








生物技术通报 ›› 2022, Vol. 38 ›› Issue (6): 120-128.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1188
高聪( ), 萧楚健, 鲁帅, 王苏蓉, 袁卉华(
), 萧楚健, 鲁帅, 王苏蓉, 袁卉华( ), 曹云英(
), 曹云英( )
)
收稿日期:2021-09-14
出版日期:2022-06-26
发布日期:2022-07-11
作者简介:高聪,男,硕士研究生,研究方向:植物生理;E-mail: 基金资助:
GAO Cong( ), XIAO Chu-jian, LU Shuai, WANG Su-rong, YUAN Hui-hua(
), XIAO Chu-jian, LU Shuai, WANG Su-rong, YUAN Hui-hua( ), CAO Yun-ying(
), CAO Yun-ying( )
)
Received:2021-09-14
Published:2022-06-26
Online:2022-07-11
摘要:
明确氧化石墨烯(graphene oxide,GO)对拟南芥生长的促进作用,为纳米材料应用于农业生产提供理论依据。采用不同浓度GO的1/2 MS培养基点拟南芥种子,测定其主根长、侧根数、根系活力、超氧阴离子自由基的产生、超氧化物歧化酶活性、根系生长相关基因的表达情况。经20-200 μg/mL GO处理后,拟南芥主根长度比对照(不加GO)提高了4.6%-43.0%,在50-200 μg/mL内,与对照相比,差异达显著水平。50 μg/mL处理显著促进了侧根形成,侧根数比对照增加了约27.1%,高于或低于50 μg/mL则不利于侧根的形成。表明50 μg/mL GO对拟南芥的主根长和侧根数均存在促进作用,同时还发现该浓度可以增加拟南芥根尖的分生区和伸长区的长度,而对根尖直径和根冠长度无影响。氯化三苯基四氮唑(TTC)和四硝基氮蓝四唑(NBT)组织染色法结果表明50 μg/mL GO浓度处理提高了根系活力和超氧化物歧化酶活性及降低了超氧阴离子的产生。基因表达分析显示ADC1和DAR2表达量下调和IQM3表达量上调,从而促进了主根的伸长;ARF7、ARF19、ERFII-1和IQM3表达量上调,从而促进了侧根数量的增加。50 μg/mL GO处理可促进拟南芥根系的生长。根系活力的增加、超氧阴离子的减少及根相关基因的表达上调是GO促进根系生长的主要原因。
高聪, 萧楚健, 鲁帅, 王苏蓉, 袁卉华, 曹云英. 氧化石墨烯对拟南芥生长的促进作用[J]. 生物技术通报, 2022, 38(6): 120-128.
GAO Cong, XIAO Chu-jian, LU Shuai, WANG Su-rong, YUAN Hui-hua, CAO Yun-ying. Promoting Effect of Graphene Oxide on the Root Growth of Arabidopsis thaliana[J]. Biotechnology Bulletin, 2022, 38(6): 120-128.
| 基因 Gene | 登录号 Locus | 引物Primer(5'-3') | 片段长度 Size/bp |
|---|---|---|---|
| ERFII-1 | At4g17490 | F:TTGTAGCAGCAGAGGAGAAGAG | 107 |
| R:CCAAACACGAGTTCCACGAC | |||
| ARF7 | At5g20730 | F: GCGGCTAAAACAAGAACTCG | 107 |
| R:CGCCTCCATCTAAACCGTAA | |||
| ARF19 | At1g19220 | F:TCCAGTGCTGCAATCAGTTC | 112 |
| R:CCTCCACCATTCATGATTCC | |||
| CKX1 | At2g41510 | F:ACAGAGGAAACAAGCCTACGAC | 102 |
| R:TGACTTTGCGAGTTGGATGG | |||
| ADC1 | At2g16500 | F:TGTGGCTTCGGTTAGGTTTG | 138 |
| R:GTCTCATGTTGTTGACCAGCTG | |||
| DAR2 | At2g39830 | F:AGCATGAGTTCTCTCTGTCAGG | 142 |
| R:CCAAAACGGATGGCATCGATAC | |||
| IQM3 | At3g52870 | F:GGAGGGTGATTGTTGACAATGG | 120 |
| R:AGGTTCACTGCGTTCTCTCTG | |||
| ACT2 | At3g18780 | F:GCCATCCAAGCTGTTCTCTC | 270 |
| R:GCTCGTAGTCAACAGCAACAA |
表1 荧光定量表达分析用的引物序列
Table 1 Primer sequences for fluorescence quantitative expression analysis
| 基因 Gene | 登录号 Locus | 引物Primer(5'-3') | 片段长度 Size/bp |
|---|---|---|---|
| ERFII-1 | At4g17490 | F:TTGTAGCAGCAGAGGAGAAGAG | 107 |
| R:CCAAACACGAGTTCCACGAC | |||
| ARF7 | At5g20730 | F: GCGGCTAAAACAAGAACTCG | 107 |
| R:CGCCTCCATCTAAACCGTAA | |||
| ARF19 | At1g19220 | F:TCCAGTGCTGCAATCAGTTC | 112 |
| R:CCTCCACCATTCATGATTCC | |||
| CKX1 | At2g41510 | F:ACAGAGGAAACAAGCCTACGAC | 102 |
| R:TGACTTTGCGAGTTGGATGG | |||
| ADC1 | At2g16500 | F:TGTGGCTTCGGTTAGGTTTG | 138 |
| R:GTCTCATGTTGTTGACCAGCTG | |||
| DAR2 | At2g39830 | F:AGCATGAGTTCTCTCTGTCAGG | 142 |
| R:CCAAAACGGATGGCATCGATAC | |||
| IQM3 | At3g52870 | F:GGAGGGTGATTGTTGACAATGG | 120 |
| R:AGGTTCACTGCGTTCTCTCTG | |||
| ACT2 | At3g18780 | F:GCCATCCAAGCTGTTCTCTC | 270 |
| R:GCTCGTAGTCAACAGCAACAA |
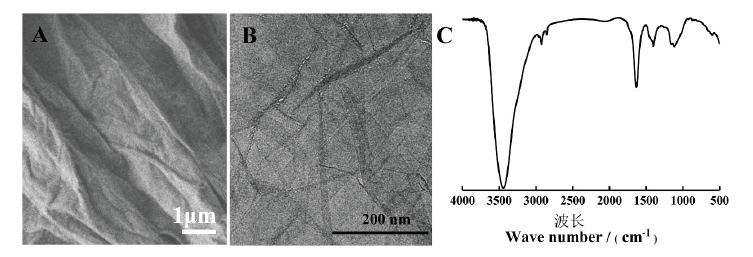
图1 GO的表征图 A:正常情况下GO的扫描电镜图;B:超声处理后GO的透射电镜图;C:GO的傅里叶红外光谱图
Fig.1 Characterization of GO A:SEM image of GO under normal condition. B:TEM image of GO after ultrasonic treatment. C:FTIR image of GO

图2 不同浓度GO处理对拟南芥主根生长的影响 A:拟南芥生长6 d后的主根代表图;B:GO对主根长的影响;n=30;CK、20、50、100和200分别指0、20、50、100和200 μg/mL GO 处理;柱上误差线为标准差,不同小写字母表示处理间差异显著(P≤0.05);试验重复3次。下同
Fig.2 Effects of different concentrations of GO on the tap-root growth of A. thaliana A:Representative photograph of A. thaliana taproot growth after 6 d of planting. B:Effects of GO on the taproot length. n=30. CK,20,50,100 and 200 refer to 0,20,50,100 and 200 μg/mL GO treatment,respectively. The error line on the column is the standard deviation,and the different small letters above the bars mean significant difference among treatments(P≤0.05). The experiment was repeated three times. The same below

图3 不同浓度GO处理对拟南芥侧根生长的影响 A:拟南芥生长11 d后的侧根代表图;B:GO对侧根数形成的影响;n=15;CK、20、50、100和200分别指0、20、50、100和200 μg/mL GO 处理
Fig.3 Effects of different concentrations of GO on the lat-eral root growth of A. thaliana A:Representative photograph of A. thaliana lateral root growth after 11 d of planting. B:Effects of GO on the lateral root. n=15. CK,20,50,100 and 200 refer to 0,20,50,100 and 200 μg/mL GO treatment,respectively
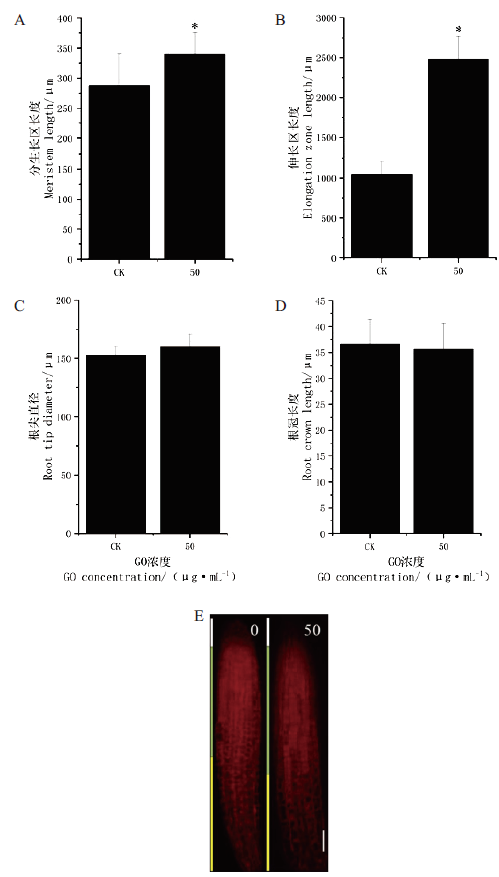
图4 不同浓度GO对拟南芥主根各部位的影响 A:拟南芥生长4 d后主根的分生区长度;B:拟南芥生长4 d后主根的伸长区长度;C:拟南芥生长4 d后主根根尖的直径;D:拟南芥生长4 d后主根根冠的大小;E:拟南芥生长4 d后主根的共聚焦显微镜图,n=30。0或CK和50分别指0和50 μg/mL GO 处理。星号表示处理间差异显著(P≤0.05)。下同。标注尺度为5 μm
Fig.4 Effects of different concentrations of GO on the different parts of taproot of A. thaliana A:Meristem zone length of taproot of A. thaliana growth after 4 d of planting. B:Extension zone length of taproot of A. thaliana growth after 4 d of planting. C:Diameter of taproot tip of A. thaliana growth after 4 d of planting. D:Length of taproot cap of A. thaliana growth after 4 d of planting. E:Representative photograph of A thaliana growth after 4 d of planting by confocal microscope staining with PID,n=30. 0 or CK and 50 refer to 0 and 50 μg/mL GO treatment,respectively. Asterisk above the bars indicate significant difference between the two treatments(P≤0.05). The same below. Scale bar is 5 μm
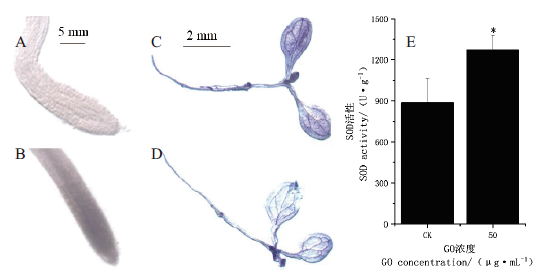
图5 不同浓度GO处理后拟南芥的组织染色及SOD活性的定量分析 A和C:对照CK,即0 μg/mL浓度GO处理;B和D:50 μg/mL浓度GO处理;A和B:TTC染色拟南芥主根根尖的代表图,标注尺度为5 mm;C和D:NBT染色拟南芥幼苗的代表图,标注尺度为2 mm;E:SOD活性的定量分析
Fig.5 Tissue staining and SOD activity quantitative analysis of A. thaliana treated with different concentrations of GO A and C:0 μg/mL GO treatment as control. B and D:Treated with GO in 50 μg/mL concentration. A and B:Representative photograph of A. thaliana taproot tip with staining with TTC,scale bar is 5 mm. C and D:Representative photograph of A. thaliana seedlings with staining with NBT,scale bar is 2 mm. E:Quantitative analysis of SOD activity
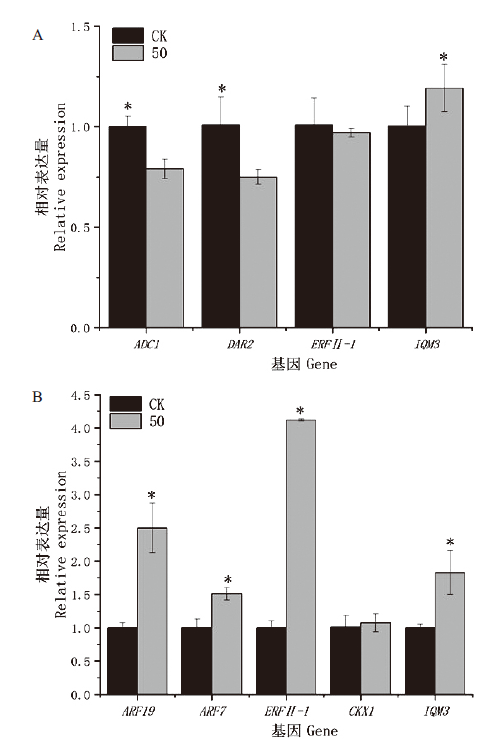
图6 不同浓度GO处理对拟南芥根生长相关基因的表达 A:调控主根相关基因的表达;B:调控侧根相关基因的表达;CK和50分别指0和50 μg/mL GO处理
Fig.6 Effects of different concentrations of GO on the gene expressions related to root growth in A. thaliana A:Regulation of root length related gene expression. B:Regulation of lateral root related gene expression. CK and 50 refer to 0 and 50 μg/mL GO treatment
| [1] |
Ruffini Castiglione M, Giorgetti L, Geri C, et al. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L[J]. J Nanoparticle Res, 2011, 13(6):2443-2449.
doi: 10.1007/s11051-010-0135-8 URL |
| [2] |
Deng YQ, White JC, Xing BS. Interactions between engineered nanomaterials and agricultural crops:implications for food safety[J]. J Zhejiang Univ Sci A, 2014, 15(8):552-572.
doi: 10.1631/jzus.A1400165 URL |
| [3] |
Syu YY, Hung JH, Chen JC, et al. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression[J]. Plant Physiol Biochem, 2014, 83:57-64.
doi: 10.1016/j.plaphy.2014.07.010 URL |
| [4] |
Sun XD, Yuan XZ, Jia Y, et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana[J]. Nature Nanotechnology, 2020, 15(9):755-760.
doi: 10.1038/s41565-020-0707-4 URL |
| [5] |
Kurepa J, Paunesku T, Vogt S, et al. Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana[J]. Nano Lett, 2010, 10(7):2296-2302.
doi: 10.1021/nl903518f URL |
| [6] | 李海涛, 沈健民, 陈骏. 石墨烯在农业中应用前景浅析[J]. 江苏农机化, 2020, (5):20-23. |
| Li HT, Shen JM, Chen J. Application prospect of graphene in agriculture[J]. Jiangsu Agric Mech, 2020, (5):20-23. | |
| [7] | 陈建伟, 余海娟, 李亮, 等. 纳米氧化钛颗粒对镉胁迫下玉米生长受抑的缓解效应[J]. 植物生理学报, 2015, 51(10):1633-1639. |
| Chen JW, Yu HJ, Li L, et al. Titanium oxide nanoparticles alleviate growth inhibition of maize under cadmium stress[J]. Plant Physiol J, 2015, 51(10):1633-1639. | |
| [8] | 吕继涛, 张淑贞. 人工纳米材料与植物的相互作用:植物毒性、吸收和传输[J]. 化学进展, 2013, 25(1):156-163. |
| Lv JT, Zhang SZ. Interactions between manufactured nanomaterials and plants:phytotoxicity, uptake and translocation[J]. Prog Chem, 2013, 25(1):156-163. | |
| [9] |
Park S, Choi KS, Kim S, et al. Graphene oxide-assisted promotion of plant growth and stability[J]. Nanomaterials, 2020, 10(4):758.
doi: 10.3390/nano10040758 URL |
| [10] | Cao Y, Zhang Q, Chen Y, et al. Identification of differential expression genes in leaves of rice(Oryza sativa L.)in response to heat stress by cDNA-AFLP analysis[J]. Biomed Res Int, 2013, 2013:576189. |
| [11] | 刘尚杰. 石墨烯对水稻种子萌发及幼苗生长的影响[D]. 荆州: 长江大学, 2013. |
| Liu SJ. The effects of graphene on the germination and seedling growth in rice[D]. Jingzhou: Yangtze University, 2013. | |
| [12] | 张鹏. 石墨烯对植物的毒性效应及机制研究[D]. 杭州: 浙江工商大学, 2015. |
| Zhang P. Mechanisms of graphene toxicity to plants[D]. Hangzhou: Zhejiang Gongshang University, 2015. | |
| [13] |
Paramo LA, Feregrino-Pérez AA, Guevara R, et al. Nanoparticles in agroindustry:applications, toxicity, challenges, and trends[J]. Nanomaterials, 2020, 10(9):1654.
doi: 10.3390/nano10091654 URL |
| [14] |
Zhang CL, Jiang HS, Gu SP, et al. Combination analysis of the physiology and transcriptome provides insights into the mechanism of silver nanoparticles phytotoxicity[J]. Environ Pollut, 2019, 252(b):1539-1549.
doi: 10.1016/j.envpol.2019.06.032 URL |
| [15] |
Maruri-López I, Jiménez-Bremont JF. Hetero- and homodimerization of Arabidopsis thaliana arginine decarboxylase AtADC1 and AtADC2[J]. Biochem Biophys Res Commun, 2017, 484(3):508-513.
doi: 10.1016/j.bbrc.2017.01.083 URL |
| [16] | Peng Y, Chen L, Lu Y, et al. DAR2 Acts as an important node connecting cytokinin, auxin, SHY2 and PLT1/2 in root meristem size control[J]. Plant Signal Behav, 2013, 8(6):e24226. |
| [17] |
Okushima Y, Overvoorde PJ, ARIMA K, et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana:unique and overlapping functions of ARF7 and ARF19[J]. Plant Cell, 2005, 17(2):444-463.
pmid: 15659631 |
| [18] |
Raspor M, Motyka V, Ninković S, et al. Overexpressing AtCKX1 in potato plants grown in vitro:the effects on cytokinin composition and tuberization[J]. J Plant Growth Regul, 2021, 40(1):37-47.
doi: 10.1007/s00344-020-10080-w URL |
| [19] | 田特. 拟南芥AP2/ERF转录因子ERFⅡ-1和ERFⅡ-2调控植物侧根生长发育的研究[D]. 济南: 山东大学, 2019. |
| Tian T. AP2/ERF transcription factors ERFⅡ-1 and ERFⅡ-2 of Arabidopsis regulate plant lateral roots growth and development[D]. Jinan: Shandong University, 2019. | |
| [20] | 徐浩, 冯奕嘉, 范甜, 等. 拟南芥IQM3基因突变减少幼苗的侧根数量和增加主根长度[J]. 植物生理学报, 2019, 55(5):629-634. |
| Xu H, Feng YJ, Fan T, et al. Disruption of IQM3 reduces the number of lateral roots and increases the length of primary root in Arabidopsis seedlings[J]. Plant Physiol J, 2019, 55(5):629-634. | |
| [21] |
Ratajczak K, Stobiecka M. Ternary interactions and energy transfer between fluorescein isothiocyanate, adenosine triphosphate, and graphene oxide nanocarriers[J]. J Phys Chem B, 2017, 121(28):6822-6830.
doi: 10.1021/acs.jpcb.7b04295 URL |
| [22] |
Ratajczak K, Krazinski B, Kowalczyk A, et al. Optical biosensing system for the detection of survivin mRNA in colorectal cancer cells using a graphene oxide carrier-bound oligonucleotide molecular beacon[J]. Nanomaterials, 2018, 8(7):510.
doi: 10.3390/nano8070510 URL |
| [1] | 杨志晓, 侯骞, 刘国权, 卢志刚, 曹毅, 芶剑渝, 王轶, 林英超. 不同抗性烟草品系Rubisco及其活化酶对赤星病胁迫的响应[J]. 生物技术通报, 2023, 39(9): 202-212. |
| [2] | 李宇, 李素贞, 陈茹梅, 卢海强. 植物bHLH转录因子调控铁稳态的研究进展[J]. 生物技术通报, 2023, 39(7): 26-36. |
| [3] | 李帜奇, 袁月, 苗荣庆, 庞秋颖, 张爱琴. 盐胁迫盐芥和拟南芥褪黑素含量及合成相关基因表达模式分析[J]. 生物技术通报, 2023, 39(5): 142-151. |
| [4] | 刘奎, 李兴芬, 杨沛欣, 仲昭晨, 曹一博, 张凌云. 青杄转录共激活因子PwMBF1c的功能研究与验证[J]. 生物技术通报, 2023, 39(5): 205-216. |
| [5] | 赖瑞联, 冯新, 高敏霞, 路喻丹, 刘晓驰, 吴如健, 陈义挺. 猕猴桃过氧化氢酶基因家族全基因组鉴定与表达分析[J]. 生物技术通报, 2023, 39(4): 136-147. |
| [6] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [7] | 陈强, 邹明康, 宋家敏, 张冲, 吴隆坤. 甜瓜LBD基因家族的鉴定和果实发育进程中的表达分析[J]. 生物技术通报, 2023, 39(3): 176-183. |
| [8] | 崔吉洁, 蔡文波, 庄庆辉, 高爱平, 黄建峰, 陈亚辉, 宋志忠. 杧果Fe-S簇装配基因MiISU1的生物学功能[J]. 生物技术通报, 2023, 39(2): 139-146. |
| [9] | 姚晓文, 梁晓, 陈青, 伍春玲, 刘迎, 刘小强, 税军, 乔阳, 毛奕茗, 陈银华, 张银东. 二斑叶螨抗性木薯木质素合成途径基因表达特性研究[J]. 生物技术通报, 2023, 39(2): 161-171. |
| [10] | 李彦霞, 王晋鹏, 冯芬, 包斌武, 董益闻, 王兴平, 罗仍卓么. 大肠杆菌型奶牛乳房炎对产奶性状相关基因表达的影响[J]. 生物技术通报, 2023, 39(2): 274-282. |
| [11] | 冯策婷, 江律, 刘鑫颖, 罗乐, 潘会堂, 张启翔, 于超. 单叶蔷薇NAC基因家族鉴定及干旱胁迫响应分析[J]. 生物技术通报, 2023, 39(11): 283-296. |
| [12] | 鄢梦雨, 韦晓薇, 曹婧, 兰海燕. 异子蓬SabHLH169基因的克隆及抗旱功能分析[J]. 生物技术通报, 2023, 39(11): 328-339. |
| [13] | 吴柏增, 何琪, 姚方杰, 赵梦然. 糙皮侧耳乳酸脱氢酶鉴定及其菌丝高温胁迫下表达特征分析[J]. 生物技术通报, 2023, 39(11): 350-359. |
| [14] | 阮航, 多浩源, 范文艳, 吕清晗, 姜述君, 朱生伟. AtERF49在拟南芥应答盐碱胁迫中的作用[J]. 生物技术通报, 2023, 39(1): 150-156. |
| [15] | 林蓉, 郑月萍, 徐雪珍, 李丹丹, 郑志富. 拟南芥ACOL8基因在乙烯合成与响应中的功能分析[J]. 生物技术通报, 2023, 39(1): 157-165. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||