








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (9): 232-241.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0064
YU Wen-jie1,2( ), FAN Si-ran2, GAO Wen-li2, XING Yu2(
), FAN Si-ran2, GAO Wen-li2, XING Yu2( ), QIN Ling1,2(
), QIN Ling1,2( )
)
Received:2025-01-16
Online:2025-09-26
Published:2025-09-24
Contact:
XING Yu, QIN Ling
E-mail:buayuwenjie@163.com;xingyu@bua.edu.cn;qinlingbac@126.com
YU Wen-jie, FAN Si-ran, GAO Wen-li, XING Yu, QIN Ling. Identification and Functional Verification of Key Genes in Riboflavin Synthesis Pathway in Chinese Chestnut[J]. Biotechnology Bulletin, 2025, 41(9): 232-241.
参数名称 Parameter name | 参数 Parameter |
|---|---|
| 色谱柱 Chromatographic column | C18柱150 mm × 4.6 mm × 5 μm |
| 流动相 Mobile phase | 乙酸钠溶液(0.05 mol/L)-甲醇(80∶20) |
| 流速 Velocity of flow (mL/min) | 1 |
| 柱温 Column temperature (℃) | 30 |
| 检测波长 Test wavelength | 激发波长462 nm,发射波长522 nm |
| 进样体积 Injection volume (μL) | 20 |
Table 1 HPLC parameters
参数名称 Parameter name | 参数 Parameter |
|---|---|
| 色谱柱 Chromatographic column | C18柱150 mm × 4.6 mm × 5 μm |
| 流动相 Mobile phase | 乙酸钠溶液(0.05 mol/L)-甲醇(80∶20) |
| 流速 Velocity of flow (mL/min) | 1 |
| 柱温 Column temperature (℃) | 30 |
| 检测波长 Test wavelength | 激发波长462 nm,发射波长522 nm |
| 进样体积 Injection volume (μL) | 20 |
引物名称 Primer name | 引物序列 Primer sequence (5'‒3') | 退火温度 Annealing temperature (℃) |
|---|---|---|
| CmRS RNAi-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCCTCTCACATCATTCTCCAA | 56.5 |
| CmRS RNAi-R | GGGGACCACTTTGTACAAGAAAGCTGGGTACGCGGCTATTCTCATATCG | 57 |
| CmLS1 RNAi-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCCCAGAACTCAGGGTCTTC | 60.3 |
| CmLS1 RNAi-R | GGGGACCACTTTGTACAAGAAAGCTGGGTACCAGCCGAATTAGCAACAG | 60.4 |
Table 2 Primers for cloning specific fragments of CmLS1 and CmRS target genes
引物名称 Primer name | 引物序列 Primer sequence (5'‒3') | 退火温度 Annealing temperature (℃) |
|---|---|---|
| CmRS RNAi-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCCTCTCACATCATTCTCCAA | 56.5 |
| CmRS RNAi-R | GGGGACCACTTTGTACAAGAAAGCTGGGTACGCGGCTATTCTCATATCG | 57 |
| CmLS1 RNAi-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCCCAGAACTCAGGGTCTTC | 60.3 |
| CmLS1 RNAi-R | GGGGACCACTTTGTACAAGAAAGCTGGGTACCAGCCGAATTAGCAACAG | 60.4 |
引物名称 Primer name | 引物序列 Primer sequence (5'‒3') | 退火温度 Annealing temperature (℃) |
|---|---|---|
| Actin-F | TTGACTATGAGCAGGAACTT | 58.9 |
| Actin-R | TTGTAGGTGGTCTCGTGAAT | 60.8 |
| CmRS QP-F | CGGCATAGTTGAAGAAGT | 56.8 |
| CmRS QP-R | GCGGCTATTCTCATATCG | 57.0 |
| CmLS1 QP-F | GCCATCCTACACCTTAACAG | 58.4 |
| CmLS1 QP-R | CTTCCTCTCAACAGCATAGC | 59.0 |
Table 3 Primers for fluorescence quantitative PCR of CmLS1 and CmRS
引物名称 Primer name | 引物序列 Primer sequence (5'‒3') | 退火温度 Annealing temperature (℃) |
|---|---|---|
| Actin-F | TTGACTATGAGCAGGAACTT | 58.9 |
| Actin-R | TTGTAGGTGGTCTCGTGAAT | 60.8 |
| CmRS QP-F | CGGCATAGTTGAAGAAGT | 56.8 |
| CmRS QP-R | GCGGCTATTCTCATATCG | 57.0 |
| CmLS1 QP-F | GCCATCCTACACCTTAACAG | 58.4 |
| CmLS1 QP-R | CTTCCTCTCAACAGCATAGC | 59.0 |
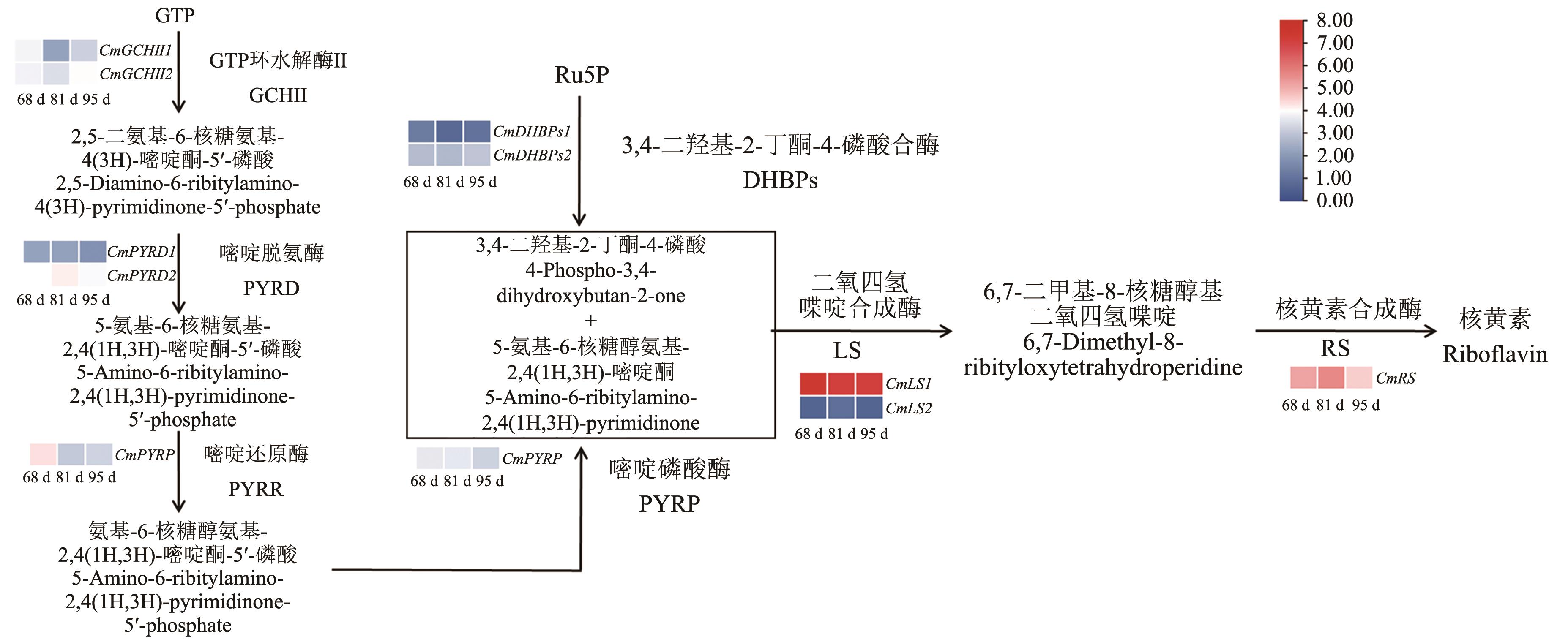
Fig. 1 Identification of riboflavin synthetase gene and analysis of gene expression at different developmental stages of Chinese chestnutGene expression in Chinese chestnut (Castanea mollissima) at 68, 81, and 95 d after anthesis
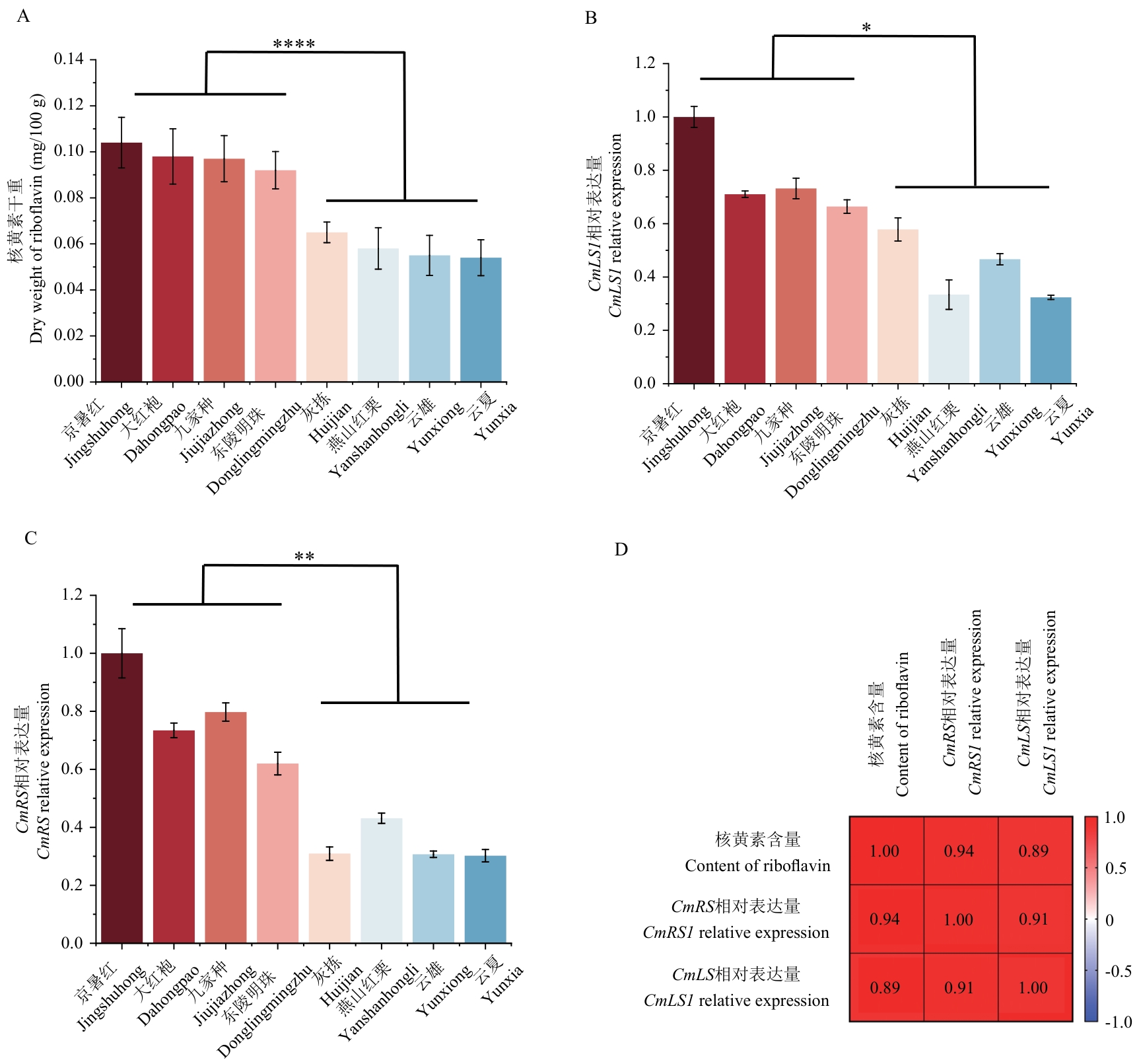
Fig. 2 Correlation analysis of riboflavin contents and expressions of CmLS1 and CmRS genes in different Chinese chestnut cultivarsA: Analysis of riboflavin content in Chinese chestnut and differences between groups. B: Differential expressions of CmLS1 in Chinese chestnut cultivars and analysis of differences between groups. C: Differential expression of CmRS in Chinese chestnut cultivars and analysis of differences between groups. D: Correlation analysis of CmLS1, CmRS and riboflavin content. **** P<0.001; ** P<0.01; * P<0.05; n=3. The same below
蛋白 Protein | 蛋白长度 Length of protein (aa) | 分子量 Molecular weight (Da) | 等电点 Isoelectric point | 不稳定系数 Coef ficient of instability | 最大亲水性 Derophilic index | 亚细胞定位 Subcel lular localization |
|---|---|---|---|---|---|---|
| CmLS1 | 230 | 24 707.02 | 8.82 | 40.54 | -0.030 | 叶绿体 |
| CmRS | 280 | 30 621.49 | 6.24 | 27.41 | 0.047 | 叶绿体 |
Table 4 Physicochemical properties of CmLS1 and CmRS proteins in Chinese chestnut
蛋白 Protein | 蛋白长度 Length of protein (aa) | 分子量 Molecular weight (Da) | 等电点 Isoelectric point | 不稳定系数 Coef ficient of instability | 最大亲水性 Derophilic index | 亚细胞定位 Subcel lular localization |
|---|---|---|---|---|---|---|
| CmLS1 | 230 | 24 707.02 | 8.82 | 40.54 | -0.030 | 叶绿体 |
| CmRS | 280 | 30 621.49 | 6.24 | 27.41 | 0.047 | 叶绿体 |
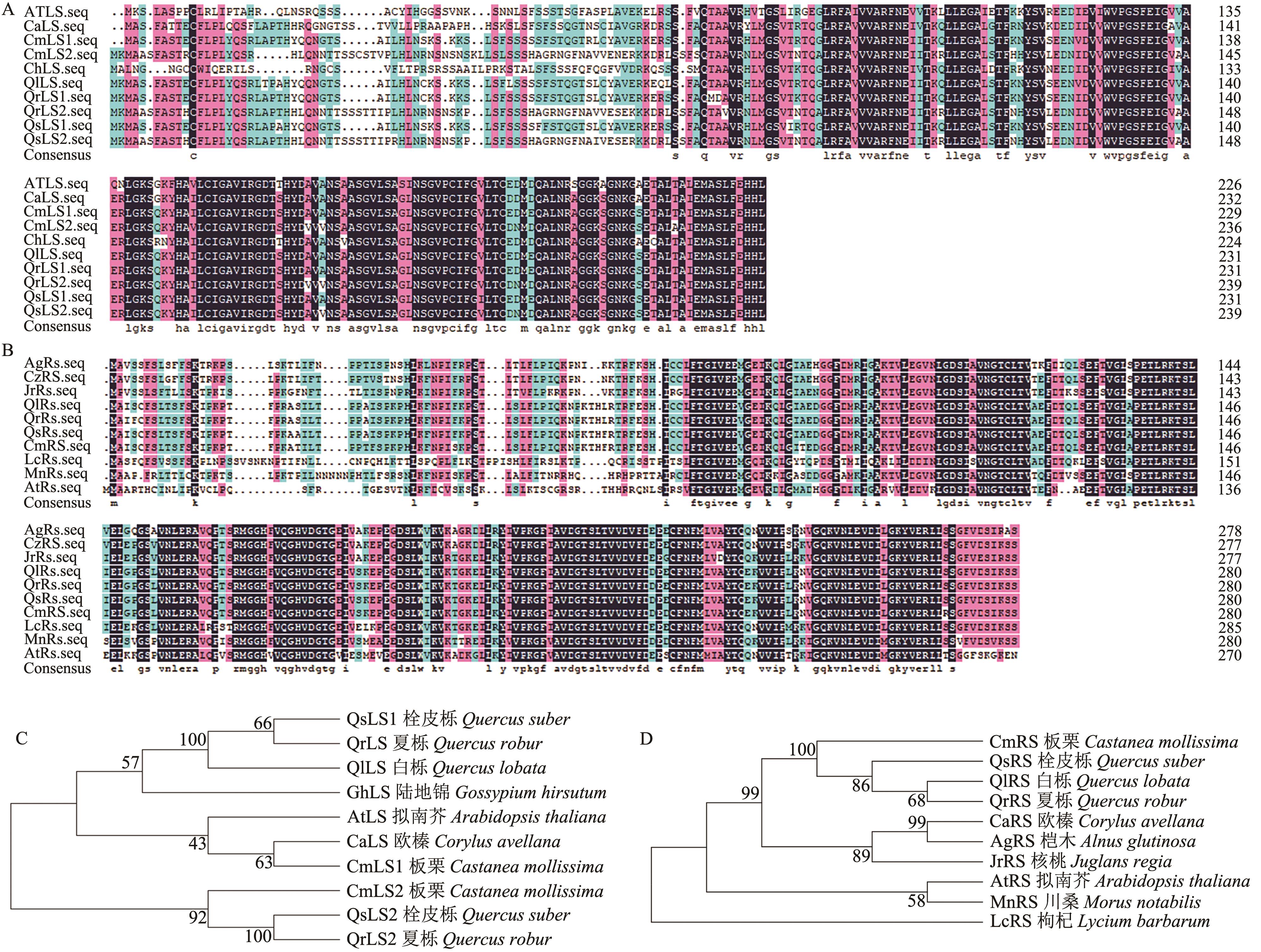
Fig. 4 Protein sequence alignment and phylogenetic tree analysis of lumazine synthase LS and riboflavin synthetase RSA: Protein sequence alignment of lumazine synthase in Chinese chestnut. B: Protein sequence alignment of riboflavin synthetase in Chinese chestnut. C: Phylogenetic tree analysis of lumazine synthas in Chinese chestnut. D: Phylogenetic tree analysis of riboflavin synthetase in Chinese chestnut
元件名称 Name of element | 元件序列 Sequence of element | 元件类型 Type of element | CmLS1启动子元件数量 Number of CmLS1 promoter element | CmRS启动子元件数量 Number of CmRS promoter element |
|---|---|---|---|---|
| G-box | CACGTT | 光响应元件 | 2 | 1 |
| AuxRE | TGTCTCAATAAG | 生长素响应元件 | 0 | 2 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应元件 | 2 | 1 |
| ABRE | ACGTG | 脱落酸响应元件 | 1 | 1 |
| TCA-element | CCATCTTTTT | 水杨酸响应元件 | 0 | 1 |
| ARE | AAACCA | 厌氧诱导响应元件 | 3 | 2 |
| LER | CCGAAA | 低温响应元件 | 2 | 0 |
Table 5 Analyses of cis-acting regulatory elements in the promotersoflumazine synthase CmLS1 andriboflavin synthetase CmRS
元件名称 Name of element | 元件序列 Sequence of element | 元件类型 Type of element | CmLS1启动子元件数量 Number of CmLS1 promoter element | CmRS启动子元件数量 Number of CmRS promoter element |
|---|---|---|---|---|
| G-box | CACGTT | 光响应元件 | 2 | 1 |
| AuxRE | TGTCTCAATAAG | 生长素响应元件 | 0 | 2 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应元件 | 2 | 1 |
| ABRE | ACGTG | 脱落酸响应元件 | 1 | 1 |
| TCA-element | CCATCTTTTT | 水杨酸响应元件 | 0 | 1 |
| ARE | AAACCA | 厌氧诱导响应元件 | 3 | 2 |
| LER | CCGAAA | 低温响应元件 | 2 | 0 |
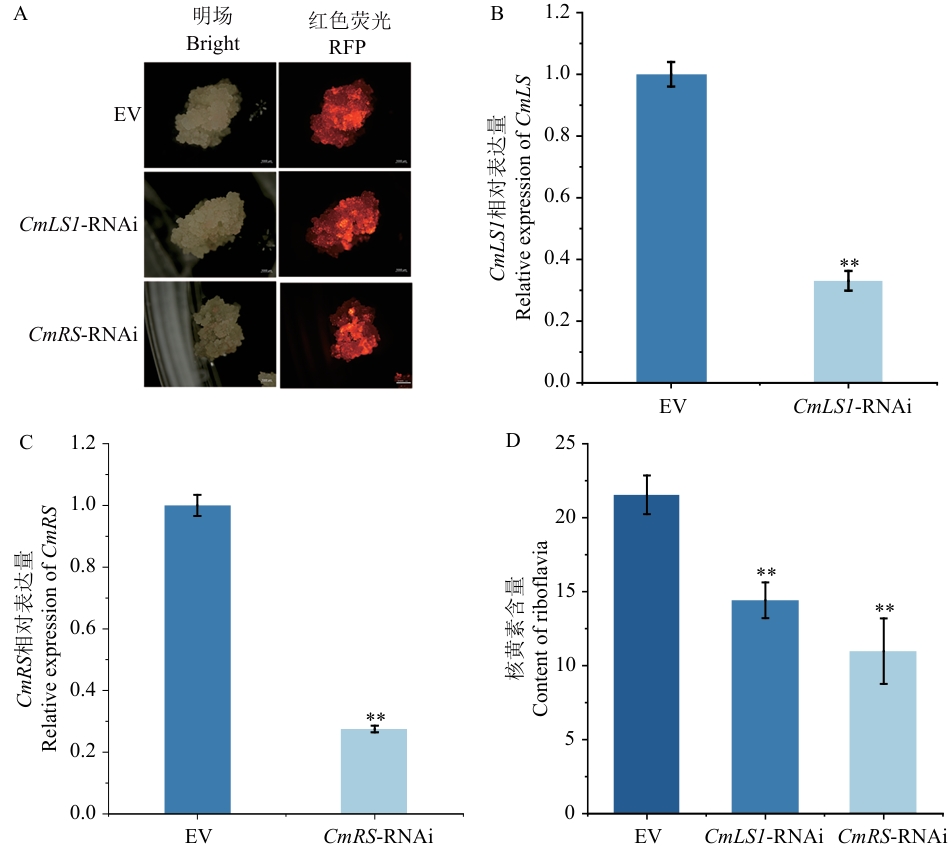
Fig. 5 CmLS1 and CmRS transient silencing and determination of riboflavin contentA: Fluorescence observation of callus. B: Relative expression of CmLS1. C: Relative expression of CmRS. D: Riboflavin content detection of positive callus. Bar =1 000 μm
| [1] | Averianova LA, Balabanova LA, Son OM, et al. Production of vitamin B2 (riboflavin) by microorganisms: an overview [J]. Front Bioeng Biotechnol, 2020, 8: 570828. |
| [2] | 李俊霖, 边祥雨, 姚站馨, 等. 膳食核黄素参考摄入量国内外研究进展 [J]. 营养学报, 2022, 44(6): 530-533. |
| Li JL, Bian XY, Yao ZX, et al. Revision of dietary riboflavin reference intake: an update [J]. Acta Nutr Sin, 2022, 44(6): 530-533. | |
| [3] | 万旻. 核黄素营养状况对骨骼健康影响的研究 [D]. 北京: 军事科学院, 2023. |
| Wan M. Study of the effect of riboflavin nutritional status on bone health [D]. Beijing: Academy of Military Science, 2023. | |
| [4] | 胡海涛, 郭龙彪. 植物核黄素的生物合成及其功能研究进展 [J]. 植物学报, 2023, 58(4): 638-655. |
| Hu HT, Guo LB. Progress in the research on riboflavin biosynthesis and function in plants [J]. Chin Bull Bot, 2023, 58(4): 638-655. | |
| [5] | Ozbekova Z, Kulmyrzaev A. Study of moisture content and water activity of rice using fluorescence spectroscopy and multivariate analysis [J]. Spectrochim Acta Part A Mol Biomol Spectrosc, 2019, 223: 117357. |
| [6] | Villareal CP, Juliano BO. Variability in contents of thiamine and riboflavin in brown rice, crude oil in brown rice and bran-Polish, and silicon in hull of IR rices [J]. Plant Foods Hum Nutr, 1989, 39(3): 287-297. |
| [7] | 武妍妍, 史文石, 石新如, 等. 板栗坚果营养物质和抗氧化成分综合评价 [J]. 林业科学研究, 2022, 35(6): 12-22. |
| Wu YY, Shi WS, Shi XR, et al. Comprehensive evaluation of nutrients and antioxidant components in nuts of chestnut [J]. For Res, 2022, 35(6): 12-22. | |
| [8] | 徐志祥, 高绘菊. 板栗营养价值及其养生保健功能 [J]. 食品研究与开发, 2004, 25(5): 118-119. |
| Xu ZX, Gao HJ. Nutritional value of chestnut and its health care function [J]. Food Res Dev, 2004, 25(5): 118-119. | |
| [9] | 张瑞菊, 孙强, 张洪坤. 板栗的营养、生产现状及前景展望 [J]. 山东商业职业技术学院学报, 2014, 14(4): 106-107. |
| Zhang RJ, Sun Q, Zhang HK. Nutrition, production status and prospect of Chinese chestnut [J]. J Shandong Inst Commer Technol, 2014, 14(4): 106-107. | |
| [10] | 乔艳杰, 刘巍, 吴瑞刚, 等. 北京市板栗产业发展问题及对策研究 [J]. 中国果树, 2024(6): 125-129. |
| Qiao YJ, Liu W, Wu RG, et al. Research on the development problems and countermeasures of Chinese chestnut industry in Beijing [J]. China Fruits, 2024(6): 125-129. | |
| [11] | 韩元顺, 许林云, 周杰. 中国板栗产业与市场发展现状及趋势 [J]. 中国果树, 2021(4): 83-88. |
| Han YS, Xu LY, Zhou J. Status and trend of the development of chestnut industry in China [J]. China Fruits, 2021(4): 83-88. | |
| [12] | 蔡荣, 虢佳花, 祁春节. 板栗产业发展现状、存在问题与对策分析 [J]. 中国果菜, 2007, 27(1): 52-53. |
| Cai R, Guo JH, Qi CJ. Present situation, existing problems and countermeasures of chestnut industry development [J]. China Fruit Veg, 2007, 27(1): 52-53. | |
| [13] | Tolar JG, Li SL, Ajo-Franklin CM. The differing roles of flavins and quinones in extracellular electron transfer in lactiplantibacillus plantarum [J]. Appl Environ Microbiol, 2023, 89(1): e0131322. |
| [14] | Bacher A, Eberhardt S, Eisenreich W, et al. Biosynthesis of riboflavin [M]//Cofactor Biosynthesis. Amsterdam: Elsevier, 2001: 1-49. |
| [15] | 郭长江, 顾景范. 核黄素 [J]. 营养学报, 2013, 35(2): 119-121. |
| Guo CJ, Gu JF. Riboflavin [J]. Acta Nutr Sin, 2013, 35(2): 119-121. | |
| [16] | 王林静, 黄亿明. 核黄素与健康 [J]. 广东药学院学报, 2000, 16(3): 223-225, 228. |
| Wang LJ, Huang YM. Riboflavin and health [J]. Acad J Guangdong Coll Pharm, 2000, 16(3): 223-225, 228. | |
| [17] | Xu ZB, Lin ZQ, Wang ZW, et al. Improvement of the riboflavin production by engineering the precursor biosynthesis pathways in Escherichia coli [J]. Chin J Chem Eng, 2015, 23(11): 1834-1839. |
| [18] | Harale B, Kidwai S, Ojha D, et al. Synthesis and evaluation of antimycobacterial activity of riboflavin derivatives [J]. Bioorg Med Chem Lett, 2021, 48: 128236. |
| [19] | Tani T, Sobajima H, Okada K, et al. Identification of the OsOPR7 gene encoding 12-oxophytodienoate reductase involved in the biosynthesis of jasmonic acid in rice [J]. Planta, 2008, 227(3): 517-526. |
| [20] | Sa N, Rawat R, Thornburg C, et al. Identification and characterization of the missing phosphatase on the riboflavin biosynthesis pathway in Arabidopsis thaliana [J]. Plant J, 2016, 88(5): 705-716. |
| [21] | Xiao S, Dai LY, Liu FQ, et al. COS1: an Arabidopsis coronatine insensitive1 suppressor essential for regulation of jasmonate-mediated plant defense and senescence [J]. Plant Cell, 2004, 16(5): 1132-1142. |
| [22] | Hu HT, Ren DY, Hu J, et al. WHITE AND LESION-MIMIC LEAF1, encoding a lumazine synthase, affects reactive oxygen species balance and chloroplast development in rice [J]. Plant J, 2021, 108(6): 1690-1703. |
| [23] | 任秀艳, 乔洁, 张江丽. 核黄素合酶的研究进展 [J]. 西北植物学报, 2011, 31(9): 1917-1926. |
| Ren XY, Qiao J, Zhang JL. Research progress of riboflavin synthase [J]. Acta Bot Boreali Occidentalia Sin, 2011, 31(9): 1917-1926. | |
| [24] | 黄伟志, 黄桂东, 钟先锋. 高效液相色谱法测定功能性饮料中维生素B1、维生素B2含量 [J]. 食品研究与开发, 2019, 40(24): 191-197. |
| Huang WZ, Huang GD, Zhong XF. Determination of vitamins B1 and vitamins B2 in energy drinks by high performance liquid chromatography [J]. Food Res Dev, 2019, 40(24): 191-197. | |
| [25] | 朱丽, 钱前. 虾青素功能米: 生物强化新思路, 优质米培育新资源 [J]. 植物学报, 2019, 54(1): 4-8. |
| Zhu L, Qian Q. Astaxanthin functional rice: new idea of biofortification, new perspectives for high-quality rice breeding [J]. Chin Bull Bot, 2019, 54(1): 4-8. | |
| [26] | Suwannasom N, Kao I, Pruß A, et al. Riboflavin: the health benefits of a forgotten natural vitamin [J]. Int J Mol Sci, 2020, 21(3): 950. |
| [27] | Tuan PA, Kim JK, Lee S, et al. Riboflavin accumulation and characterization of cDNAs encoding lumazine synthase and riboflavin synthase in bitter melon (Momordica charantia) [J]. J Agric Food Chem, 2012, 60(48): 11980-11986. |
| [28] | Zhao YY, Wang DF, Wu TQ, et al. Transgenic expression of a rice riboflavin synthase gene in tobacco enhances plant growth and resistance to Tobacco mosaic virus [J]. Can J Plant Pathol, 2014, 36(1): 100-109. |
| [29] | Namba J, Harada M, Shibata R, et al. AtDREB2G is involved in the regulation of riboflavin biosynthesis in response to low-temperature stress and abscisic acid treatment in Arabidopsis thaliana [J]. Plant Sci, 2024, 347: 112196. |
| [30] | Wang TZ, Wang J, Zhang D, et al. Protein kinase MtCIPK12 modulates iron reduction in Medicago truncatula by regulating riboflavin biosynthesis [J]. Plant Cell Environ, 2023, 46(3): 991-1003. |
| [31] | Xu XP, Zhang CY, Xu XQ, et al. Riboflavin mediates m6A modification targeted by miR408, promoting early somatic embryogenesis in Longan [J]. Plant Physiol, 2023, 192(3): 1799-1820. |
| [32] | Bosch G, Fuentes M, Erro J, et al. Hydrolysis of riboflavins in root exudates under iron deficiency and alkaline stress [J]. Plant Physiol Biochem, 2024, 210: 108573. |
| [33] | Deng BL, Dong HS. Ectopic expression of riboflavin-binding protein gene TsRfBP paradoxically enhances both plant growth and drought tolerance in transgenic Arabidopsis thaliana [J]. J Plant Growth Regul, 2013, 32(1): 170-181. |
| [34] | Wu YZ, Cheng SR, Ding XF, et al. Exogenous riboflavin application at different growth stages regulates photosynthetic accumulation and grain yield in fragrant rice [J]. Agriculture, 2024, 14(11): 1979. |
| [1] | SONG Hui-yang, SU Bao-jie, LI Jing-hao, MEI Chao, SONG Qian-na, CUI Fu-zhu, FENG Rui-yun. Cloning and Functional Analysis of the StAS2-15 Gene in Potato under Salt Stress [J]. Biotechnology Bulletin, 2025, 41(5): 119-128. |
| [2] | ZHANG Yu, SHI Lei, GONG Lei, NIE Feng-jie, YANG Jiang-wei, LIU Xuan, YANG Wen-jing, ZHANG Guo-hui, XIE Rui-xia, ZHANG Li. Genome-wide Identification of Potato WOX Gene Family and Its Expression Analysis in in vitro Regeneration and Abiotic Stress [J]. Biotechnology Bulletin, 2024, 40(3): 170-180. |
| [3] | ZHAO Zhong-ying, LI Qing-miao, TIAN Meng-liang, YANG Xiao-qian, KANG Yao, QIU Yu-jie, ZHANG Qing-ling, LIU Fan. Effects of 60Co-γ Radiation on the Seedling Rate and Plant Characteristics of Pinellia ternata Callus [J]. Biotechnology Bulletin, 2021, 37(9): 142-151. |
| [4] | WANG Jian-yong, ZOU Yong-mei, GE Yan-bin, WANG Kai, XI Meng-li. Advance on Epigenetic Modification During Plant Callus Induction [J]. Biotechnology Bulletin, 2021, 37(8): 253-262. |
| [5] | WU Li-fang, WEI Xiao-mei, LU Wei-dong. Embryonic Callus Induction of Sophora davidii and Their Somatic Embryogenesis and Germination [J]. Biotechnology Bulletin, 2019, 35(4): 13-19. |
| [6] | FANG Luo, WU Xiao-qin. Suspension Culture of Somatic Cells of Pinus elliottii Against Brown Spot Needle Blight of Pine [J]. Biotechnology Bulletin, 2019, 35(3): 13-18. |
| [7] | WANG Jun-yi, DONG Jin-jin, LIU Wei, CAO Fu-liang, WANG Gui-bin, WANG Yi-qiang. Research on the Growth,Browning and Flavonoid Accumulation of Ginkgo biloba Callus [J]. Biotechnology Bulletin, 2019, 35(2): 16-22. |
| [8] | WANG Ling, LI Yan, DAI Wei-na, YAN Jing, ZHANG Chao-hong. Establishment and Optimization of Cell Suspension Culture System for Vitis vinifera [J]. Biotechnology Bulletin, 2018, 34(8): 80-86. |
| [9] | MAO Pei-qi, LI Hou-hua, LI Ai, CAO Zhi-xiu, HAN Mei-ling, ZHANG Yan-long. Effects of Silver Nitrate on the Synthesis of Phenolic Compounds and the Expression Levels of Related Genes in Callus Browning Process of Paeonia ostii ‘Fengdan’ [J]. Biotechnology Bulletin, 2018, 34(8): 101-107. |
| [10] | ZHANG Zheng-xue, LAN Zeng-quan, WU Tian. Establishment of Cell Suspension Culture System Based on the Leaf Callus in Noni [J]. Biotechnology Bulletin, 2018, 34(5): 142-147. |
| [11] | ZHANG Ya-fang, HE Gang, RONG Guang-tian, LIU Xian-gui, NI Shang-ge, ZHANG Shi-liang. Changes of Endogenous Hormones During the Culture of Callus from Sopatholobus suberechtus [J]. Biotechnology Bulletin, 2017, 33(3): 66-70. |
| [12] | LI Zhi-liang,WU Zhong-yi,YANG Qing,ZHANG Xi-tai,YE Jia,XING Hao-chun,CHEN Jian-zhong,HUANG Cong-lin,. The Transformation of Drought-resistant Gene into Maize by Microprojectile Bombardment [J]. Biotechnology Bulletin, 2016, 32(5): 61-68. |
| [13] | DING Xi-lian, QU Yan-ying, LI Qiong, GUO Jia-yan, CHEN Quan-jia. The Study of Regeneration System from Different Sea Island Cotton (Gossypium barbadense L.)Cultivars [J]. Biotechnology Bulletin, 2016, 32(1): 97-102. |
| [14] | Guo Shuangshuang, Shi Ce, Han Mei, Yang Limin. The Effect of the Fermented Liquid and Mycelium Extract of FX-139 on the Growth and Defense Enzymes of Ginseng callus [J]. Biotechnology Bulletin, 2015, 31(5): 128-133. |
| [15] | Liu Xiaodan, Zhang Keqin, Liu Lian, Li Wenchang. Determination the Content of Total Flavonoids and Hypericin in Callus from Hypericum attenuatum Choisy [J]. Biotechnology Bulletin, 2015, 31(1): 98-103. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||