








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (7): 49-59.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0022
Previous Articles Next Articles
GAO Jing( ), CHENG Yi-cun, GAO Ming, ZHAO Yun-xiao(
), CHENG Yi-cun, GAO Ming, ZHAO Yun-xiao( ), WANG Yang-dong(
), WANG Yang-dong( )
)
Received:2025-01-08
Online:2025-07-26
Published:2025-07-22
Contact:
ZHAO Yun-xiao, WANG Yang-dong
E-mail:gaojing_19990306@163.com;zyx_yunxiao@caf.ac.cn;wangyangdong@caf.ac.cn
GAO Jing, CHENG Yi-cun, GAO Ming, ZHAO Yun-xiao, WANG Yang-dong. Regulation of Plant Tannin Synthesis and Mechanisms of Its Responses to Environment[J]. Biotechnology Bulletin, 2025, 41(7): 49-59.
来源植物 Plants of source | 单宁类型 Type of tannins | 主要功能 Main functions | 单宁含量 Tannin content (%) | 参考文献 Reference |
|---|---|---|---|---|
| 柿Diospyros kaki | 缩合单宁 | 抗氧化、抗炎、抗辐射、抗菌、抗病毒、抗高血压、降血脂、降血糖、解毒 | 4 | [ |
| 杨属Populus | 缩合单宁 | 抗氧化、抗菌、抗紫外线、抗病原体 | 15-25 | [ |
| 茶叶Camellia sinensis | 水解单宁和缩合单宁 | 抗氧化、抗菌、抗过敏、抗紫外线、消炎、除臭、抗虫、细胞防御、促进免疫 | 20-35 | [ |
| 葡萄Vitis vinifera | 水解单宁和缩合单宁 | 抗氧化、抗菌、抗病毒、抗衰老、降血糖、抑制络氨酸酶活性、抑制呼吸道炎症、保护心血管、保护神经元、保护软骨组织 | 40-50 | [ |
| 栎属Quercus | 水解单宁和缩合单宁 | 抗氧化、抗炎、抗菌、抗增殖、免疫调节、降血糖、降胆固醇、保胃保肝 | 25-35 | [ |
| 石榴Punica granatum | 水解单宁 | 抗氧化应激、抗炎、抗癌、抗真菌、抗病毒、促进伤口愈合 | 10-20 | [ |
| 核桃Juglans regia | 水解单宁和缩合单宁 | 抗氧化、抗菌、抗肿瘤、镇痛 | 20-30 | [ |
| 五倍子Rhus chinensis | 水解单宁 | 抗氧化、抗病原微生物、抗癌、抗龋齿、抗肥胖、抗炎、止血、降血糖 | 65-70 | [ |
| 麻黄Ephedrae Herba | 缩合单宁 | 抗炎、抗氧化、抗增殖、抗菌、促进伤口愈合、酶抑制活性 | 10-20 | [ |
| 杨梅Morella rubra | 水解单宁和缩合单宁 | 抗氧化、抗炎、抗过敏、抗癌、抗菌、止泻 | 10-20 | [ |
| 桉属Eucalyptus | 缩合单宁和水解单宁 | 抗氧化、抗菌、抗炎、抑制酶活性 | 40-50 | [ |
| 油茶Camellia oleifera | 水解单宁和缩合单宁 | 抗氧化、抗炎、抗菌、抗肿瘤、降血糖 | 10-20 | [ |
| 黑荆Acacia mearnsii | 缩合单宁 | 抗氧化、抗菌、抗糖尿病、抗虫、抗肥胖、抑制酶活性 | 30-45 | [ |
Table 1 Chemical structures and functions of common plant being rich in tannins
来源植物 Plants of source | 单宁类型 Type of tannins | 主要功能 Main functions | 单宁含量 Tannin content (%) | 参考文献 Reference |
|---|---|---|---|---|
| 柿Diospyros kaki | 缩合单宁 | 抗氧化、抗炎、抗辐射、抗菌、抗病毒、抗高血压、降血脂、降血糖、解毒 | 4 | [ |
| 杨属Populus | 缩合单宁 | 抗氧化、抗菌、抗紫外线、抗病原体 | 15-25 | [ |
| 茶叶Camellia sinensis | 水解单宁和缩合单宁 | 抗氧化、抗菌、抗过敏、抗紫外线、消炎、除臭、抗虫、细胞防御、促进免疫 | 20-35 | [ |
| 葡萄Vitis vinifera | 水解单宁和缩合单宁 | 抗氧化、抗菌、抗病毒、抗衰老、降血糖、抑制络氨酸酶活性、抑制呼吸道炎症、保护心血管、保护神经元、保护软骨组织 | 40-50 | [ |
| 栎属Quercus | 水解单宁和缩合单宁 | 抗氧化、抗炎、抗菌、抗增殖、免疫调节、降血糖、降胆固醇、保胃保肝 | 25-35 | [ |
| 石榴Punica granatum | 水解单宁 | 抗氧化应激、抗炎、抗癌、抗真菌、抗病毒、促进伤口愈合 | 10-20 | [ |
| 核桃Juglans regia | 水解单宁和缩合单宁 | 抗氧化、抗菌、抗肿瘤、镇痛 | 20-30 | [ |
| 五倍子Rhus chinensis | 水解单宁 | 抗氧化、抗病原微生物、抗癌、抗龋齿、抗肥胖、抗炎、止血、降血糖 | 65-70 | [ |
| 麻黄Ephedrae Herba | 缩合单宁 | 抗炎、抗氧化、抗增殖、抗菌、促进伤口愈合、酶抑制活性 | 10-20 | [ |
| 杨梅Morella rubra | 水解单宁和缩合单宁 | 抗氧化、抗炎、抗过敏、抗癌、抗菌、止泻 | 10-20 | [ |
| 桉属Eucalyptus | 缩合单宁和水解单宁 | 抗氧化、抗菌、抗炎、抑制酶活性 | 40-50 | [ |
| 油茶Camellia oleifera | 水解单宁和缩合单宁 | 抗氧化、抗炎、抗菌、抗肿瘤、降血糖 | 10-20 | [ |
| 黑荆Acacia mearnsii | 缩合单宁 | 抗氧化、抗菌、抗糖尿病、抗虫、抗肥胖、抑制酶活性 | 30-45 | [ |
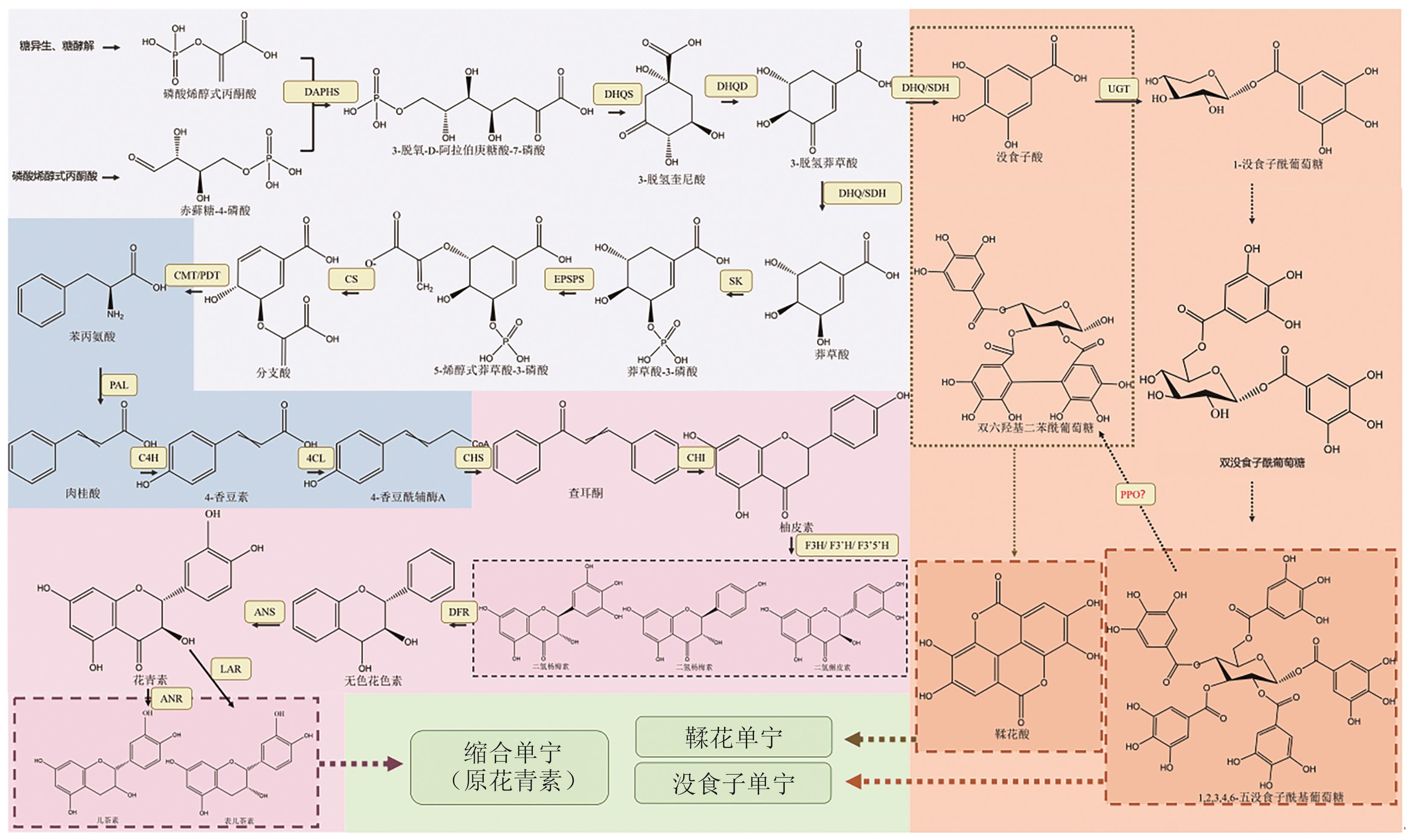
Fig. 1 Tannins biosynthesis pathwaysDAPHS: 3-deoxy-D-arabino-heptulosonate7-phosphate synthase. DHQS: 3-dehydroquinate synthase. DHQD: 3-dehydroquinate dehydratase. DHQ/SDH: Dehydroquinate dehydratase/shikimate dehydrogenase. SK: Shikimate kinase. EPSPS: Enolpyruvylshikimate-3-phosphate synthase. CS: Chorismate synthase. CMT/PDT: Chorismate mutase/prephenate dehydratase. PAL: Phenylalanine ammonia-lyase. C4H: Cinnamic acid 4-hydroxylase. 4CL: 4-coumarate CoA ligase. CHS: Chalcone synthase. CHI: Chalcone isomerase. F3H: Flavanone 3-hydroxylase. F3'H: Flavonoid 3'-hydroxylase. F3'5'H: Flavonoid 3', 5'-hydroxylase. DFR: Dihydroflavonol 4-reductase. ANS: Anthocyanin synthase. ANR: Anthocyanin reductase. UGT: UDP-glycosyl transferases. PPO: Polyphenol oxidase. The dashed line refers to the synthesis pathways that have not yet been clarified
环境因子 Environmental factors | 响应因子 Response factors | 关键酶及转录因子 Key enzymes and transcription factors | 对单宁合成的影响 Effects on tannin synthesis | 参考文献 Reference |
|---|---|---|---|---|
| 光照Light | 苯丙氨酸解氨酶和过氧化物酶活性增加 | PAL、C4H | 促进 | [ |
| 紫外线Ultraviolet radiation | 抗氧化酶活性增加 | MYB、DFR、ANS | 促进 | [ |
| 高温High temperature | 增强热应激信号的转导 | HSPs、MBW | 短时高温抑制;长时高温促进 | [ |
| 寒冷Cold | 水杨酸响应同时抗氧化酶活性提高 | SOD、CAT、POX、bHLH | 促进 | [ |
| 干旱Drought | 改变苯丙烷途径提高抗氧化酶活性 | MBW、WRKY、UGT、 | 促进 | [ |
| 水分Water | 抗氧化酶活性 | MBW、C4H、CHI | 随着胁迫程度的增加先促进后抑制 | [ |
| 重金属Heavy metals | 生物合成酶活性增加,基因表达增强 | SDH、PAL、CHI、PPO | 促进 | [ |
| 盐分Salt | 基因表达增强 | FNSII、bHLH、CHS | 促进 | [ |
| 昆虫Insects | 基因表达增强 | DAPHS、UGT、CHS、LAR | 促进 | [ |
| 病原体Pathogens | 触发免疫反应 | NBS-LRR、SDH | 促进 | [ |
Table 2 Regulatory mechanisms of tannin synthesis in response to environmental factors
环境因子 Environmental factors | 响应因子 Response factors | 关键酶及转录因子 Key enzymes and transcription factors | 对单宁合成的影响 Effects on tannin synthesis | 参考文献 Reference |
|---|---|---|---|---|
| 光照Light | 苯丙氨酸解氨酶和过氧化物酶活性增加 | PAL、C4H | 促进 | [ |
| 紫外线Ultraviolet radiation | 抗氧化酶活性增加 | MYB、DFR、ANS | 促进 | [ |
| 高温High temperature | 增强热应激信号的转导 | HSPs、MBW | 短时高温抑制;长时高温促进 | [ |
| 寒冷Cold | 水杨酸响应同时抗氧化酶活性提高 | SOD、CAT、POX、bHLH | 促进 | [ |
| 干旱Drought | 改变苯丙烷途径提高抗氧化酶活性 | MBW、WRKY、UGT、 | 促进 | [ |
| 水分Water | 抗氧化酶活性 | MBW、C4H、CHI | 随着胁迫程度的增加先促进后抑制 | [ |
| 重金属Heavy metals | 生物合成酶活性增加,基因表达增强 | SDH、PAL、CHI、PPO | 促进 | [ |
| 盐分Salt | 基因表达增强 | FNSII、bHLH、CHS | 促进 | [ |
| 昆虫Insects | 基因表达增强 | DAPHS、UGT、CHS、LAR | 促进 | [ |
| 病原体Pathogens | 触发免疫反应 | NBS-LRR、SDH | 促进 | [ |
| [1] | Erb M, Kliebenstein DJ. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy [J]. Plant Physiol, 2020, 184(1): 39-52. |
| [2] | Bhatla SC, Lal MA. Secondary metabolites [M]//Plant Physiology, Development and Metabolism. Singapore: Springer Nature Singapore, 2023: 765-808. |
| [3] | Hartmann T. From waste products to ecochemicals: fifty years research of plant secondary metabolism [J]. Phytochemistry, 2007, 68(22/23/24): 2831-2846. |
| [4] | Hassanpour S, MaheriSis N, Eshratkhah B. Plants and secondary metabolites (Tannins): A Review[J]. International Journal of Forest, Soil and Erosion, 2011, 1(1):47-53. |
| [5] | Manzoor F, Nisa MU, Hussain HA, et al. Effect of different levels of hydrolysable tannin intake on the reproductive hormones and serum biochemical indices in healthy female rats [J]. Sci Rep, 2020, 10(1): 20600. |
| [6] | 王隆基. 掌叶覆盆子多酚化合物的鉴定及水解单宁代谢基因簇的发掘 [D]. 合肥: 安徽农业大学, 2021. |
| Wang LJ. Identification of polyphenols compounds and discovery of hydrolysable tannin metabolism gene cluster in R . chingii [D]. Hefei: Anhui Agricultural University, 2021. | |
| [7] | Qin Z, Liu HM, Ma YX, et al. Developments in extraction, purification, and structural elucidation of proanthocyanidins (2000-2019) [M]//Bioactive Natural Products. Amsterdam: Elsevier, 2021: 347-391. |
| [8] | Oluwole O, Fernando WB, Lumanlan J, et al. Role of phenolic acid, tannins, stilbenes, lignans and flavonoids in human health-a review [J]. Int J Food Sci Tech, 2022, 57(10): 6326-6335. |
| [9] | Ye MH, Nan YL, Ding MM, et al. Effects of dietary tannic acid on the growth, hepatic gene expression, and antioxidant enzyme activity in Brandt’s voles (Microtus brandti) [J]. Comp Biochem Physiol B Biochem Mol Biol, 2016, 196/197: 19-26. |
| [10] | El-Aswad AF, Aisu J, Khalifa MH. Biological activity of tannins extracts from processed Camellia sinensis (black and green tea), Vicia faba and Urtica dioica and Allium cepa essential oil on three economic insects [J]. J Plant Dis Prot, 2023, 130(3): 495-508. |
| [11] | Afroz M, Rahman MM, Amin MR. Insect plant interaction with reference to secondary metabolites: a review [J]. Agric Rev, 2021, 42(4): 427-433. |
| [12] | Chung KT, Wei CI, Johnson MG. Are tannins a double-edged sword in biology and health? [J]. Trends Food Sci Technol, 1998, 9(4): 168-175. |
| [13] | Jiao TM, Huang YP, Wu YL, et al. Functional diversity of subgroup 5 R2R3-MYBs promoting proanthocyanidin biosynthesis and their key residues and motifs in tea plant [J]. Hortic Res, 2023, 10(8): uhad135. |
| [14] | Constabel CP, Yoshida K, Walker V. Diverse ecological roles of plant tannins: plant defense and beyond [J]. Recent Adv Polyphenol Res, 2014, 4: 115-142. |
| [15] | Kraus TEC, Dahlgren RA, Zasoski RJ. Tannins in nutrient dynamics of forest ecosystems-a review [J]. Plant Soil, 2003, 256(1): 41-66. |
| [16] | Dehghanian Z, Habibi K, Dehghanian M, et al. Reinforcing the bulwark: unravelling the efficient applications of plant phenolics and tannins against environmental stresses [J]. Heliyon, 2022, 8(3): e09094. |
| [17] | Lang T, Tam NF, Hussain M, et al. Dynamics of heavy metals during the development and decomposition of leaves of Avicennia marina and Kandelia obovata in a subtropical mangrove swamp [J]. Sci Total Environ, 2023, 855: 158700. |
| [18] | Melo LFM, Aquino-Martins VGQ, Silva APD, et al. Biological and pharmacological aspects of tannins and potential biotechnological applications [J]. Food Chem, 2023, 414: 135645. |
| [19] | Joanisse GD, Bradley RL, Preston CM, et al. Soil enzyme inhibition by condensed litter tannins may drive ecosystem structure and processes: the case of Kalmia angustifolia [J]. New Phytol, 2007, 175(3): 535-546. |
| [20] | Lalany F, Arcand MM. Alteration of the phenylpropanoid pathway through breeding of zero-tannin lentil modifies rhizosphere microbial communities and carbon cycling processes [J]. Rhizosphere, 2020, 13: 100183. |
| [21] | Lang T, Wei PP, Chen XX, et al. Microcosm study on allelopathic effects of leaf litter leachates and purified condensed tannins from Kandelia obovata on germination and growth of Aegiceras corniculatum [J]. Forests, 2021, 12(8): 1000. |
| [22] | Wang RF, Shi X, Li KK, et al. Activity and potential mechanisms of action of persimmon tannins according to their structures: a review [J]. Int J Biol Macromol, 2023, 242(Pt 3): 125120. |
| [23] | Gourlay G, Hawkins BJ, Albert A, et al. Condensed tannins as antioxidants that protect poplar against oxidative stress from drought and UV-B [J]. Plant Cell Environ, 2022, 45(2): 362-377. |
| [24] | Gourlay G, Peter Constabel C. Condensed tannins are inducible antioxidants and protect hybrid poplar against oxidative stress [J]. Tree Physiol, 2019, 39(3): 345-355. |
| [25] | Dai XL, Liu YJ, Zhuang JH, et al. Discovery and characterization of tannase genes in plants: roles in hydrolysis of tannins [J]. New Phytol, 2020, 226(4): 1104-1116. |
| [26] | Unusan N. Proanthocyanidins in grape seeds: an updated review of their health benefits and potential uses in the food industry [J]. J Funct Foods, 2020, 67: 103861. |
| [27] | Moldovan ML, Carpa R, Fizeşan I, et al. Phytochemical profile and biological activities of tendrils and leaves extracts from a variety of Vitis vinifera L [J]. Antioxidants, 2020, 9(5): 373. |
| [28] | Morales D. Oak trees (Quercus spp.) as a source of extracts with biological activities: a narrative review [J]. Trends Food Sci Technol, 2021, 109: 116-125. |
| [29] | 孙雨晴. 石榴皮多酚及其酶解产物的抗氧化活性和对氧化应激小鼠的保护作用研究 [D]. 南京: 南京农业大学, 2016. |
| Sun YQ. The Antioxidant activity of pomegranate peel polyphenols and its enzymatic hydrolysates and their protective effects on oxidatively stressed mice [D]. Nanjing: Nanjing Agricultural University, 2016. | |
| [30] | Li BG, Cui CQ, Zhang CF, et al. Traditional applications, ethnopharmacology, and phytochemistry of walnut green husk (Juglans regia L.): a review [J]. Nat Prod Commun, 2024, 19(6): 1934578X241262156. |
| [31] | Zhang Y, Zhang YY, Yi JJ, et al. Phytochemical characteristics and biological activities of Rhus chinensis Mill.: a review [J]. Curr Opin Food Sci, 2022, 48: 100925. |
| [32] | Osmic N, Culum D, Ibragic S. Catechins and other phenolic compounds in herb of eight Ephedra species in comparison to Camellia sinensis [J]. Nat Prod Res, 2024, 38(8): 1457-1462. |
| [33] | Sun CD, Huang HZ, Xu CJ, et al. Biological activities of extracts from Chinese bayberry (Myrica rubra Sieb. et Zucc.): a review [J]. Plant Foods Hum Nutr, 2013, 68(2): 97-106. |
| [34] | 王妙玲. 桉树单宁对土壤呼吸及微生物群落结构的影响 [D]. 南宁: 广西大学, 2022. |
| Wang ML. Effects of eucalyptus tannins on soilrespiration and microbial communit [D]. Nanning: Guangxi University, 2022. | |
| [35] | Pinto PCR, Sousa G, Crispim F, et al. Eucalyptus globulus Bark as source of tannin extracts for application in leather industry [J]. ACS Sustainable Chem Eng, 2013, 1(8): 950-955. |
| [36] | 徐曼, 陈笳鸿, 汪咏梅, 等. 油茶果壳单宁成分的提取及其分析试验初报 [J]. 林产化学与工业, 2009, 29(S1): 187-191. |
| Xu M, Chen JH, Wang YM, et al. Preliminary report of extraction and analysis of tannins from Camellia oleifera fruit shell [J]. Chem Ind For Prod, 2009, 29(S1): 187-191. | |
| [37] | Peng YQ, Zhou H, Zhang AL, et al. Natural products from Camellia oleifera fruit and its comprehensive utilisation [J]. Nat Prod Res, 2024: 1-17. |
| [38] | Ogawa S, Yazaki Y. Tannins from Acacia mearnsii de wild. bark: tannin determination and biological activities [J]. Molecules, 2018, 23(4): 837. |
| [39] | 张亮亮, 侯学良. 中国单宁植物 [M]. 北京: 化学工业出版社, 2023. |
| Zhang LL, Hou XL. China tannin vegetable [M]. Beijing: Chemical Industry Press, 2023. | |
| [40] | Ji YZ, Xu QH, Jin LQ, et al. Cellulosic paper with high antioxidative and barrier properties obtained through incorporation of tannin into kraft pulp fibers [J]. Int J Biol Macromol, 2020, 162: 678-684. |
| [41] | Herrmann KM. The shikimate pathway: early steps in the biosynthesis of aromatic compounds [J]. Plant Cell, 1995, 7(7): 907-919. |
| [42] | Sheng Q, Yi LX, Zhong B, et al. Shikimic acid biosynthesis in microorganisms: current status and future direction [J]. Biotechnol Adv, 2023, 62: 108073. |
| [43] | Entus R, Poling M, Herrmann KM. Redox regulation of Arabidopsis 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase [J]. Plant Physiol, 2002, 129(4): 1866-1871. |
| [44] | Bontpart T, Marlin T, Vialet S, et al. Two shikimate dehydrogenases, VvSDH3 and VvSDH4, are involved in Gallic acid biosynthesis in grapevine [J]. J Exp Bot, 2016, 67(11): 3537-3550. |
| [45] | Tahara K, Milkowski C, Oda-Yamamizo C. Elucidation and reconstitution of hydrolyzable tannin biosynthesis [J]. Plant Biotechnol, 2024, 41(3): 203-212. |
| [46] | Ossipov V, Salminen JP, Ossipova S, et al. Gallic acid and hydrolysable tannins are formed in birch leaves from an intermediate compound of the shikimate pathway [J]. Biochem Syst Ecol, 2003, 31(1): 3-16. |
| [47] | Mir R, Jallu S, Singh TP. The shikimate pathway: review of amino acid sequence, function and three-dimensional structures of the enzymes [J]. Crit Rev Microbiol, 2015, 41(2): 172-189. |
| [48] | Akagi T, Ikegami A, Suzuki Y, et al. Expression balances of structural genes in shikimate and flavonoid biosynthesis cause a difference in proanthocyanidin accumulation in persimmon (Diospyros kaki Thunb.) fruit [J]. Planta, 2009, 230(5): 899-915. |
| [49] | Fotovvat M, Najafi F, Ali Khavari-Nejad R, et al. Investigating the simultaneous effect of chitosan and arbuscular mycorrhizal fungi on growth, phenolic compounds, PAL enzyme activity and lipid peroxidation in Salvia nemorosa L [J]. Plant Physiol Biochem, 2024, 210: 108617. |
| [50] | Heleno SA, Martins A, Queiroz MJRP, et al. Bioactivity of phenolic acids: metabolites versus parent compounds: a review [J]. Food Chem, 2015, 173: 501-513. |
| [51] | Liu CG, Wang XQ, Shulaev V, et al. A role for leucoanthocyanidin reductase in the extension of proanthocyanidins [J]. Nat Plants, 2016, 2: 16182. |
| [52] | Gomes CP, Bzainia A, Almeida A, et al. Chemical routes for the transformation of bio-monomers into polymers[M]//Plant Biomass Derived Materials: Sources, Extractions, and Applications. Weinheim: WILEY‐VCH, 2024: 329-361. |
| [53] | Wang H, Asker K, Zhan C, et al. Transcriptomic and metabolic analysis of fruit development and identification of genes involved in raffinose and hydrolysable tannin biosynthesis in walnuts [J]. J Agric Food Chem, 2021, 69(28): 8050-8062. |
| [54] | Yang QS, Chen XN, Li JJ, et al. HB26, a member of HD-Zip I subfamily, is involved in the regulation of hydrolysable tannin biosynthesis in the cupules of Quercus variabilis by transactivation of UGT84A13 [J]. Ind Crops Prod, 2023, 200: 116866. |
| [55] | Yang QS, Li JJ, Wang Y, et al. Genomic basis of the distinct biosynthesis of β-glucogallin, a biochemical marker for hydrolyzable tannin production, in three oak species [J]. New Phytol, 2024, 242(6): 2702-2718. |
| [56] | Wang ZH, Chen XX, Zhao Y, et al. A serine carboxypeptidase-like acyltransferase catalyzes consecutive four-step reactions of hydrolyzable tannin biosynthesis in Camellia oleifera [J]. Plant J, 2024, 119(3): 1299-1312. |
| [57] | Gonzalez A, Zhao MZ, Leavitt JM, et al. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings [J]. Plant J, 2008, 53(5): 814-827. |
| [58] | Zhou HP, He JX, Zhang YY, et al. RHA2b-mediated MYB30 degradation facilitates MYB75-regulated, sucrose-induced anthocyanin biosynthesis in Arabidopsis seedlings [J]. Plant Commun, 2024, 5(3): 100744. |
| [59] | Parker T, Bolt T, Williams T, et al. Seed color patterns in domesticated common bean are regulated by MYB-bHLH-WD40 transcription factors and temperature [J]. Plant J, 2024, 119(6): 2765-2781. |
| [60] | Verweij W, Spelt CE, Bliek M, et al. Functionally similar WRKY proteins regulate vacuolar acidification in Petunia and hair development in Arabidopsis [J]. Plant Cell, 2016, 28(3): 786-803. |
| [61] | Zhang YY, Pu YY, Zhang YM, et al. Tuber transcriptome analysis reveals a novel WRKY transcription factor StWRKY70 potentially involved in potato pigmentation [J]. Plant Physiol Biochem, 2024, 213: 108792. |
| [62] | Bi MM, Liang R, Wang JW, et al. Multifaceted roles of LhWRKY44 in promoting anthocyanin accumulation in Asiatic hybrid lilies (Lilium spp.) [J]. Hortic Res, 2023, 10(9): uhad167. |
| [63] | Chen SY, Wang SC. GLABRA2, A common regulator for epidermal cell fate determination and anthocyanin biosynthesis in Arabidopsis [J]. Int J Mol Sci, 2019, 20(20): 4997. |
| [64] | Watanabe Y, Otsuka Y, Hinata K, et al. Distribution of tannins in the leaves of Siebold’s beech (Fagus crenata) grown under different light regimes [J]. Acta Physiol Plant, 2022, 44(10): 100. |
| [65] | Huyskens-Keil S, Eichholz-Dündar I, Hassenberg K, et al. Impact of light quality (white, red, blue light and UV-C irradiation) on changes in anthocyanin content and dynamics of PAL and POD activities in apical and basal spear sections of white Asparagus after harvest [J]. Postharvest Biol Technol, 2020, 161: 111069. |
| [66] | Germ M, Stibilj V, Kreft S, et al. Flavonoid, tannin and hypericin concentrations in the leaves of St. John’s wort (Hypericum perforatum L.) are affected by UV-B radiation levels [J]. Food Chem, 2010, 122(3): 471-474. |
| [67] | Lees GL, Hinks CF, Suttill NH. Effect of high temperature on condensed tannin accumulation in leaf tissues of big trefoil (Lotus uliginosus Schkuhr) [J]. J Sci Food Agric, 1994, 65(4): 415-421. |
| [68] | Gouot JC, Smith JP, Holzapfel BP, et al. Impact of short temperature exposure of Vitis vinifera L. cv. Shiraz grapevine bunches on berry development, primary metabolism and tannin accumulation [J]. Environ Exp Bot, 2019, 168: 103866. |
| [69] | Ozfidan-Konakci C, Yildiztugay E, Yildiztugay A, et al. Cold stress in soybean (Glycine max L.) roots: exogenous gallic acid promotes water status and increases antioxidant activities [J]. Bot Serb, 2019, 43(1): 59-71. |
| [70] | Tharayil N, Suseela V, Triebwasser DJ, et al. Changes in the structural composition and reactivity of Acer rubrum leaf litter tannins exposed to warming and altered precipitation: climatic stress-induced tannins are more reactive [J]. New Phytol, 2011, 191(1): 132-145. |
| [71] | 任敏. 水分胁迫对达乌里胡枝子单宁和黄酮类物质的影响 [D]. 太谷: 山西农业大学, 2018. |
| Ren M. Efferts of wayer stress on tannin content and flavonoid content of Lespedeza davurica [D]. Taigu: Shanxi Agricultural University, 2018. | |
| [72] | Ma H, Xin CY, Xu YY, et al. Effect of salt stress on secondary metabolites of cotton and biological characteristics and detoxification enzyme activity of cotton spider mites [J]. Crop Prot, 2021, 141: 105498. |
| [73] | Chen H, Liu J, Cui K, et al. Molecular mechanisms of tannin accumulation in Rhus galls and genes involved in plant-insect interactions [J]. Sci Rep, 2018, 8(1): 9841. |
| [74] | Kumar S, Bhushan B, Wakchaure GC, et al. Plant phenolics under water-deficit conditions: biosynthesis, accumulation, and physiological roles in water stress alleviation [M]//Plant Phenolics in Sustainable Agriculture. Singapore: Springer Singapore, 2020: 451-465. |
| [75] | Sharma KP. Tannin degradation by phytopathogen's tannase: a plant’s defense perspective [J]. Biocatal Agric Biotechnol, 2019, 21: 101342. |
| [76] | Zhang XY, Bian ZH, Li S, et al. Comparative analysis of phenolic compound profiles, antioxidant capacities, and expressions of phenolic biosynthesis-related genes in soybean microgreens grown under different light spectra [J]. J Agric Food Chem, 2019, 67(49): 13577-13588. |
| [77] | Shao CY, Chen JJ, Lv ZD, et al. Staged and repeated drought-induced regulation of phenylpropanoid synthesis confers tolerance to a water deficit environment in Camellia sinensis [J]. Ind Crops Prod, 2023, 201: 116843. |
| [78] | Celeste Varela M, Arslan I, Reginato MA, et al. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina) [J]. Plant Physiol Biochem, 2016, 104: 81-91. |
| [79] | Dusenge ME, Duarte AG, Way DA. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration [J]. New Phytol, 2019, 221(1): 32-49. |
| [80] | Naikoo MI, Dar MI, Raghib F, et al. Role and regulation of plants phenolics in abiotic stress tolerance [M]//Plant Signaling Molecules. Amsterdam: Elsevier, 2019: 157-168. |
| [81] | Glaubitz U, Erban A, Kopka J, et al. High night temperature strongly impacts TCA cycle, amino acid and polyamine biosynthetic pathways in rice in a sensitivity-dependent manner [J]. J Exp Bot, 2015, 66(20): 6385-6397. |
| [82] | Barbehenn RV, Peter Constabel C. Tannins in plant-herbivore interactions [J]. Phytochemistry, 2011, 72(13): 1551-1565. |
| [83] | Ahmad P, Ahanger MA, Singh VP, et al. Plant metabolites and regulation under environmental stress [M]. Chantilly: Elsevier Science & Technology, 2018. |
| [84] | Kopper BJ, Jakobi VN, Osier TL, et al. Effects of paper birch condensed tannin on whitemarked tussock moth (Lepidoptera: Lymantriidae) performance [J]. Environ Entomol, 2002, 31(1): 10-14. |
| [85] | Ni BB, Liu H, Wang ZS, et al. A chromosome-scale genome of Rhus chinensis Mill. provides new insights into plant-insect interaction and gallotannins biosynthesis [J]. Plant J, 2024, 118(3): 766-786. |
| [86] | Elderd BD, Rehill BJ, Haynes KJ, et al. Induced plant defenses, host-pathogen interactions, and forest insect outbreaks [J]. Proc Natl Acad Sci USA, 2013, 110(37): 14978-14983. |
| [87] | Preminger M. Changes in bark chemistry across beech bark disease development [D]. New York : State University of New York, 2019. |
| [88] | Liebeke M, Strittmatter N, Fearn S, et al. Unique metabolites protect earthworms against plant polyphenols [J]. Nat Commun, 2015, 6: 7869. |
| [89] | Solaiman ZM, Senoo K. Arbuscular mycorrhizal fungus causes increased condensed tannins concentrations in shoots but decreased in roots of Lotus japonicus L [J]. Rhizosphere, 2018, 5: 32-37. |
| [1] | HUANG Xu-sheng, ZHOU Ya-li, CHAI Xu-dong, WEN Jing, WANG Ji-ping, JIA Xiao-yun, LI Run-zhi. Cloning of Plastidial PfLPAT1B Gene from Perilla frutescens and Its Functional Analysis in Oil Biosynthesis [J]. Biotechnology Bulletin, 2025, 41(7): 226-236. |
| [2] | ZAN Shu-wen, XIE Huan-huan, ZHANG Yu-qin, WANG Wen-Juan, ZHANG Peng-fei, LIANG Jin-jun, WEN Peng-fei. VvAGAMOUS Regulates Carpel Development through VvCRABS CLAW in Grape [J]. Biotechnology Bulletin, 2025, 41(5): 208-217. |
| [3] | WU Ya, YAO Run, YANG Han-ting, LIU Wei, YANG Shuai, SONG Chi, CHEN Shi-lin. Genome-wide Identification and Expression Analysis of SDR Gene Family in Mentha suaveolens ‘Variegata’ [J]. Biotechnology Bulletin, 2025, 41(5): 175-185. |
| [4] | LU Tian-yi, LI Ai-peng, FEI Qiang. Research Progress in the Biosynthesis of Polylactic Acid [J]. Biotechnology Bulletin, 2025, 41(4): 47-60. |
| [5] | LI Xiao-ming, SHANG Xiu-hua, WANG You-shuang, WU Zhi-hua. Research Progress in Benzoxazinoids in Plants [J]. Biotechnology Bulletin, 2025, 41(4): 9-20. |
| [6] | YU Ting, HUANG Dan-dan, ZHU Yan-hui, YANG Mei-hong, AI Ju, GAO Dong-li. Screening and Interaction Verification of Transcription Factors Stpatatin 05 Gene in Potato [J]. Biotechnology Bulletin, 2025, 41(3): 137-145. |
| [7] | NIE Zhu-xin, GUO Jin, QIAO Zi-yang, LI Wei-wei, ZHANG Xue-yan, LIU Chun-yang, WANG Jing. Transcriptome Analysis of the Anthocyanin Biosynthesis in the Fruit Development Processes of Lycium ruthenicum Murr. [J]. Biotechnology Bulletin, 2024, 40(8): 106-117. |
| [8] | MA Xiao-xiang, MA Ze-yuan, LIU Ya-yue, ZHOU Long-jian, HE Yi-fan, ZHANG Yi. Effects of Simulated Mutational Biosynthetic Regulation on the Secondary Metabolites of Aspergillus terreus C23-3 [J]. Biotechnology Bulletin, 2024, 40(8): 275-287. |
| [9] | SHEN Zhen-hui, CAO Yao, YANG Lin-lei, LUO Xiang-ying, ZI Ling-shan, LU Qing-qing, LI Rong-chun. Cloning and Bioinformatics Analysis of the Ergothioneine Biosynthesis Genes in Naematelia aurantialba and Stereum hirsutum [J]. Biotechnology Bulletin, 2024, 40(7): 259-272. |
| [10] | HE Yu-bing, FU Zhen-hao, LI Ren-han, LIU Xiu-xia, LIU Chun-li, YANG Yan-kun, LI Ye, BAI Zhong-hu. Efficient Biosynthesis of 2-Naphthaleneethanol in Metabolically Engineered Saccharomyces cerevisiae [J]. Biotechnology Bulletin, 2024, 40(7): 99-107. |
| [11] | HU Jin-jin, LI Su-zhen, MA Xu-hui, LIU Xiao-qing, XIE Shan-shan, JIANG Hai-yang, CHEN Ru-mei. Regulation of Maize Anthocyanin Biosynthesis Metabolism [J]. Biotechnology Bulletin, 2024, 40(6): 34-44. |
| [12] | HU Ya-dan, WU Guo-qiang, LIU Chen, WEI Ming. Roles of MYB Transcription Factor in Regulating the Responses of Plants to Stress [J]. Biotechnology Bulletin, 2024, 40(6): 5-22. |
| [13] | YANG Yan, HU Yang, LIU Ni-ru, YIN Lu, YANG Rui, WANG Peng-fei, MU Xiao-peng, ZHANG Shuai, CHENG Chun-zhen, ZHANG Jian-cheng. Cloning and Functional Analysis of MbbZIP43 Gene in ‘Hongmantang’ Red-flesh Apple [J]. Biotechnology Bulletin, 2024, 40(2): 146-159. |
| [14] | WANG Jun-fang, HUANG Qiu-bin, ZHANG Piao-dan, ZHANG Peng-pai. Structure and Biosynthesis of Surfactin as well as Its Role in Biological Control [J]. Biotechnology Bulletin, 2024, 40(1): 100-112. |
| [15] | CHEN Zhi-min, LI Cui, WEI Ji-tian, LI Xin-ran, LIU Yi, GUO Qiang. Research Progress in the Regulation of Chlorogenic Acid Biosynthesis and Its Application [J]. Biotechnology Bulletin, 2024, 40(1): 57-71. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||