








生物技术通报 ›› 2021, Vol. 37 ›› Issue (8): 141-151.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0068
马亚男1( ), 卢旭2, 魏云春1, 李康1, 魏若男1, 李胜1(
), 卢旭2, 魏云春1, 李康1, 魏若男1, 李胜1( ), 马绍英3(
), 马绍英3( )
)
收稿日期:2021-01-17
出版日期:2021-08-26
发布日期:2021-09-10
作者简介:马亚男,女,硕士研究生,研究方向:农艺与种业;E-mail: 基金资助:
MA Ya-nan1( ), LU Xu2, WEI Yun-chun1, LI Kang1, WEI Ruo-nan1, LI Sheng1(
), LU Xu2, WEI Yun-chun1, LI Kang1, WEI Ruo-nan1, LI Sheng1( ), MA Shao-ying3(
), MA Shao-ying3( )
)
Received:2021-01-17
Published:2021-08-26
Online:2021-09-10
摘要:
醛酮还原酶(aldo-keto reductase,AKR)是一类NADP-依赖型氧化还原酶,通过对葡萄AKR基因鉴定分析,旨在揭示葡萄AKR基因分子生物学功能和遗传调控机制。基于2020年最新组装的葡萄参考基因组数据,利用生物信息学对葡萄AKR基因进行理化性质、基因结构、Motif分析、顺式作用元件、亚细胞定位、二级结构预测以及组织特异性表达分析,最后用荧光定量分析了葡萄AKR基因在盐胁迫下表达水平变化。从全基因组水平上共鉴定出13个葡萄AKR基因家族成员,分为2个亚族;基因结构分析显示,不同成员编码的氨基酸序列外显子数目及位置存在差异;motif分析表明,AKR蛋白保守结构域高度相似;顺式作用元件分析显示,该基因上游启动子元件不仅存在核心元件和增强元件,同时还存在各种激素及胁迫响应元件,器官特异性元件和光调控元件;二级结构和亚细胞定位预测表明,该基因家族主要在细胞核中表达,并以α-螺旋和不规则卷曲为主。基因芯片表达谱分析显示,AKR家族成员间表达水平差异大且存在组织特异性。荧光定量结果显示,盐胁迫条件下VvAKR02、VvAKR05、VvAKR07、VvAKR11、VvAKR12和VvAKR13表达量显著上调。从葡萄中鉴定了13个AKR家族基因,并分析其盐胁迫下的表达水平,对于挖掘葡萄AKR家族中耐盐基因提供了一定的理论依据和实验基础。
马亚男, 卢旭, 魏云春, 李康, 魏若男, 李胜, 马绍英. 葡萄AKR基因家族的鉴定和组织特异性表达分析[J]. 生物技术通报, 2021, 37(8): 141-151.
MA Ya-nan, LU Xu, WEI Yun-chun, LI Kang, WEI Ruo-nan, LI Sheng, MA Shao-ying. Identification and Tissue Specific Expression Analysis of AKR Gene Family in Grape[J]. Biotechnology Bulletin, 2021, 37(8): 141-151.
| 基因Gene | 上游引物Forward primer(5'-3') | 下游引物Reverse primer(5'-3') |
|---|---|---|
| VvAKR01 | AGGAGGCAGCAGTGTCAAAATGG | CCAGCCACCGCATCATCATCTC |
| VvAKR02 | ACCATTGAAAGCCTCAGCCAGTG | GGCGTTGTCAGAGGGTTATCGTC |
| VvAKR03 | TTGTGCTGAGGAAGCTGGGTAAAC | GCCGCAGAGTGGGAACAAGAAG |
| VvAKR04 | AGCGGTGATTTGAATGCCTTTTGC | GGCGGTCAATACGGTGGAGATG |
| VvAKR05 | AGCGGGTTCATCTCCACTTGATTG | GGAAGGCATGGAAGAGTGTCAGAG |
| VvAKR06 | TCTGCTCTGTCTTGTGCGAAACC | GAACCAACCCCATACCCTAAAGCC |
| VvAKR07 | TGCTGCCAAAGTGGGTTCATCTC | GTCAGAAGCTCGGTCTCACCAAA |
| VvAKR08 | AAAGCTTTGGTGCACCGACG | TCCCAGGCTTCAAGCTCACC |
| VvAKR09 | TAGCCGCCTTAAGAGCCTGTCC | ACGCTGTCTTCACCGGCAATCG |
| VvAKR10 | GACACTGTGACCGCTCTGAATACC | CTCTCGGCGTACTCTCCTCTTGG |
| VvAKR11 | CTTGGATGACAGCAGGAGGAACAC | AGCACATGGAGAGCAATGGAAGC |
| VvAKR12 | AGTGCTTCCATTGCTCTCCATGTG | CTTATCCATTGGCCGGTCCGTATG |
| VvAKR13 | CTGCCTGCTCTTGCCAGATGTG | CCGTGTTCCTCCTGCTGTCAATC |
| UBI | GCTCGCTGTTTTTGCAGTTCTAC | AACATAGGTGAGGCCGCACTT |
表1 葡萄AKR基因家族实时荧光定量引物
Table 1 Real-time fluorescence quantitative primers of AKR gene family in grape
| 基因Gene | 上游引物Forward primer(5'-3') | 下游引物Reverse primer(5'-3') |
|---|---|---|
| VvAKR01 | AGGAGGCAGCAGTGTCAAAATGG | CCAGCCACCGCATCATCATCTC |
| VvAKR02 | ACCATTGAAAGCCTCAGCCAGTG | GGCGTTGTCAGAGGGTTATCGTC |
| VvAKR03 | TTGTGCTGAGGAAGCTGGGTAAAC | GCCGCAGAGTGGGAACAAGAAG |
| VvAKR04 | AGCGGTGATTTGAATGCCTTTTGC | GGCGGTCAATACGGTGGAGATG |
| VvAKR05 | AGCGGGTTCATCTCCACTTGATTG | GGAAGGCATGGAAGAGTGTCAGAG |
| VvAKR06 | TCTGCTCTGTCTTGTGCGAAACC | GAACCAACCCCATACCCTAAAGCC |
| VvAKR07 | TGCTGCCAAAGTGGGTTCATCTC | GTCAGAAGCTCGGTCTCACCAAA |
| VvAKR08 | AAAGCTTTGGTGCACCGACG | TCCCAGGCTTCAAGCTCACC |
| VvAKR09 | TAGCCGCCTTAAGAGCCTGTCC | ACGCTGTCTTCACCGGCAATCG |
| VvAKR10 | GACACTGTGACCGCTCTGAATACC | CTCTCGGCGTACTCTCCTCTTGG |
| VvAKR11 | CTTGGATGACAGCAGGAGGAACAC | AGCACATGGAGAGCAATGGAAGC |
| VvAKR12 | AGTGCTTCCATTGCTCTCCATGTG | CTTATCCATTGGCCGGTCCGTATG |
| VvAKR13 | CTGCCTGCTCTTGCCAGATGTG | CCGTGTTCCTCCTGCTGTCAATC |
| UBI | GCTCGCTGTTTTTGCAGTTCTAC | AACATAGGTGAGGCCGCACTT |
| 基因Gene | 登录号 Gene accession No. | 染色体定位 Chromosomal location | 全长Full length/bp | CDS/bp | 氨基酸 Amino acid | 分子量 Molecular weight/bp | 等电点 pI | 不稳定系数 Instability index | 脂肪系数Aliphatic index | 平均亲水性Hydropathicity |
|---|---|---|---|---|---|---|---|---|---|---|
| VvAKR01 | GSVIVT01009396001 | chr18:7983438..7986889 | 3452 | 969 | 322 | 36192.49 | 5.9 | 38.98 | 89.66 | -0.238 |
| VvAKR02 | GSVIVT01010238001 | chr1:18082562..18106134 | 23573 | 1002 | 333 | 36674.09 | 7.07 | 34.21 | 90.75 | -0.213 |
| VvAKR03 | GSVIVT01011576001 | chr1:6306239..6307845 | 1607 | 867 | 288 | 32083.49 | 9.01 | 42.94 | 96.88 | -0.086 |
| VvAKR04 | GSVIVT01011577001 | chr1:6298552..6305704 | 7153 | 912 | 303 | 34017.51 | 6.54 | 47.47 | 89.8 | -0.164 |
| VvAKR05 | GSVIVT01011579001 | chr1:6289243..6290820 | 1578 | 951 | 316 | 35455.01 | 5.85 | 51.55 | 95.73 | -0.206 |
| VvAKR06 | GSVIVT01011582001 | chr1:6274423..6285766 | 11344 | 1293 | 430 | 48655.51 | 7.1 | 43.48 | 94.12 | -0.184 |
| VvAKR07 | GSVIVT01011583001 | chr1:6264420..6272529 | 8110 | 909 | 302 | 34322.71 | 6.34 | 27.01 | 92.02 | -0.274 |
| VvAKR08 | GSVIVT01011585001 | chr1:6241008..6248273 | 7266 | 1092 | 363 | 40730.95 | 5.98 | 49.92 | 95.92 | -0.228 |
| VvAKR09 | GSVIVT01030263001 | chr8:9745143..9747339 | 2197 | 969 | 322 | 35975.24 | 6.17 | 45.07 | 83.57 | -0.319 |
| VvAKR10 | GSVIVT01034098001 | chr8:15157629..15161115 | 3487 | 948 | 315 | 35068.99 | 5.83 | 50.57 | 89.46 | -0.243 |
| VvAKR11 | GSVIVT01034099001 | chr8:15153012..15157096 | 4085 | 828 | 275 | 30440 | 6.45 | 46.14 | 87.56 | -0.204 |
| VvAKR12 | GSVIVT01034101001 | chr8:15144714..15149849 | 5136 | 903 | 300 | 33663.65 | 6.76 | 42.71 | 88.37 | -0.317 |
表2 葡萄AKR基因理化性质
Table 2 Physical and chemical property of AKR genes in grape
| 基因Gene | 登录号 Gene accession No. | 染色体定位 Chromosomal location | 全长Full length/bp | CDS/bp | 氨基酸 Amino acid | 分子量 Molecular weight/bp | 等电点 pI | 不稳定系数 Instability index | 脂肪系数Aliphatic index | 平均亲水性Hydropathicity |
|---|---|---|---|---|---|---|---|---|---|---|
| VvAKR01 | GSVIVT01009396001 | chr18:7983438..7986889 | 3452 | 969 | 322 | 36192.49 | 5.9 | 38.98 | 89.66 | -0.238 |
| VvAKR02 | GSVIVT01010238001 | chr1:18082562..18106134 | 23573 | 1002 | 333 | 36674.09 | 7.07 | 34.21 | 90.75 | -0.213 |
| VvAKR03 | GSVIVT01011576001 | chr1:6306239..6307845 | 1607 | 867 | 288 | 32083.49 | 9.01 | 42.94 | 96.88 | -0.086 |
| VvAKR04 | GSVIVT01011577001 | chr1:6298552..6305704 | 7153 | 912 | 303 | 34017.51 | 6.54 | 47.47 | 89.8 | -0.164 |
| VvAKR05 | GSVIVT01011579001 | chr1:6289243..6290820 | 1578 | 951 | 316 | 35455.01 | 5.85 | 51.55 | 95.73 | -0.206 |
| VvAKR06 | GSVIVT01011582001 | chr1:6274423..6285766 | 11344 | 1293 | 430 | 48655.51 | 7.1 | 43.48 | 94.12 | -0.184 |
| VvAKR07 | GSVIVT01011583001 | chr1:6264420..6272529 | 8110 | 909 | 302 | 34322.71 | 6.34 | 27.01 | 92.02 | -0.274 |
| VvAKR08 | GSVIVT01011585001 | chr1:6241008..6248273 | 7266 | 1092 | 363 | 40730.95 | 5.98 | 49.92 | 95.92 | -0.228 |
| VvAKR09 | GSVIVT01030263001 | chr8:9745143..9747339 | 2197 | 969 | 322 | 35975.24 | 6.17 | 45.07 | 83.57 | -0.319 |
| VvAKR10 | GSVIVT01034098001 | chr8:15157629..15161115 | 3487 | 948 | 315 | 35068.99 | 5.83 | 50.57 | 89.46 | -0.243 |
| VvAKR11 | GSVIVT01034099001 | chr8:15153012..15157096 | 4085 | 828 | 275 | 30440 | 6.45 | 46.14 | 87.56 | -0.204 |
| VvAKR12 | GSVIVT01034101001 | chr8:15144714..15149849 | 5136 | 903 | 300 | 33663.65 | 6.76 | 42.71 | 88.37 | -0.317 |
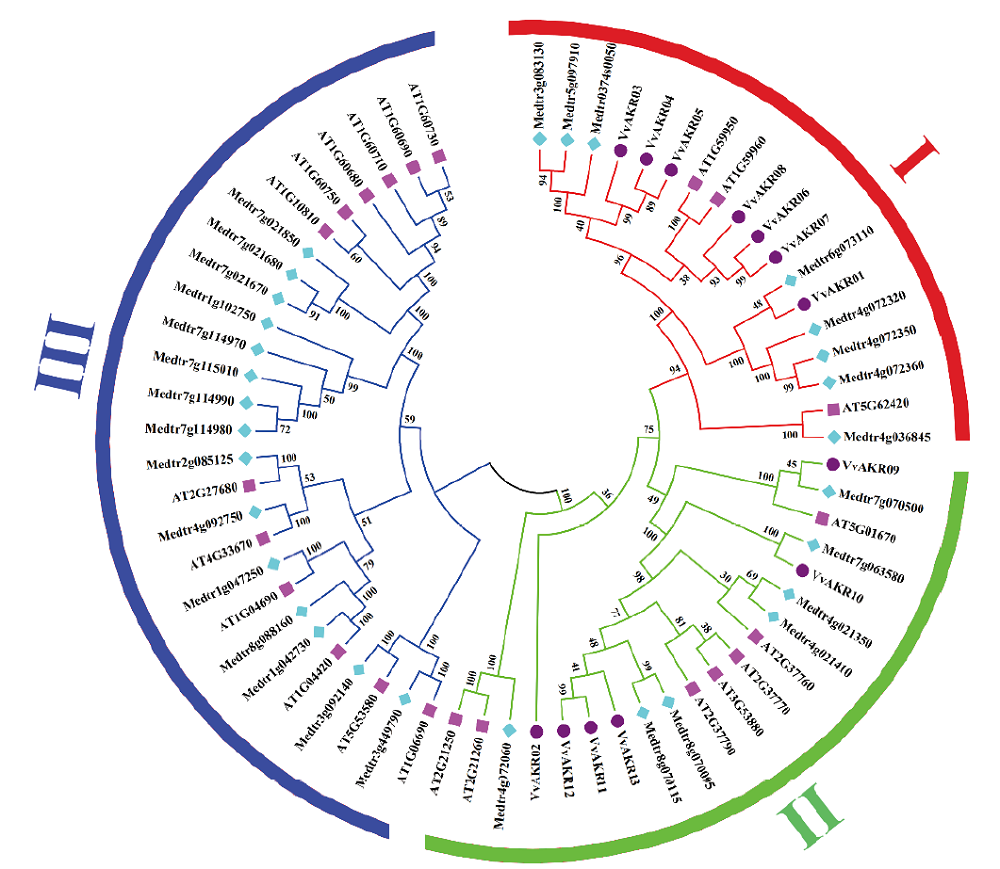
图1 拟南芥、苜蓿和葡萄AKR基因的进化树 粉色正方形代表拟南芥,浅蓝色菱形代表苜蓿,紫色圆圈代表葡萄
Fig.1 Phylogenetic tree of AKR genes in Arabidopsis,alfalfa and grape Pink squares represent Arabidopsis, light blue diamonds represent alfalfa, and purple circles refer to grapes
| 基因 Gene | 分子式 Formula | α-螺旋 Alpha helix/% | β-转角 Beta turn/% | 扩展链结构 Hydropathicity/% | 不规则卷曲 Random coil/% | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|
| VvAKR01 | C1621H2533N437O473S15 | 42.55 | 4.04 | 14.29 | 39.13 | Nucleus,cytosol,cytoskeleton and chloroplast |
| VvAKR02 | C1630H2584N452O480S15 | 37.84 | 6.01 | 15.32 | 40.84 | Nucleus,cytosol,cytoskeleton and chloroplast |
| VvAKR03 | C1437H2314N388O409S16 | 41.32 | 4.51 | 18.06 | 36.11 | Nucleus,membrane,cytosol,chloroplast and mitochondrion,peroxisome |
| VvAKR04 | C1533H2418N402O437S17 | 42.57 | 4.62 | 13.86 | 38.94 | Nucleus,cytosol,cytoskeleton and chloroplast |
| VvAKR05 | C1584H2534N424O466S15 | 43.04 | 3.80 | 15.51 | 37.66 | Extracellular,cytosol,chloroplast,mitochondrion and endoplasmic reticulum |
| VvAKR06 | C2207H3481N571O627S19 | 34.42 | 3.95 | 17.44 | 44.19 | Nucleus,membrane,cytosol,cytoskeleton,chloroplast and mitochondrion |
| VvAKR07 | C1560H2445N401O445S12 | 41.39 | 5.30 | 13.58 | 39.74 | Nucleus,extracellular,cytosol and cytoskeleton |
| VvAKR08 | C1829H2900N490O533S14 | 42.15 | 4.41 | 13.50 | 39.94 | Nucleus,membrane,cytosol and cytoskeleton |
| VvAKR09 | C1601H2518N442O469S16 | 40.37 | 8.39 | 14.60 | 36.65 | Nucleus,membrane,cytosol,cytoskeleton and chloroplast |
| VvAKR10 | C1582H2451N419O463S10 | 42.54 | 6.67 | 13.97 | 36.83 | Nucleus,cytosol and chloroplast |
| VvAKR11 | C1378H2147N363O395S10 | 43.27 | 5.09 | 14.18 | 37.45 | Nucleus,cytosol,cytoskeleton,endoplasmic reticulum and vacuole |
| VvAKR12 | C1518H2375N411O435S10 | 43.00 | 6.67 | 12.00 | 38.33 | Nucleus,membrane,cytosol,cytoskeleton and vacuole |
| VvAKR013 | C1872H2916N492O531S11 | 48.91 | 6.25 | 11.41 | 33.42 | Nucleus,cytosol and chloroplast |
表3 葡萄AKR基因二级结构及亚细胞定位预测
Table 3 Secondary structure and Subcellular location prediction of AKR genes in grape
| 基因 Gene | 分子式 Formula | α-螺旋 Alpha helix/% | β-转角 Beta turn/% | 扩展链结构 Hydropathicity/% | 不规则卷曲 Random coil/% | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|
| VvAKR01 | C1621H2533N437O473S15 | 42.55 | 4.04 | 14.29 | 39.13 | Nucleus,cytosol,cytoskeleton and chloroplast |
| VvAKR02 | C1630H2584N452O480S15 | 37.84 | 6.01 | 15.32 | 40.84 | Nucleus,cytosol,cytoskeleton and chloroplast |
| VvAKR03 | C1437H2314N388O409S16 | 41.32 | 4.51 | 18.06 | 36.11 | Nucleus,membrane,cytosol,chloroplast and mitochondrion,peroxisome |
| VvAKR04 | C1533H2418N402O437S17 | 42.57 | 4.62 | 13.86 | 38.94 | Nucleus,cytosol,cytoskeleton and chloroplast |
| VvAKR05 | C1584H2534N424O466S15 | 43.04 | 3.80 | 15.51 | 37.66 | Extracellular,cytosol,chloroplast,mitochondrion and endoplasmic reticulum |
| VvAKR06 | C2207H3481N571O627S19 | 34.42 | 3.95 | 17.44 | 44.19 | Nucleus,membrane,cytosol,cytoskeleton,chloroplast and mitochondrion |
| VvAKR07 | C1560H2445N401O445S12 | 41.39 | 5.30 | 13.58 | 39.74 | Nucleus,extracellular,cytosol and cytoskeleton |
| VvAKR08 | C1829H2900N490O533S14 | 42.15 | 4.41 | 13.50 | 39.94 | Nucleus,membrane,cytosol and cytoskeleton |
| VvAKR09 | C1601H2518N442O469S16 | 40.37 | 8.39 | 14.60 | 36.65 | Nucleus,membrane,cytosol,cytoskeleton and chloroplast |
| VvAKR10 | C1582H2451N419O463S10 | 42.54 | 6.67 | 13.97 | 36.83 | Nucleus,cytosol and chloroplast |
| VvAKR11 | C1378H2147N363O395S10 | 43.27 | 5.09 | 14.18 | 37.45 | Nucleus,cytosol,cytoskeleton,endoplasmic reticulum and vacuole |
| VvAKR12 | C1518H2375N411O435S10 | 43.00 | 6.67 | 12.00 | 38.33 | Nucleus,membrane,cytosol,cytoskeleton and vacuole |
| VvAKR013 | C1872H2916N492O531S11 | 48.91 | 6.25 | 11.41 | 33.42 | Nucleus,cytosol and chloroplast |
| [1] |
Mittler R. Oxidative stress, antioxidants and stress tolerance[J]. Trends Plant Sci, 2002, 7(9):405-410.
pmid: 12234732 |
| [2] |
Hegedüs A, Erdei S, Janda T, et al. Transgenic tobacco plants overproducing alfalfa aldose/aldehyde reductase show higher tolerance to low temperature and cadmium stress[J]. Plant Sci, 2004, 166(5):1329-1333.
doi: 10.1016/j.plantsci.2004.01.013 URL |
| [3] |
Simpson PJ, Tantitadapitak C, Reed AM, et al. Characterization of two novel aldo-keto reductases from Arabidopsis:expression patterns, broad substrate specificity, and an open active-site structure suggest a role in toxicant metabolism following stress[J]. J Mol Biol, 2009, 392(2):465-480.
doi: 10.1016/j.jmb.2009.07.023 pmid: 19616008 |
| [4] |
Spite M, Baba SP, Ahmed Y, et al. Substrate specificity and catalytic efficiency of aldo-keto reductases with phospholipid aldehydes[J]. Biochem J, 2007, 405(1):95-105.
doi: 10.1042/BJ20061743 URL |
| [5] |
Lee EH, Song DG, Lee JY, et al. Inhibitory effect of the compounds isolated from Rhus verniciflua on aldose reductase and advanced glycation endproducts[J]. Biol Pharm Bull, 2008, 31(8):1626-1630.
doi: 10.1248/bpb.31.1626 URL |
| [6] |
Mudalkar S, Sreeharsha RV, Reddy AR. A novel aldo-keto reductase from Jatropha curcas L. (JcAKR)plays a crucial role in the detoxification of methylglyoxal, a potent electrophile[J]. J Plant Physiol, 2016, 195:39-49.
doi: 10.1016/j.jplph.2016.03.005 URL |
| [7] | Éva C, Zelenyánszki H, Tömösközi-Farkas R, et al. Transgenic barley expressing the Arabidopsis AKR4C9 aldo-keto reductase enzyme exhibits enhanced freezing tolerance and regenerative capacity[J]. S Afr N J Bot, 2014, 93:179-184. |
| [8] | 蔡秋香, 廖端芳, 祖旭宇, 等. 醛酮还原酶超家族的研究进展[J]. 医学综述, 2008, 14(24):3693-3695. |
| Cai QX, Liao DF, Zu XY, et al. Research trends of alketose-reductase[J]. Med Recapitul, 2008, 14(24):3693-3695. | |
| [9] |
Gallego O, Ruiz FX, Ardèvol A, et al. Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10[J]. PNAS, 2007, 104(52):20764-20769.
pmid: 18087047 |
| [10] |
Jez JM, Bennett MJ, Schlegel BP, et al. Comparative anatomy of the aldo-keto reductase superfamily[J]. Biochem J, 1997, 326(Pt 3):625-636.
doi: 10.1042/bj3260625 URL |
| [11] |
Petschacher B, Leitgeb S, Kavanagh KL, et al. The coenzyme specificity of Candida tenuis xylose reductase(AKR2B5)explored by site-directed mutagenesis and X-ray crystallography[J]. Biochem J, 2005, 385(pt 1):75-83.
pmid: 15320875 |
| [12] |
Alban C, Baldet P, Douce R. Localization and characterization of two structurally different forms of acetyl-CoA carboxylase in young pea leaves, of which one is sensitive to aryloxyphenoxypropionate herbicides[J]. Biochem J, 1994, 300(Pt 2):557-565.
doi: 10.1042/bj3000557 URL |
| [13] |
Sengupta D, Naik D, Reddy AR. Plant aldo-keto reductases (AKRs)as multi-tasking soldiers involved in diverse plant metabolic processes and stress defense:a structure-function update[J]. J Plant Physiol, 2015, 179:40-55.
doi: 10.1016/j.jplph.2015.03.004 URL |
| [14] |
Éva C, Tóth G, Oszvald M, et al. Overproduction of an Arabidopsis aldo-keto reductase increases barley tolerance to oxidative and cadmium stress by an in vivo reactive aldehyde detoxification[J]. Plant Growth Regul, 2014, 74(1):55-63.
doi: 10.1007/s10725-014-9896-x URL |
| [15] |
Suekawa M, Fujikawa Y, et al. Gene expression and promoter analysis of a novel tomato aldo-keto reductase in response to environmental stresses[J]. J Plant Physiol, 2016, 200:35-44.
doi: 10.1016/j.jplph.2016.05.015 URL |
| [16] | 李立威. 甘蓝醛还原酶基因克隆及其功能分析[D]. 福州:福建农林大学, 2014. |
| Li LW. Cloning and functional analysis of aldehyde reductase gene in Brassica oleracea[D]. Fuzhou:Fujian Agriculture and Forestry University, 2014. | |
| [17] | 张飞雪, 虞章红, 刘坤宇, 等. 不结球白菜醛酮还原酶BcAKR4C9基因的克隆及表达分析[J]. 南京农业大学学报, 2018, 41(2):240-247. |
| Zhang FX, Yu ZH, Liu KY, et al. Cloning and expression analysis of aldo-keto reductase gene BcAKR4C9 from non-heading Chinese cabbage[J]. J Nanjing Agric Univ, 2018, 41(2):240-247. | |
| [18] | 杨洋, 李鸿彬. 棉纤维醛酮还原酶基因促进拟南芥主根伸长[J]. 石河子大学学报:自然科学版, 2017, 35(5):612-617. |
| Yang Y, Li HB. A cotton fiber aldehyde ketone reductase gene Gh AKR promotes elongation of main root in Arabidopsis thaliana[J]. J Shihezi Univ:Nat Sci, 2017, 35(5):612-617. | |
| [19] |
Cai XF, Zhang CJ, Ye J, et al. Ectopic expression of FaGalUR leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato[J]. Plant Growth Regul, 2015, 76(2):187-197.
doi: 10.1007/s10725-014-9988-7 URL |
| [20] |
Kanayama Y, Mizutani R, Yaguchi S, et al. Characterization of an uncharacterized aldo-keto reductase gene from peach and its role in abiotic stress tolerance[J]. Phytochemistry, 2014, 104:30-36.
doi: 10.1016/j.phytochem.2014.04.008 URL |
| [21] |
Wilkins MR, Gasteiger E, Bairoch A, et al. Protein identification and analysis tools in the ExPASy server[J]. Methods Mol Biol, 1999, 112:531-552.
pmid: 10027275 |
| [22] |
Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2. 0[J]. Bioinform Oxf Engl, 2007, 23(21):2947-2948.
doi: 10.1093/bioinformatics/btm404 URL |
| [23] |
Kumar S, Stecher G, Tamura K. MEGA7:molecular evolutionary genetics analysis version 7. 0 for bigger datasets[J]. Mol Biol Evol, 2016, 33(7):1870-1874.
doi: 10.1093/molbev/msw054 URL |
| [24] |
Zhang ZB, Zhang JW, Chen YJ, et al. Genome-wide analysis and identification of HAK potassium transporter gene family in maize(Zea mays L.)[J]. Mol Biol Rep, 2012, 39(8):8465-8473.
doi: 10.1007/s11033-012-1700-2 URL |
| [25] |
Bailey TL, Boden M, Buske FA, et al. MEME SUITE:tools for motif discovery and searching[J]. Nucleic Acids Res, 2009, 37(web server issue):W202-W208.
doi: 10.1093/nar/gkp335 URL |
| [26] |
Higo K, Ugawa Y, Iwamoto M, et al. Plant Cis-acting regulatory DNA elements(PLACE)database:1999[J]. Nucleic Acids Res, 1999, 27(1):297-300.
pmid: 9847208 |
| [27] |
Yu J, Sun H, Zhang JJ, et al. Analysis of aldo-keto reductase gene family and their responses to salt, drought, and abscisic acid stresses in Medicago truncatula[J]. Int J Mol Sci, 2020, 21(3):754.
doi: 10.3390/ijms21030754 URL |
| [28] |
Penning TM. The aldo-keto reductases(AKRs):Overview[J]. Chem Biol Interactions, 2015, 234:236-246.
doi: 10.1016/j.cbi.2014.09.024 URL |
| [29] |
Gavidia I, Pérez-Bermúdez P, Seitz HU. Cloning and expression of two novel aldo-keto reductases from Digitalis purpurea leaves[J]. Eur J Biochem, 2002, 269(12):2842-2850.
pmid: 12071946 |
| [30] |
Hideg, Nagy T, Oberschall A, et al. Detoxification function of aldose/aldehyde reductase during drought and ultraviolet-B(280-320 nm)stresses[J]. Plant Cell Environ, 2003, 26(4):513-522.
doi: 10.1046/j.1365-3040.2003.00982.x URL |
| [31] | Oberschall A, Deák M, Török K, et al. A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chemical and drought stresses[J]. The Plant Journal, 2000, 24(4):437-446. |
| [32] |
Colrat S, Latché A, Guis M, et al. Purification and characterization of a NADPH-dependent aldehyde reductase from mung bean that detoxifies eutypine, a toxin from Eutypa lata1[J]. Plant Physiol, 1999, 119(2):621-626.
pmid: 9952458 |
| [33] |
Guillén P, Guis M, Martínez-Reina G, et al. A novel NADPH-dependent aldehyde reductase gene from Vigna radiata confers resistance to the grapevine fungal toxin eutypine[J]. The Plant Journal, 1998, 16(3):335-343.
doi: 10.1046/j.1365-313x.1998.00303.x URL |
| [1] | 赵光绪, 杨合同, 邵晓波, 崔志豪, 刘红光, 张杰. 一株高效溶磷产红青霉培养条件优化及其溶磷特性[J]. 生物技术通报, 2023, 39(9): 71-83. |
| [2] | 宋志忠, 徐维华, 肖慧琳, 唐美玲, 陈景辉, 管雪强, 刘万好. 酿酒葡萄铁调节转运蛋白基因VvIRT1的克隆、表达与功能[J]. 生物技术通报, 2023, 39(8): 234-240. |
| [3] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [4] | 魏茜雅, 秦中维, 梁腊梅, 林欣琪, 李映志. 褪黑素种子引发处理提高朝天椒耐盐性的作用机制[J]. 生物技术通报, 2023, 39(7): 160-172. |
| [5] | 张路阳, 韩文龙, 徐晓雯, 姚健, 李芳芳, 田效园, 张智强. 烟草TCP基因家族的鉴定及表达分析[J]. 生物技术通报, 2023, 39(6): 248-258. |
| [6] | 董聪, 高庆华, 王玥, 罗同阳, 王庆庆. 基于联合策略提高FAD依赖的葡萄糖脱氢酶的酵母表达[J]. 生物技术通报, 2023, 39(6): 316-324. |
| [7] | 李敬蕊, 王育博, 解紫薇, 李畅, 吴晓蕾, 宫彬彬, 高洪波. 甜瓜PIN基因家族的鉴定及高温胁迫表达分析[J]. 生物技术通报, 2023, 39(5): 192-204. |
| [8] | 陈晓萌, 张雪静, 张欢, 张宝江, 苏艳. 重组牛乳源金黄色葡萄球菌GapC蛋白优势B细胞抗原表位的预测和筛选[J]. 生物技术通报, 2023, 39(5): 306-313. |
| [9] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [10] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [11] | 王艺清, 王涛, 韦朝领, 戴浩民, 曹士先, 孙威江, 曾雯. 茶树SMAS基因家族的鉴定及互作分析[J]. 生物技术通报, 2023, 39(4): 246-258. |
| [12] | 杨岚, 张晨曦, 樊学伟, 王阳光, 王春秀, 李文婷. 鸡 BMP15 基因克隆、表达模式及启动子活性分析[J]. 生物技术通报, 2023, 39(4): 304-312. |
| [13] | 宋海娜, 吴心桐, 杨鲁豫, 耿喜宁, 张华敏, 宋小龙. 葱鳞葡萄孢菌诱导下韭菜RT-qPCR内参基因的筛选和验证[J]. 生物技术通报, 2023, 39(3): 101-115. |
| [14] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [15] | 陈强, 邹明康, 宋家敏, 张冲, 吴隆坤. 甜瓜LBD基因家族的鉴定和果实发育进程中的表达分析[J]. 生物技术通报, 2023, 39(3): 176-183. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||