








生物技术通报 ›› 2022, Vol. 38 ›› Issue (11): 90-96.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0087
钟菁( ), 孙玲玲, 张姝, 蒙园, 支怡飞, 涂黎晴, 徐天鹏, 濮黎萍, 陆阳清(
), 孙玲玲, 张姝, 蒙园, 支怡飞, 涂黎晴, 徐天鹏, 濮黎萍, 陆阳清( )
)
收稿日期:2022-01-19
出版日期:2022-11-26
发布日期:2022-12-01
作者简介:钟菁,女,硕士研究生,研究方向:动物繁殖新技术;E-mail:基金资助:
ZHONG Jing( ), SUN Ling-ling, ZHANG Shu, MENG Yuan, ZHI Yi-fei, TU Li-qing, XU Tian-peng, PU Li-ping, LU Yang-qing(
), SUN Ling-ling, ZHANG Shu, MENG Yuan, ZHI Yi-fei, TU Li-qing, XU Tian-peng, PU Li-ping, LU Yang-qing( )
)
Received:2022-01-19
Published:2022-11-26
Online:2022-12-01
摘要:
采用CRISPR/Cas9技术敲除DF-1的黑色素瘤分化相关基因5(melanoma-differentiation-associated gene 5,Mda5),探索Mda5基因对新城疫病毒(Newcastle disease virus,NDV)和传染性法氏囊病毒(infectious bursal disease virus,IBDV)复制的影响。通过CRISPR/Cas9技术构建Mda5敲除的DF-1细胞系;实时荧光定量PCR和免疫荧光法检测病毒感染Mda5敲除细胞后病毒RNA水平、细胞病变情况及抗病毒相关基因mRNA水平等。获得Mda5基因敲除的DF-1细胞;与对照组细胞相比,细胞形态、贴壁能力和增殖能力均无显著性差异;与对照组细胞相比,Mda5基因敲除的DF-1细胞感染IBDV后,IFN-β和PKR表达显著性下调,CH25H的表达和病毒复制水平均无显著性差异;感染NDV后,IFN-β表达和病毒复制水平显著性下调,CH25H表达量显著性上调,PKR表达无显著性差异。CRISPR/Cas9技术建立Mda5敲除的DF-1中,IFN-β虽然显著应答IBDV和NDV感染,但不能显著抑制病毒的复制,说明Mda5并非抗病毒复制的关键模式识别受体。
钟菁, 孙玲玲, 张姝, 蒙园, 支怡飞, 涂黎晴, 徐天鹏, 濮黎萍, 陆阳清. 应用CRISPR/Cas9技术敲除Mda5基因对新城疫及传染性法氏囊病毒复制的影响[J]. 生物技术通报, 2022, 38(11): 90-96.
ZHONG Jing, SUN Ling-ling, ZHANG Shu, MENG Yuan, ZHI Yi-fei, TU Li-qing, XU Tian-peng, PU Li-ping, LU Yang-qing. Effect of Knocking Out the Mda5 Gene by CRISPR/Cas9 Technology on the Replication of Newcastle Disease and Infectious Bursal Virus[J]. Biotechnology Bulletin, 2022, 38(11): 90-96.
| 引物名称Primer name | 序列Sequence(5'-3') |
|---|---|
| sg RNA1 | F:CACCGTGTAGAGGAAGCGCTCGTCT R:AAACAGACGAGCGCTTCCTCTACAC |
| sg RNA2 | F:CACCGCTGCTATGCGCCGTGGAACG R:AAACCGTTCCACGGCGCATAGCAGC |
| sg RNA3 | F:CACCGTCGCGGCGGCCACGTTCCA |
| R:AAACTGGAACGTGGCCGCCGCGAC | |
| sg RNA4 | F:CACCGTGAACCATCCGGGGTCGCGG R:AAACCCGCGACCCCGGATGGTTCAC |
| sg RNA5 | F:CACCgCAGTGAACCATCCGGGGTCG R:AAACCGACCCCGGATGGTTCACTGC |
| sg RNA6 | F:CACCGCTGGGGTTCACGTAGCAAG R:AAACCTTGCTACGTGAACCCCAGC |
表1 sgRNA1-6引物序列
Table 1 sgRNA1-6 primer sequences
| 引物名称Primer name | 序列Sequence(5'-3') |
|---|---|
| sg RNA1 | F:CACCGTGTAGAGGAAGCGCTCGTCT R:AAACAGACGAGCGCTTCCTCTACAC |
| sg RNA2 | F:CACCGCTGCTATGCGCCGTGGAACG R:AAACCGTTCCACGGCGCATAGCAGC |
| sg RNA3 | F:CACCGTCGCGGCGGCCACGTTCCA |
| R:AAACTGGAACGTGGCCGCCGCGAC | |
| sg RNA4 | F:CACCGTGAACCATCCGGGGTCGCGG R:AAACCCGCGACCCCGGATGGTTCAC |
| sg RNA5 | F:CACCgCAGTGAACCATCCGGGGTCG R:AAACCGACCCCGGATGGTTCACTGC |
| sg RNA6 | F:CACCGCTGGGGTTCACGTAGCAAG R:AAACCTTGCTACGTGAACCCCAGC |
| 引物名称Primer name | 序列Sequence(5'-3') |
|---|---|
| IFN-β qPCR F | TGCAACCATCTTCGTCACCA |
| IFN-β qPCR R | GGAGGTGGAGCCGTATTCTG |
| PKR qPCR F | CCTATGCAATCAAACGAGTTGAG |
| PKR qPCR R | GTCCCTTCCCAGCTGCAATA |
| qPCR(CH25H)F qPCR(CH25H)R | ATCCATTCCTCCTCGGATGC AAAGGCACAAGTCGGTGAGT |
表2 qPCR引物序列
Table 2 qPCR primer sequences
| 引物名称Primer name | 序列Sequence(5'-3') |
|---|---|
| IFN-β qPCR F | TGCAACCATCTTCGTCACCA |
| IFN-β qPCR R | GGAGGTGGAGCCGTATTCTG |
| PKR qPCR F | CCTATGCAATCAAACGAGTTGAG |
| PKR qPCR R | GTCCCTTCCCAGCTGCAATA |
| qPCR(CH25H)F qPCR(CH25H)R | ATCCATTCCTCCTCGGATGC AAAGGCACAAGTCGGTGAGT |
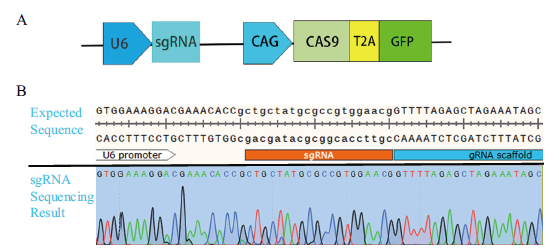
图1 重组质粒构建结果 A:sgRNA骨架;B:sgRNA expression vector测序骨架
Fig. 1 Results of recombinant plasmid construction A:sgRNA skeleton;B:sgRNA expression vector sequencing framework
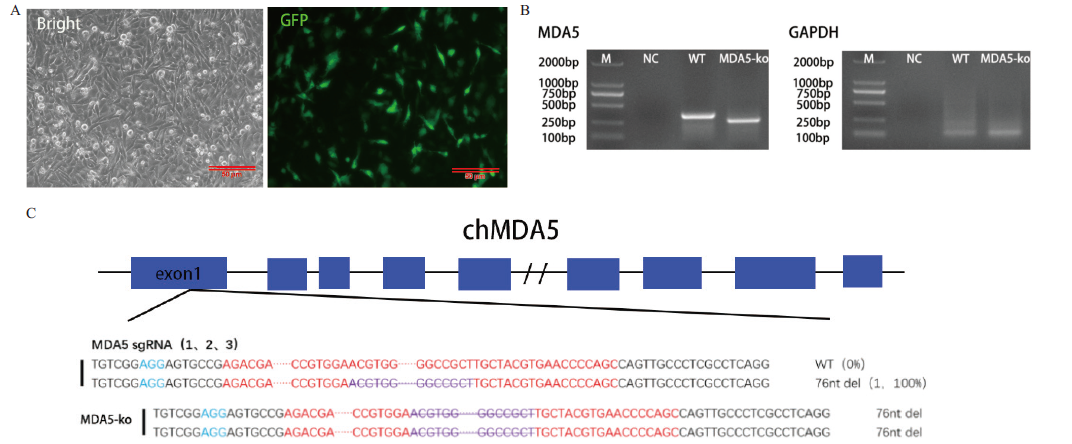
图2 MDA5 KO细胞系建立 A:MDA5 KO转染后24 h;B:MDA5 KO细胞PCR鉴定结果图;C:MDA5 KO细胞PCR产物测序结果图
Fig. 2 Establishment of MDA5 KO cell line A:24 h after MDA5 KO transfection;B:PCR identification results graph of MDA5 KO cells;C:sequencing results of PCR products from MDA5 KO cells
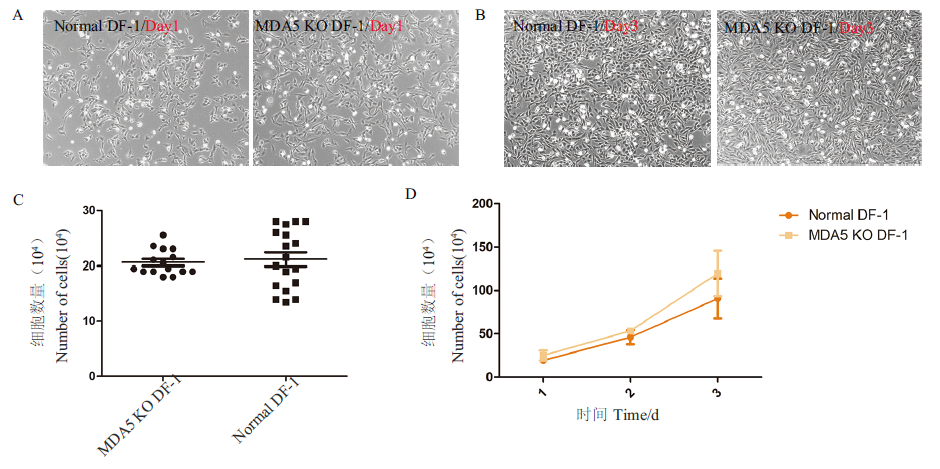
图3 MDA5 KO与普通DF-1形态及生长速度比较 A:普通DF-1和MDA5 KO解冻后24 h;B:普通DF-1和MDA5 KO解冻后72 h;C:普通DF-1和MDA5 KO解冻后24 h计数结果;D:普通DF-1和MDA5 KO解冻后1-3 d生长曲线
Fig. 3 Comparison of morphology and growth rate between MDA5 KO and ordinary DF-1 A:24 h after conventional DF-1 and MDA5 KO were thawed;B:72 h after conventional DF-1 and MDA5 KO were thawed;C:count results of conventional DF-1 and MDA5 KO at 24 h after thawing;D:growth curves of ordinary DF-1 and MDA5 KO 1-3 d after thawing
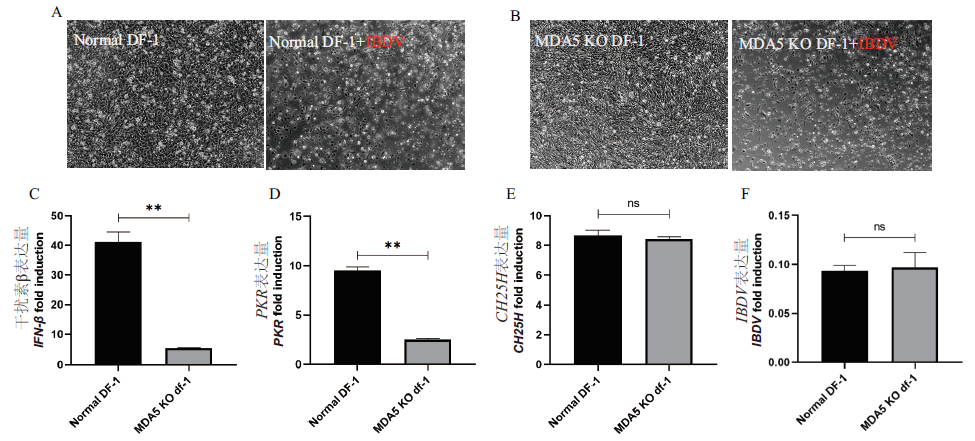
图4 IBDV感染MDA5 KO后结果 A:普通DF-1感染IBDV前后对比;B:MDA5 KO感染IBDV前后对比;C:IBDV感染后干扰素IFN-β基因检测结果;D:IBDV感染后PKR基因检测结果;E:IBDV感染后CH25H基因检测结果;F:IBDV感染后IBDV基因检测结果
Fig. 4 Results of IBDV infection with MDA5 KO A:Comparison of normal DF-1 before and after infection with IBDV.B:Comparison of MDA5 KO before and after infection with IBDV. C:Results of IFN-β gene detection after IBDV infection. D:PKR gene test results after IBDV infection. E:Results of CH25H gene detection after IBDV infection. F:Results of IBDV gene detection after IBDV infection

图5 NDV感染MDA5 KO后结果 A:普通DF-1感染NDV前后对比;B:MDA5 KO感染NDV前后对比;C:NDV感染后干扰素IFN-β基因检测结果;D:NDV感染后PKR基因检测结果;E:NDV感染后CH25H基因检测结果;F:NDV感染后NDV基因检测结果
Fig. 5 Results of NDV infection with MDA5 KO A:Comparison of normal DF-1 before and after infection with NDV. B:Comparison of MDA5 KO before and after infection with NDV. C:Results of IFN-β gene detection after NDV infection. D:PKR gene test results after NDV infection. E:Results of CH25H gene detection after NDV infection. F:Results of NDV gene detection after NDV infection
| [1] | 文开. 鸡新城疫的病原学、流行病学及综合防控措施[J]. 中国畜禽种业, 2021, 17(9):180-181. |
| Wen K. Etiology, epidemiology and comprehensive prevention and control measures of Newcastle disease in chicken[J]. Chin Livest Poult Breed, 2021, 17(9):180-181. | |
| [2] | 覃周岚. 鸡传染性法氏囊炎病因及防控[J]. 中国畜禽种业, 2017, 13(3):159. |
| Qin ZL. Etiology and prevention and control of infectious bursal disease in chickens[J]. Chin Livest Poult Breed, 2017, 13(3):159. | |
| [3] |
Zhang WX, Zuo EW, He Y, et al. Promoter structures and differential responses to viral and non-viral inducers of chicken melanoma differentiation-associated gene 5[J]. Mol Immunol, 2016, 76:1-6.
doi: 10.1016/j.molimm.2016.06.006 URL |
| [4] |
de Oliveira Mann CC, Hornung V. Molecular mechanisms of nonself nucleic acid recognition by the innate immune system[J]. Eur J Immunol, 2021, 51(8):1897-1910.
doi: 10.1002/eji.202049116 URL |
| [5] | Yin X, Riva L, Pu Y, et al. MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells[J]. Cell Rep, 2021, 34(2):108628. |
| [6] |
Lee CC, Wu CC, Lin TL. Characterization of chicken melanoma differentiation-associated gene 5(MDA5)from alternative translation initiation[J]. Comp Immunol Microbiol Infect Dis, 2012, 35(4):335-343.
doi: 10.1016/j.cimid.2012.02.004 URL |
| [7] |
Zhang WX, Zuo EW, He Y, et al. Promoter structures and differential responses to viral and non-viral inducers of chicken melanoma differentiation-associated gene 5[J]. Mol Immunol, 2016, 76:1-6.
doi: 10.1016/j.molimm.2016.06.006 URL |
| [8] |
Karpala AJ, Stewart C, McKay J, et al. Characterization of chicken Mda5 activity:regulation of IFN-β in the absence of RIG-I functionality[J]. J Immunol, 2011, 186(9):5397-5405.
doi: 10.4049/jimmunol.1003712 pmid: 21444763 |
| [9] |
Kint J, Fernandez-Gutierrez M, Maier HJ, et al. Activation of the chicken type I interferon response by infectious bronchitis coronavirus[J]. J Virol, 2015, 89(2):1156-1167.
doi: 10.1128/JVI.02671-14 pmid: 25378498 |
| [10] |
Xiang B, Zhu WX, Li YL, et al. Immune responses of mature chicken bone-marrow-derived dendritic cells infected with Newcastle disease virus strains with differing pathogenicity[J]. Arch Virol, 2018, 163(6):1407-1417.
doi: 10.1007/s00705-018-3745-6 pmid: 29397456 |
| [11] | 韩青松. 鸡MDA5调控新城疫病毒免疫反应机制研究[D]. 杨凌: 西北农林科技大学, 2019. |
| Han QS. Study on the mechanism of chicken MDA5 regulating chicken immune response to Newcastle disease virus[D]. Yangling: Northwest A & F University, 2019. | |
| [12] |
Lee CC, Wu CC, Lin TL. Chicken melanoma differentiation-associated gene 5(MDA5)recognizes infectious bursal disease virus infection and triggers MDA5-related innate immunity[J]. Arch Virol, 2014, 159(7):1671-1686.
doi: 10.1007/s00705-014-1983-9 URL |
| [13] | 张蕾, 张海波, 章敬旗, 等. CRISPR/Cas9系统在家禽中应用研究进展[J]. 中国畜牧兽医, 2020, 47(1):140-147. |
| Zhang L, Zhang HB, Zhang JQ, et al. Research progress on application of CRISPR/Cas9 system in poultry[J]. China Animal Husb Vet Med, 2020, 47(1):140-147. | |
| [14] |
Cheng YQ, Lun MX, Liu YX, et al. CRISPR/Cas9-mediated chicken TBK1 gene knockout and its essential role in STING-mediated IFN-β induction in chicken cells[J]. Front Immunol, 2019, 9:3010.
doi: 10.3389/fimmu.2018.03010 URL |
| [15] | 刘欢欢, 谢丽君, 邵志勇, 等. MDA5在先天性免疫抗病毒作用中的研究进展[J]. 中国畜牧兽医, 2015, 42(1):230-233. |
| Liu HH, Xie LJ, Shao ZY, et al. Research progress on antiviral effect of MDA5 in innate immunity[J]. China Animal Husb Vet Med, 2015, 42(1):230-233. | |
| [16] |
Liao ZH, Dai ZK, Cai CY, et al. Knockout of Atg5 inhibits proliferation and promotes apoptosis of DF-1 cells[J]. In Vitro Cell Dev Biol Anim, 2019, 55(5):341-348.
doi: 10.1007/s11626-019-00342-7 URL |
| [17] |
Kasumba DM, Grandvaux N. Therapeutic targeting of RIG-I and MDA5 might not lead to the same Rome[J]. Trends Pharmacol Sci, 2019, 40(2):116-127.
doi: S0165-6147(18)30228-1 pmid: 30606502 |
| [18] | Barber Megan R W, Aldridge Jerry R, Webster Robert G, Magor Katharine E. Association of RIG-I with innate immunity of ducks to influenza.[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(13). |
| [19] |
Thompson MR, Sharma S, Atianand M, et al. Interferon γ-inducible protein(IFI)16 transcriptionally regulates type i interferons and other interferon-stimulated genes and controls the interferon response to both DNA and RNA viruses[J]. J Biol Chem, 2014, 289(34):23568-23581.
doi: 10.1074/jbc.M114.554147 pmid: 25002588 |
| [20] | 翟景波, 吕昌龙. RLRs家族中RIG-I和MDA-5的研究进展[J]. 微生物学免疫学进展, 2017, 45(1):54-59. |
| Zhai J. B.; Lv, C. L. Progress on study of RIG-I and MDA5 in RLRs family[J]Proceedings in Microbiology Immunology, 2017, 45(1):54-59. | |
| [21] | Carty M, Guy C, Bowie AG. Detection of viral infections by innate immunity[J]. Biochem Pharmacol, 2021, 183:114316. |
| [22] | 王振兴. 黄芪甲苷经AMPK/mTOR信号通路调控细胞自噬与凋亡保护PM2. 5诱导急性肺损伤的机制研究[D]. 成都: 成都中医药大学, 2019. |
| Wang ZX. Astragaloside Ⅳ regulates autophagy and apoptosis in PM2. 5-induced acute lung injury via AMPK/mTOR pathway[D]. Chengdu: Chengdu University of TCM, 2019. | |
| [23] | 孔正茹. 禽网状内皮组织增生病病毒诱导宿主天然免疫反应及感染法氏囊转录组学的研究[D]. 扬州: 扬州大学, 2019. |
| Kong ZR. Host innate immune response induced by avian reticuloendotheliosis virus and transcriptomics study of virus infected Bursa[D]. Yangzhou: Yangzhou University, 2019. |
| [1] | 陈小玲, 廖东庆, 黄尚飞, 陈英, 芦志龙, 陈东. 利用CRISPR/Cas9系统改造酿酒酵母的研究进展[J]. 生物技术通报, 2023, 39(8): 148-158. |
| [2] | 杨玉梅, 张坤晓. 应用CRISPR/Cas9技术建立ERK激酶相分离荧光探针定点整合的稳定细胞株[J]. 生物技术通报, 2023, 39(8): 159-164. |
| [3] | 施炜涛, 姚春鹏, 魏文康, 王蕾, 房元杰, 仝钰洁, 马晓姣, 蒋文, 张晓爱, 邵伟. 利用CRISPR/Cas9技术构建MDH2敲除细胞株及抗呕吐毒素效应研究[J]. 生物技术通报, 2023, 39(7): 307-315. |
| [4] | 刘晓燕, 祝振亮, 史广宇, 华梓宇, 杨晨, 张涌, 刘军. 乳腺生物反应器的表达优化策略[J]. 生物技术通报, 2023, 39(5): 77-91. |
| [5] | 程静雯, 曹磊, 张艳敏, 叶倩, 陈敏, 谭文松, 赵亮. CHO细胞多基因工程改造策略的建立及应用[J]. 生物技术通报, 2023, 39(2): 283-291. |
| [6] | 黄文莉, 李香香, 周炆婷, 罗莎, 姚维嘉, 马杰, 张芬, 沈钰森, 顾宏辉, 王建升, 孙勃. 利用CRISPR/Cas9技术靶向编辑青花菜BoZDS[J]. 生物技术通报, 2023, 39(2): 80-87. |
| [7] | 王兵, 赵会纳, 余婧, 陈杰, 骆梅, 雷波. 利用CRISPR/Cas9系统研究REVOLUTA参与烟草叶芽发育的调控[J]. 生物技术通报, 2023, 39(10): 197-208. |
| [8] | 李双喜, 华进联. 抗猪繁殖与呼吸障碍综合征基因编辑猪研究进展[J]. 生物技术通报, 2023, 39(10): 50-57. |
| [9] | 林蓉, 郑月萍, 徐雪珍, 李丹丹, 郑志富. 拟南芥ACOL8基因在乙烯合成与响应中的功能分析[J]. 生物技术通报, 2023, 39(1): 157-165. |
| [10] | 史亚楠, 王德培, 王一川, 周昊, 薛鲜丽. 敲除msn2对米曲霉生长和发酵产曲酸的影响[J]. 生物技术通报, 2022, 38(8): 188-197. |
| [11] | 刘静静, 刘晓蕊, 李琳, 王盈, 杨海元, 戴一凡. 利用CRISPR/Cas9技术建立OXTR基因敲除猪胎儿成纤维细胞系[J]. 生物技术通报, 2022, 38(6): 272-278. |
| [12] | Olalekan Amoo, 胡利民, 翟云孤, 范楚川, 周永明. 利用基因编辑技术研究BRANCHED1参与油菜分枝过程的调控[J]. 生物技术通报, 2022, 38(4): 97-105. |
| [13] | 丁亚群, 丁宁, 谢深民, 黄梦娜, 张昱, 张勤, 姜力. Vps28基因敲除小鼠模型的构建及其对泌乳和免疫性状影响的研究[J]. 生物技术通报, 2022, 38(3): 164-172. |
| [14] | 燕炯, 冯晨毅, 高学坤, 许祥, 杨佳敏, 陈朝阳. 基于CRISPR/Cas9技术构建Plin1基因敲除小鼠模型及表型分析[J]. 生物技术通报, 2022, 38(3): 173-180. |
| [15] | 宗梅, 韩硕, 郭宁, 段蒙蒙, 刘凡, 王桂香. 利用真空渗透和CRISPR/Cas9系统获得非转基因菜薹突变体[J]. 生物技术通报, 2022, 38(10): 159-163. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||