 叶柯, 李洁, 张洋子, 许文涛, 朱龙佼, 李相阳. 基于抗体-适配体夹心生物传感器检测大肠杆菌O157: H7[J]. 生物技术通报, 2023, 39(12): 81-89. |
| [14] | 李奕雅, 吴一凡, 丁能水, 范小萍, 陈凡. 荧光素酶辅助定量大肠杆菌破碎效果的方法[J]. 生物技术通报, 2023, 39(12): 90-98. |
| [15] | 唐瑞琪, 赵心清, 朱笃, 汪涯. 大肠杆菌对木质纤维素水解液抑制物的胁迫耐受性[J]. 生物技术通报, 2023, 39(11): 205-216. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||