








生物技术通报 ›› 2022, Vol. 38 ›› Issue (2): 237-244.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0611
收稿日期:2021-05-11
出版日期:2022-02-26
发布日期:2022-03-09
作者简介:贾海红,女,博士,助理研究员,研究方向:抗氧化酶的结构和功能;E-mail: 基金资助:
JIA Hai-hong( ), LI Bing-qing(
), LI Bing-qing( )
)
Received:2021-05-11
Published:2022-02-26
Online:2022-03-09
摘要:
超氧化物歧化酶(superoxide dismutase,SOD)是生物体内存在的一种抗氧化金属酶,它能够催化超氧阴离子自由基歧化生成氧(O2)和过氧化氢(H2O2),在机体氧化与抗氧化平衡中起到至关重要的作用,且与很多疾病的发生、发展密不可分。对SOD的活性调节一直是研究热点,大多数研究都集中在转录水平(基因表达)和翻译水平(酶蛋白合成)两个方面。随着研究的深入,发现蛋白质翻译后修饰(PTM)对SOD的酶活性有重要影响。近年来,研究蛋白质翻译后修饰对SOD的酶活性的影响越来越受到重视。总结了硝基化、磷酸化、S-谷胱甘肽化、糖基化、乙酰化、次磺酸化、亚磺酸化、SUMO化等几种SOD翻译后的修饰方式,讨论了修饰后对SOD酶活性的影响和生理意义,并对SOD翻译后修饰的发展及面临的挑战进行了展望,为相关疾病的研究、治疗及靶向药物的研制提供了理论基础。
贾海红, 李冰清. 超氧化物歧化酶翻译后修饰的研究进展[J]. 生物技术通报, 2022, 38(2): 237-244.
JIA Hai-hong, LI Bing-qing. Research Progress in the Post-translational Modification of Superoxide Dismutase[J]. Biotechnology Bulletin, 2022, 38(2): 237-244.
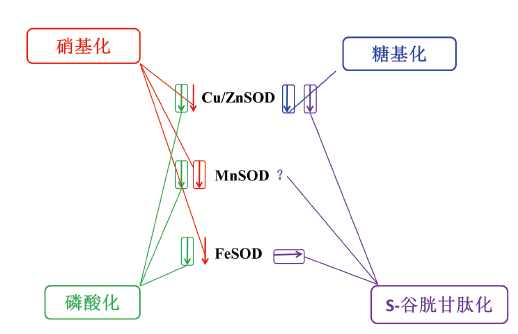
图1 翻译后修饰对SOD活性的影响 向下箭头表示SOD活性下降。方框覆盖的箭头表示修饰发生在体内。水平箭头表示修饰后的酶活性没有改变
Fig. 1 Effect of post-translational modification on SOD activity The downward arrow shows decreased SOD activity. The arrows covered with boxes indicate that the modification occurred in the body. The horizontal arrow indicates that the enzyme activity did not change after modification
| [1] |
Flohé L. Looking back at the early stages of redox biology[J]. Antioxidants, 2020, 9(12):1254.
doi: 10.3390/antiox9121254 URL |
| [2] |
Hatinguais R, Pradhan A, Brown GD, et al. Mitochondrial reactive oxygen species regulate immune responses of macrophages to Aspergillus fumigatus[J]. Front Immunol, 2021, 12:641495.
doi: 10.3389/fimmu.2021.641495 pmid: 33841423 |
| [3] |
Candas D, Li JJ. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx[J]. Antioxid Redox Signal, 2014, 20(10):1599-1617.
doi: 10.1089/ars.2013.5305 URL |
| [4] |
Sheng Y, Abreu IA, Cabelli DE, et al. Superoxide dismutases and superoxide reductases[J]. Chem Rev, 2014, 114(7):3854-3918.
doi: 10.1021/cr4005296 URL |
| [5] |
Zhao HQ, Zhang RF, Yan XY, et al. Superoxide dismutase nanozymes:an emerging star for anti-oxidation[J]. J Mater Chem B, 2021. DOI: 10. 1039/d1tb00720c.
doi: 10. 1039/d1tb00720c |
| [6] |
Perry JJ, Shin DS, Getzoff ED, et al. The structural biochemistry of the superoxide dismutases[J]. Biochim Biophys Acta, 2010, 1804(2):245-262.
doi: 10.1016/j.bbapap.2009.11.004 pmid: 19914407 |
| [7] |
Tak YJ, Park JH, Rhim H, et al. ALS-related mutant SOD1 aggregates interfere with mitophagy by sequestering the autophagy receptor optineurin[J]. Int J Mol Sci, 2020, 21(20):7525.
doi: 10.3390/ijms21207525 URL |
| [8] |
Yan Z, Spaulding HR. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise[J]. Redox Biol, 2020, 32:101508.
doi: 10.1016/j.redox.2020.101508 URL |
| [9] |
Basak D, Uddin MN, Hancock J. The role of oxidative stress and its counteractive utility in colorectal cancer(CRC)[J]. Cancers, 2020, 12(11):3336.
doi: 10.3390/cancers12113336 URL |
| [10] |
Lei Y, Wang K, Deng L, et al. Redox regulation of inflammation:old elements, a new story[J]. Med Res Rev, 2015, 35(2):306-340.
doi: 10.1002/med.21330 URL |
| [11] |
Dhar SK, Tangpong J, Chaiswing L, et al. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages[J]. Cancer Res, 2011, 71(21):6684-6695.
doi: 10.1158/0008-5472.CAN-11-1233 URL |
| [12] |
Chang KY, Hsu TI, Hsu CC, et al. Specificity protein 1-modulated superoxide dismutase 2 enhances temozolomide resistance in glioblastoma, which is independent of O6-methylguanine-DNA methyltransferase[J]. Redox Biol, 2017, 13:655-664.
doi: 10.1016/j.redox.2017.08.005 URL |
| [13] |
Dhar SK, Zhang J, Gal J, et al. FUsed in sarcoma is a novel regulator of manganese superoxide dismutase gene transcription[J]. Antioxid Redox Signal, 2014, 20(10):1550-1566.
doi: 10.1089/ars.2012.4984 URL |
| [14] | Yamakura F, Kawasaki H. Post-translational modifications of superoxide dismutase[J]. Biochim Biophys Acta, 2010, 1804(2):318-325. |
| [15] |
Xu WC, Liang JZ, Li C, et al. Pathological hydrogen peroxide triggers the fibrillization of wild-type SOD1 via sulfenic acid modification of Cys-111[J]. Cell Death Dis, 2018, 9(2):67.
doi: 10.1038/s41419-017-0106-4 URL |
| [16] |
Ahmad R, Hussain A, Ahsan H. Peroxynitrite:cellular pathology and implications in autoimmunity[J]. J Immunoassay Immunochem, 2019, 40(2):123-138.
doi: 10.1080/15321819.2019.1583109 URL |
| [17] | Bila I, Dzydzan O, Brodyak I, et al. Agmatine prevents oxidative-nitrative stress in blood leukocytes under streptozotocin-induced diabetes mellitus[J]. Open Life Sci, 2019, 14:299-310. |
| [18] |
Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration[J]. Redox Biol, 2018, 14:618-625.
doi: S2213-2317(17)30621-3 pmid: 29154193 |
| [19] |
Demicheli V, Moreno DM, Radi R. Human Mn-superoxide dismutase inactivation by peroxynitrite:a paradigm of metal-catalyzed tyrosine nitration in vitro and in vivo[J]. Metallomics, 2018, 10(5):679-695.
doi: 10.1039/c7mt00348j pmid: 29737331 |
| [20] |
Smith CD, Carson M, van der Woerd M, et al. Crystal structure of peroxynitrite-modified bovine Cu, Zn superoxide dismutase[J]. Arch Biochem Biophys, 1992, 299(2):350-355.
pmid: 1444476 |
| [21] | Yamakura F, Matsumoto T, Fujimura T, et al. Modification of a single tryptophan residue in human Cu, Zn-superoxide dismutase by peroxynitrite in the presence of bicarbonate[J]. Biochim et Biophys Acta BBA Protein Struct Mol Enzymol, 2001, 1548(1):38-46. |
| [22] |
Yamakura F, Matsumoto T, Ikeda K, et al. Nitrated and oxidized products of a single tryptophan residue in human Cu, Zn-superoxide dismutase treated with either peroxynitrite-carbon dioxide or myeloperoxidase-hydrogen peroxide-nitrite[J]. J Biochem, 2005, 138(1):57-69.
doi: 10.1093/jb/mvi095 URL |
| [23] |
Alvarez B, Demicheli V, Durán R, et al. Inactivation of human Cu, Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical[J]. Free Radic Biol Med, 2004, 37(6):813-822.
doi: 10.1016/j.freeradbiomed.2004.06.006 URL |
| [24] |
Taylor DM, Gibbs BF, Kabashi E, et al. Tryptophan 32 potentiates aggregation and cytotoxicity of a copper/zinc superoxide dismutase mutant associated with familial amyotrophic lateral sclerosis[J]. J Biol Chem, 2007, 282(22):16329-16335.
pmid: 17389599 |
| [25] |
MacMillan-Crow LA, Crow JP, Kerby JD, et al. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts[J]. PNAS, 1996, 93(21):11853-11858.
pmid: 8876227 |
| [26] |
MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues[J]. Biochemistry, 1998, 37(6):1613-1622.
pmid: 9484232 |
| [27] |
MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant(Y34F)by peroxynitrite[J]. Arch Biochem Biophys, 1999, 366(1):82-88.
pmid: 10334867 |
| [28] |
Yamakura F, Taka H, Fujimura T, et al. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine[J]. J Biol Chem, 1998, 273(23):14085-14089.
doi: 10.1074/jbc.273.23.14085 pmid: 9603906 |
| [29] |
Quijano C, Hernandez-Saavedra D, Castro L, et al. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration[J]. J Biol Chem, 2001, 276(15):11631-11638.
doi: 10.1074/jbc.M009429200 pmid: 11152462 |
| [30] |
Quint P, Reutzel R, Mikulski R, et al. Crystal structure of nitrated human manganese superoxide dismutase:mechanism of inactivation[J]. Free Radic Biol Med, 2006, 40(3):453-458.
doi: 10.1016/j.freeradbiomed.2005.08.045 URL |
| [31] |
Neumann H, Hazen JL, Weinstein J, et al. Genetically encoding protein oxidative damage[J]. J Am Chem Soc, 2008, 130(12):4028-4033.
doi: 10.1021/ja710100d pmid: 18321101 |
| [32] |
Guan Y, Hickey MJ, Borgstahl GE, et al. Crystal structure of Y34F mutant human mitochondrial manganese superoxide dismutase and the functional role of tyrosine 34[J]. Biochemistry, 1998, 37(14):4722-4730.
pmid: 9537987 |
| [33] | Larrainzar E, Urarte E, Auzmendi I, et al. Use of recombinant iron-superoxide dismutase as a marker of nitrative stress[J]. Methods Enzymol, 2008, 437:605-618. |
| [34] |
Martinez A, Peluffo G, Petruk AA, et al. Structural and molecular basis of the peroxynitrite-mediated nitration and inactivation of Trypanosoma cruzi iron-superoxide dismutases(Fe-SODs)A and B:disparate susceptibilities due to the repair of Tyr35 radical by Cys83 in Fe-SODB through intramolecular electron transfer[J]. J Biol Chem, 2014, 289(18):12760-12778.
doi: 10.1074/jbc.M113.545590 URL |
| [35] |
Csar XF, Wilson NJ, Strike P, et al. Copper/zinc superoxide dismutase is phosphorylated and modulated specifically by granulocyte-colony stimulating factor in myeloid cells[J]. Proteomics, 2001, 1(3):435-443.
pmid: 11680888 |
| [36] |
Archambaud C, Nahori MA, Pizarro-Cerda J, et al. Control of Listeria superoxide dismutase by phosphorylation[J]. J Biol Chem, 2006, 281(42):31812-31822.
doi: 10.1074/jbc.M606249200 pmid: 16905535 |
| [37] |
Voisin S, Watson DC, Tessier L, et al. The cytoplasmic phosphoproteome of the Gram-negative bacterium Campylobacter jejuni:evidence for modification by unidentified protein kinases[J]. Proteomics, 2007, 7(23):4338-4348.
doi: 10.1002/(ISSN)1615-9861 URL |
| [38] |
Bykova NV, Egsgaard H, Møller IM. Identification of 14 new phosphoproteins involved in important plant mitochondrial processes[J]. FEBS Lett, 2003, 540(1/2/3):141-146.
doi: 10.1016/S0014-5793(03)00250-3 URL |
| [39] |
Hopper RK, Carroll S, Aponte AM, et al. Mitochondrial matrix phosphoproteome:effect of extra mitochondrial calcium[J]. Biochemistry, 2006, 45(8):2524-2536.
doi: 10.1021/bi052475e URL |
| [40] |
Castellano I, Cecere F, De Vendittis A, et al. Rat mitochondrial manganese superoxide dismutase:amino acid positions involved in covalent modifications, activity, and heat stability[J]. Biopolymers, 2009, 91(12):1215-1226.
doi: 10.1002/bip.v91:12 URL |
| [41] |
Rashdan NA, Shrestha B, Pattillo CB. S-glutathionylation, friend or foe in cardiovascular health and disease[J]. Redox Biol, 2020, 37:101693.
doi: 10.1016/j.redox.2020.101693 pmid: 32912836 |
| [42] |
Banks CJ, Andersen JL. Mechanisms of SOD1 regulation by post-translational modifications[J]. Redox Biol, 2019, 26:101270.
doi: S2213-2317(19)30584-1 pmid: 31344643 |
| [43] |
Bruijn LI. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1[J]. Science, 1998, 281(5384):1851-1854.
pmid: 9743498 |
| [44] | Castellano I, Ruocco MR, Cecere F, et al. Glutathionylation of the iron superoxide dismutase from the psychrophilic Eubacterium Pseudoalteromonas haloplanktis[J]. Biochim et Biophys Acta BBA Proteins Proteom, 2008, 1784(5):816-826. |
| [45] |
Anastasiou IA, Eleftheriadou I, Tentolouris A, et al. The effect of oxidative stress and antioxidant therapies on pancreatic β-cell dysfunction:results from in vitro and in vivo studies[J]. Curr Med Chem, 2021, 28(7):1328-1346.
doi: 10.2174/0929867327666200526135642 URL |
| [46] | 徐玉英. 乙酰化对大肠杆菌SodB蛋白功能的影响研究[D]. 福州:福建农林大学, 2012. |
| Xu YY. Effect of acetylation on the iorn-containing superoxide dismutase(SodB)of Escherichia coli[D]. Fuzhou:Fujian Agriculture and Forestry University, 2012. | |
| [47] | Vidimar V, Gius D, Chakravarti D, et al. Dysfunctional MnSOD leads to redox dysregulation and activation of prosurvival AKT signaling in uterine leiomyomas[J]. Sci Adv, 2016, 2(11):e1601132. |
| [48] |
Chen YH, Zhang JY, Lin Y, et al. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS[J]. EMBO Rep, 2011, 12(6):534-541.
doi: 10.1038/embor.2011.65 URL |
| [49] | 赵作辉, 李翠玲, 王道光, 等. MnSOD乙酰化对肾透明细胞癌786-O细胞增殖、凋亡的影响[J]. 山东大学学报:医学版, 2017, 55(9):31-35. |
| Zhao ZH, Li CL, Wang DG, et al. Effect of MnSOD acetylation on the proliferation and apoptosis of clear cell renal cell carcinoma cell line 786-O[J]. J Shandong Univ:Heal Sci, 2017, 55(9):31-35. | |
| [50] |
Cohen TJ, Hwang AW, Restrepo CR, et al. An acetylation switch controls TDP-43 function and aggregation propensity[J]. Nat Commun, 2015, 6:5845.
doi: 10.1038/ncomms6845 pmid: 25556531 |
| [51] |
Niikura T, Kita Y, Abe Y. SUMO3 modification accelerates the aggregation of ALS-linked SOD1 mutants[J]. PLoS One, 2014, 9(6):e101080.
doi: 10.1371/journal.pone.0101080 URL |
| [52] | Xie GS, Zhu L, Zhang YH, et al. Sulfinylation on superoxide dismutase 1 Cys111:novel mechanism for 1-nitropyrene to promote acute reactive oxygen species generation[J]. Small Struct, 2021, 2(3):2000123. |
| [53] | Li J, Dai X, He X, et al. Effect of SOD2 methylation on mitochondrial DNA4834-bp deletion mutation in marginal cells under oxidative stress[J]. Bosn J Basic Med Sci, 2020, 20(1):70-77. |
| [1] | 周恒, 谢彦杰. 植物氧化胁迫信号应答的研究进展[J]. 生物技术通报, 2023, 39(11): 36-43. |
| [2] | 赵忠娟, 杨凯, 扈进冬, 魏艳丽, 李玲, 徐维生, 李纪顺. 盐胁迫条件下哈茨木霉ST02对椒样薄荷生长及根区土壤理化性质的影响[J]. 生物技术通报, 2022, 38(7): 224-235. |
| [3] | 王小琴, 黄银萍, 王蔚倩, 吴萍, 全舒. 含非天然氨基酸定点突变的MLL3SET蛋白表达与纯化[J]. 生物技术通报, 2022, 38(3): 194-202. |
| [4] | 武杞蔓, 田诗涵, 李昀烨, 潘英杰, 张颖. 微生物菌肥对设施黄瓜生长、产量及品质的影响[J]. 生物技术通报, 2022, 38(1): 125-131. |
| [5] | 陈晓雨, 张建, 张新亚, 唐雨婷, 邵钰晨, 罗志丹, 卢辰. 一种快速精确测定Tth DNA聚合酶活性的方法[J]. 生物技术通报, 2021, 37(5): 281-286. |
| [6] | 谢果珍, 唐圆, 吴仪, 黄莉莉, 谭周进. 七味白术散总苷对菌群失调腹泻小鼠肠道微生物及酶活性的影响[J]. 生物技术通报, 2021, 37(12): 124-131. |
| [7] | 田庚, 高伟强, 陈晓波, 张春晓. 地衣芽孢杆菌KD-1β-甘露聚糖酶定点突变提高酶活性及稳定性[J]. 生物技术通报, 2021, 37(10): 100-109. |
| [8] | 武欢, 卢珍红, 郝向阳, 王斌, 焦元辰, 杨春梅, 程春振. 非洲菊GjMnSOD基因的克隆及表达分析[J]. 生物技术通报, 2021, 37(10): 17-25. |
| [9] | 袁亮. 微生物碳酸酐酶诱导CaCO3沉淀的影响因素及生成机理[J]. 生物技术通报, 2020, 36(8): 79-68. |
| [10] | 殷金瑶, 王义, 徐良向, 朱利, 王晨, 刘文波, 缪卫国. 橡胶树白粉菌(HO-73)启动子WY172不同长度片段的克隆及表达活性分析[J]. 生物技术通报, 2020, 36(1): 29-36. |
| [11] | 胡楚霄, 雷善钰, 秦艳平, 赵奕锦, 向泉桔. 蒽对3株灵芝菌株漆酶活性及其转录表达水平的影响[J]. 生物技术通报, 2019, 35(9): 112-117. |
| [12] | 岳鑫, 杨爱江, 徐鹏, 胡霞, 朱桓毅, 包欣. 锑胁迫对斑马鱼酶活性的影响研究[J]. 生物技术通报, 2019, 35(6): 99-106. |
| [13] | 周敏雅, 陆睿, 张婷, 袁婷婷, 卢瑶瑶, 严坤宁, 袁玉国, 成勇. 重组人SOD1/3转基因山羊的制备及表达产物的检测[J]. 生物技术通报, 2019, 35(5): 85-92. |
| [14] | 郭珺, 樊芳芳, 王立革, 武爱莲, 郑军. 固碳微生物菌株的分离鉴定及其固碳能力测定[J]. 生物技术通报, 2019, 35(1): 90-97. |
| [15] | 孙雯, 郑峰. S. suis 2 中国强毒株烯醇化酶 Enolase 基因的分子克隆及蛋白生物功能研究[J]. 生物技术通报, 2017, 33(4): 222-230. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||